Recently, we demonstrated a marked reduction in the expression of the thrombopoietin receptor, Mpl, in polycythemia vera (PV) platelets and megakaryocytes using an antiserum against the Mpl extracellular domain. To further examine this abnormality, we raised an antibody to the Mpl C-terminus. Immunologic analysis of PV platelets with this antiserum confirmed the reduction in Mpl expression. However, the C-terminal antiserum detected 2 forms of Mpl in PV platelets in contrast to normal platelets, in which a single form of Mpl was detected by both the extracellular domain and C-terminal antisera. Two-dimensional gel electrophoresis studies with isoelectric focusing in the first dimension identified normal platelet Mpl as an 85 to 92 kD protein with an isoelectric point (pI) of 5.5. PV platelets contained an additional 80 to 82 kD Mpl Mpl isoform with a pI of 6.5. Analysis of Mpl expressed by the human megakaryocytic cell line, Dami, showed 2 isoforms similar to those found in PV platelets suggesting a precursor-product relationship. Digestion of Dami cell and normal platelet lysates with neuraminidase converted the more acidic Mpl isoform to the more basic one, indicating that the 2 isoforms differed with respect to posttranslational glycosylation. Futhermore, in contrast to normal platelet Mpl, PV platelet Mpl was susceptible to endoglycosidase H digestion, indicating defective Mpl processing by PV megakaryocytes. The glycosylation defect was specific for Mpl, as 2 other platelet membrane glycoproteins, glycoprotein IIb and multimerin, were processed normally. Importantly, the extent of the PV platelet Mpl glycosylation defect correlated with disease duration and extramedullary hematopoiesis.
POLYCYTHEMIA VERA (PV) is a clonal disorder of unknown etiology, involving a multipotent hematopoietic progenitor cell that causes the accumulation of phenotypically normal red blood cells, white blood cells, and platelets and their precursors in the absence of a known physiologic stimulus.1 Although trilineage hyperplasia is the hallmark of PV, erythrocytosis is its most prominent clinical manifestation and, as a consequence, most investigations of PV have focused on erythropoiesis and in particular, on the erythropoietin receptor. To date, however, structural studies of the erythropoietin receptor gene, its protein expression, and ligand binding have been normal in PV.2-4 Most recently, PV erythroid progenitor cells cultured in vitro have been shown to be resistant to apoptosis in the absence of erythropoietin, a behavior that may be related to the hypersensitivity of these cells to other growth factors such as insulin-like growth factor-1 (IGF-1), interleu- kin-3 (IL-3), stem cell factor (SCF), and granulocyte-macrophage colony-stimulating factor (GM-CSF).5-9
Because neither acquired nor congenital abnormalities of the erythropoietin receptor can be implicated in the pathogenesis of PV and because the disorder involves all of the major hematopoietic cell lineages, if a receptor abnormality is involved, that receptor must initially be both expressed and active in primitive multipotent hematopoietic progenitor cells. The thrombopoietin receptor, Mpl, is such a candidate receptor.10 For example, thrombopoietin (TPO) or Mpl knock-out mice have reduced numbers of multilineage and committed hematopoietic progenitor cells, while TPO augments the proliferation of primitive hematopoietic stem cells in vitro.11,12 Importantly, forced expression studies of either TPO or Mpl recapitulate many clinical aspects of PV: overexpression of TPO in mice causes myelofibrosis and extramedullary hematopoiesis, while ectopic expression of Mpl induces a fatal erythroblastosis.13,14 Furthermore, a truncated Mpl gene is the oncogenic protein of the myeloproliferative leukemia virus (MPLV), which immortalizes hematopoietic progenitor cells in vitro and causes polycythemia when administered to mice in vivo.15,16Finally, TPO has been observed to prevent apoptosis of erythroid progenitor cells deprived of erythropoietin in vitro.17
Circulating platelets express a functional TPO receptor. To our surprise, in contrast to normal platelets, TPO failed to stimulate PV platelet protein tyrosine phosphorylation, a defect specific for PV and a related blood disorder, idiopathic myelofibrosis (IMF). Impaired TPO signaling in PV platelets was associated with a reduction in platelet and megakaryocyte Mpl expression as determined by immunologic analysis using an antiserum raised against the Mpl extracytoplasmic domain.18 The diminished function and expression of a hematopoietic cytokine receptor in patients with a disorder characterized by growth factor hypersensitivity and excessive production of hematopoietic cells was an unique and unexpected finding.
To further examine the defective expression of PV platelet Mpl, we raised an antiserum to the C-terminal domain of Mpl. We now report both quantitative and qualitative PV platelet Mpl abnormalities. In contrast to normal platelet Mpl, PV platelet Mpl is incompletely glycosylated. Furthermore, the degree of impairment of Mpl glycosylation correlated with disease duration and the extent of extramedullary hematopoiesis. This is the first report of a qualitative defect in a growth factor receptor in a myeloproliferative disorder.
MATERIALS AND METHODS
Antisera.
Rabbit polyclonal antiglycoprotein IIb was kindly supplied by Dr Paul Bray (Johns Hopkins University School of Medicine, Baltimore, MD). An affinity-purified, polyclonal rabbit IgG antibody to the soluble extracellular domain of human Mpl was a gift from the Kirin Brewery (Maebashi, Gunma, Japan). Monoclonal antimultimerin antiserum was provided by Dr Catherine Hayward (McMaster University, Ontario, Canada). Polyclonal rabbit antiserum was raised to the C-terminus of human Mpl after immunization with a KLH-linked peptide of the terminal 14 amino acids of full-length human Mpl (ANHSYLPLSYWQQP).
Subjects.
The study protocol was approved by our Joint Committee on Clinical Investigation and informed consent was obtained from each patient. The diagnosis of PV was based on the criteria of the Polycythemia Vera Study Group and included an elevated red blood cell mass. The diagnosis of the other chronic myeloproliferative disorders was based on standard clinical criteria.19
Reagents.
N-glycosidase F, neuraminidase, and endoglycosidase H were purchased from Boehringer-Manheim (Indianapolis, IN). All other reagents were purchased from Sigma (St Louis, MO).
Dami cell culture.
The human megakaryocytic cell line, Dami, was supplied by Dr Paul F. Bray. Dami cells were grown in Iscove’s modified Dulbecco’s medium containing 10% fetal bovine serum. Dami cell lysis buffer consisted of 10% glycerol, 1% Triton x-100, 20 mmol/L Tris pH 7.4, 75 mmol/L NaCl, 0.5 mmol/L EDTA, 50 mmol/L NaF, 1 mmol/L Na Vanadate, 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF), 20 ng/mL leupeptin, 20 ng/mL pepstatin, and 20 ng/mL aprotinin.
Platelet preparation.
Whole blood was drawn from healthy volunteers and patients after informed consent. Platelet-rich plasma, obtained after centrifugation at 12,000 rpm for 12 minutes, was centrifuged for 15 minutes at 2,000 rpm to form a platelet pellet. The platelets were washed 2 times with phosphate-buffered saline supplemented with 0.6% sodium citrate and 0.1% bovine serum albumin and then lysed in the Dami cell lysis buffer. Protein concentrations of the platelet lysates were obtained with the Pierce protein assay kit (Rockford, IL).
2D-gel electrophoresis.
For 2D-gel electrophoresis analysis, 25 μg of platelet lysate was applied to the IPGphor system (Amersham, Arlington Heights, IL) and isoelectric focusing was performed according to the manufacturer’s specifications (500 V for 1 hour, 1,000 V for 1 hour followed by 8,000 V for 2 hours). Second dimension separation was performed with 10% acrylamide sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were electroblotted to nitrocellulose membranes at 450 mA for 2 hours.
Glycosidase digestion.
A total of 25 μg of Dami cell lysate was incubated with 20 mU neuraminidase at 37°C for 1 hour. For both N-glycosidase F and endoglycosidase H digestions, 25 μg of platelet lysate were first denatured by boiling in SDS in the presence of a reducing agent, and then incubated for 18 hours at 30°C in 0.5 mol/L Tris-HCl pH 8.0 (N-glycosidase F, 5 U) or 0.5 mol/L sodium citrate pH 5.5 (endoglycosidase H, 2.5 mU).
Immunoblotting.
Immunoblotting protocols were performed as reported elsewhere.18 Dilutions for the antisera were as follows: 1:2,500 for the N-terminal antiserum, 1:5,000 for the C-terminal peptide antiserum, and 1:20,000 for the antiglycoprotein IIb.
RESULTS
PV Mpl expression is diminished in PV platelets.
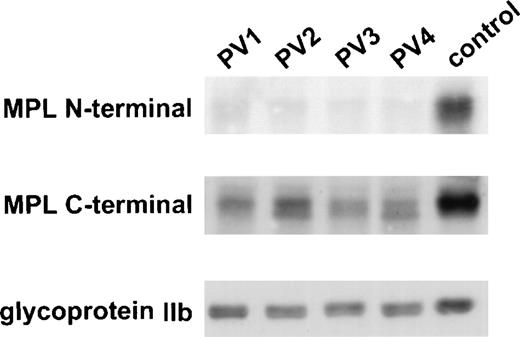
In a previous study, total and surface expression of Mpl appeared diminished in PV platelets as determined by 3 different assays: TPO-induced protein tyrosine phosphorylation, flow cytometry, and immunoblotting using an affinity-purified antiserum against the Mpl extracytoplasmic domain.18 21 To confirm these results, we examined the expression of Mpl in normal and PV platelet lysates by immunoblotting with a polyclonal antiserum raised against the 14 C-terminal amino acids of Mpl (ANHSYLPLSYWQQP). As shown in Fig 1, virtually no PV platelet Mpl was detected by immunoblotting using the extracytoplasmic domain Mpl antiserum. By contrast, Mpl was present in the lysates of all PV patients when the C-terminal Mpl antiserum was used. However, not only was the quantity of PV platelet Mpl still diminished, but 2 forms of Mpl were present in PV platelets.
C-terminal antiserum detects Mpl in PV platelets. PV and control platelet lysates were probed with an antiserum against the N-terminus and one against the C-terminus of Mpl. The membrane was reprobed with an antiserum against glycoprotein IIb to control for equal loading of platelet protein.
C-terminal antiserum detects Mpl in PV platelets. PV and control platelet lysates were probed with an antiserum against the N-terminus and one against the C-terminus of Mpl. The membrane was reprobed with an antiserum against glycoprotein IIb to control for equal loading of platelet protein.
PV platelets express 2 Mpl isoforms.
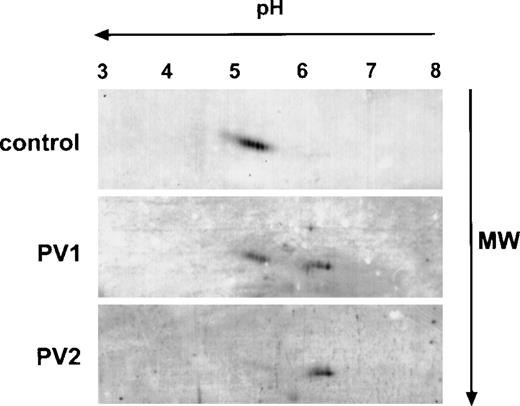
To further examine the differences in Mpl expression in normal and PV platelets, we analyzed Mpl in platelet lysates by 2-dimensional gel electrophoresis using isoelectric focusing in the first dimension and 10% SDS-PAGE in the second followed by immunoblotting with the Mpl C-terminal antiserum. As shown in Fig 2, Mpl from control platelets migrated with an apparent molecular mass of 85 to 92 kD and as a series of differently charged species with isoelectric points spanning a pH range between 5.1 and 5.5, presumably due to differences in sialation. In contrast, as suggested by 1-dimensional electrophoresis (Fig 1), 2 major Mpl isoforms were present in PV platelets. One, which we designated the A form, migrated in the same fashion as normal platelet Mpl, but was reduced in quantity; the second isoform, designated the B form, migrated with an apparent molecular mass of 80 to 82 kD and with isoelectric points ranging from 6.2 to 6.5. In some PV patients, we could detect only the B form (Fig 2, lowest panel).
Two-dimensional gel electrophoresis of PV and control platelet Mpl. A total of 25 μg of platelet lysate protein was subjected to isoelectric focusing in the first dimension (horizontal axis) followed by 10% acrylamide SDS-PAGE in the second dimension (vertical axis), transferred to a nitrocellulose membrane, and immunoblotted with the Mpl C-terminal peptide antiserum.
Two-dimensional gel electrophoresis of PV and control platelet Mpl. A total of 25 μg of platelet lysate protein was subjected to isoelectric focusing in the first dimension (horizontal axis) followed by 10% acrylamide SDS-PAGE in the second dimension (vertical axis), transferred to a nitrocellulose membrane, and immunoblotted with the Mpl C-terminal peptide antiserum.
Mpl isoforms represent differences in sialic acid content.
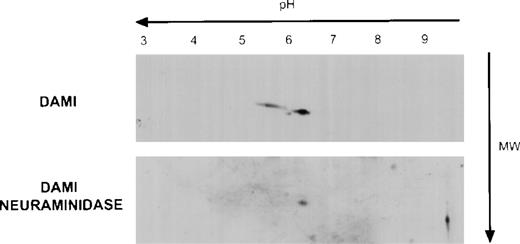
The extracellular domain of Mpl contains a reduplicated cytokine binding domain and 4 potential sites for asparagine-linked glycosylation.22 Because the theoretical molecular mass of Mpl based on its amino acid sequence is 68 kD, we postulated that the Mpl A and B isoforms differed with respect to their degree of glycosylation and, in particular, their sialic acid content. To evaluate this, because platelets do not synthesize protein efficiently, we first examined Mpl expression in the human megakaryocyte cell line, Dami. As shown in Fig 3, analysis of Dami cell Mpl expression by 2-dimensional gel electrophoresis showed 2 Mpl isoforms with both isoelectric points (pI) and apparent molecular masses similar to the A and B Mpl isoforms present in normal and PV platelet lysates, respectively. Furthermore, when Dami cell lysates or normal platelet lysates were digested with neuraminidase before 2-dimensional gel electrophoresis, only the B isoform was observed, suggesting that sialic acid accounts for the differences between the A and B forms. Additionally, in vitro translation of full-length Mpl cDNA in a system capable of core, but not complex glycosylation produced a protein identical in pI and molecular weight as the B form (data not shown) that was recognized by the Mpl C-terminal antiserum, but not the Mpl extracytoplasmic domain antiserum, indicating that the latter antiserum was sensitive to conformation or carbohydrate epitopes.
Mpl isoforms in Dami cell lysates. A total of 20 μg of Dami cell lysate protein was subjected to 2-dimensional gel electrophoresis and immunblotting with the Mpl C-terminal peptide antiserum as described in Fig 2.
Mpl isoforms in Dami cell lysates. A total of 20 μg of Dami cell lysate protein was subjected to 2-dimensional gel electrophoresis and immunblotting with the Mpl C-terminal peptide antiserum as described in Fig 2.
PV platelet Mpl is susceptible to digestion by endoglycosidase H.
The data cited above suggested that PV platelet Mpl was incompletely glycosylated and, in particular, lacked its full complement of sialic acid. Protein glycosylation in the endoplasmic reticulum is a cotranslational process. However, carbohydrate processing to create complex oligosaccharide side chains takes place in the Golgi apparatus where incompletely processed proteins can be distinguished by their sensitivity to digestion with endoglycosidase H.20Therefore, we examined the sensitivity of Mpl in normal and PV platelet lysates to endoglycosidases H and F. As shown in Fig 4, normal platelet Mpl was resistant to digestion with endoglycosidase H, while a portion of PV platelet Mpl was not. Both normal and PV platelet Mpl could be digested with N-glycosidase F, yielding products of identical molecular weight, indicating similarity of normal and PV platelet Mpl at the polypeptide level.
Specific and nonspecific endoglycosidase digestion of control and PV platelet lysates. Platelet lysates from a control and 3 different patients were untreated (lanes 1 to 4), digested with endoglycosidase H (lanes 5 to 8), or digested with N-glycosidase F (lanes 9 to 12), and subsequently probed with the Mpl C-terminal antiserum.
Specific and nonspecific endoglycosidase digestion of control and PV platelet lysates. Platelet lysates from a control and 3 different patients were untreated (lanes 1 to 4), digested with endoglycosidase H (lanes 5 to 8), or digested with N-glycosidase F (lanes 9 to 12), and subsequently probed with the Mpl C-terminal antiserum.
Expression of 2 other platelet membrane glycoproeins, GPIIb and multimerin, is normal in PV platelets.
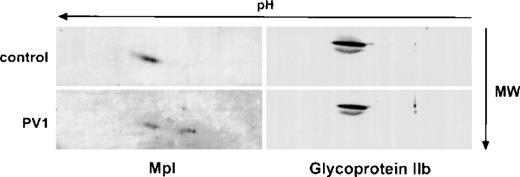
Studies with isolated Dami cell membranes (not shown) also indicated that the A isoform of Mpl was the predominant Mpl species present in the plasma membrane, which is consistent with the notion that the B isoform represents incompletely processed Mpl. To determine if defective posttranslational processing was specific for PV platelet Mpl, we examined the expression of 2 other platelet membrane glycoproteins, glycoprotein IIb and multimerin. These glycoproteins were chosen because we were unable to identify by immunoblotting in platelets expression of the cytokine receptor subunits gp130 and IL-3 beta, which share homology with Mpl. As shown in Fig 5, based on 2-dimensional gel electrophoresis, qualitative and quantitative analysis of glycoprotein IIb was identical in normal and PV platelets. Similar results (not shown) were obtained with multimerin.
Two-dimensional electrophoresis analysis of glycoprotein IIb in control and PV platelets. Platelet lysates from a control (upper panels) and a PV patient (lower panels) were analyzed by 2-dimensional gel electrophoresis and immunoblotted with the Mpl C-terminal antiserum (left) or antiserum to glycoprotein IIb (right).
Two-dimensional electrophoresis analysis of glycoprotein IIb in control and PV platelets. Platelet lysates from a control (upper panels) and a PV patient (lower panels) were analyzed by 2-dimensional gel electrophoresis and immunoblotted with the Mpl C-terminal antiserum (left) or antiserum to glycoprotein IIb (right).
Expression of the PV platelet Mpl B isoform correlates with disease progression.
To understand the significance of the Mpl B isoform in PV platelets, we performed 2-dimensional gel electrophoresis and immunoblotting using the C-terminal Mpl antiserum on platelet lysates from 24 PV patients who fulfilled the diagnostic criteria of the Polycythemia Vera Study Group.23 These studies yielded the following results: first, as we previously reported using the Mpl extracytoplasmic domain antiserum and as shown in Fig 1 for 3 of the patients, all 24 patients had reduced amounts of platelet Mpl. Second, 4 patterns of PV platelet Mpl expression emerged: the presence of the A isoform alone (but diminished in total amount compared with normal), equal quantities of the A and B isoforms, a lesser quantity of A compared with B, or B only. Third, as shown in Table 1, the 4 isoform patterns segregated according to disease duration and degree of splenomegaly, which is a measure of extramedullary hematopoiesis.24 Thus, in the patients with a relatively short disease duration from the time of diagnosis, only the A isoform was present. In the patients with either extended disease duration and/or the development of clinically evident splenomegaly, the B isoform was predominant.
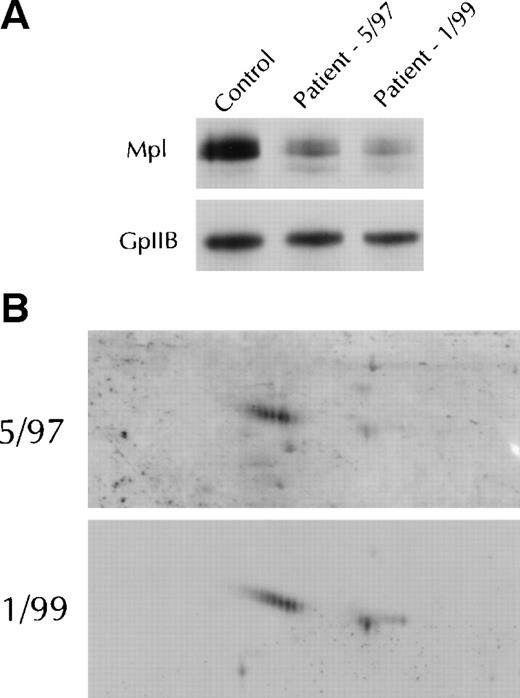
Study of 3 PV patients over time supported the contention that Mpl isoform expression was a function of disease progression. As shown in Fig 6 for a representative patient, by 1-and 2-dimensional gel electrophoresis and immunoblotting, there was a reduction of platelet Mpl expression and an evolution from primarily the A isoform at diagnosis to emergence of the B isoform 20 months after diagnosis, with phlebotomy as the only therapy.
Mpl isoform evolution in a single patient. (A) Immunoblots after 1-dimensional electrophoresis of patient platelet lysate obtained at diagnosis in May 1997 and again in January 1999 were probed with the Mpl C-terminal antiserum. The immunoblot was reprobed with the glycoprotein IIb antiserum to verify equal protein loading. (B) Two-dimensional gel electrophoresis of the platelet lysates shown in (A) were probed with the C-terminal antiserum.
Mpl isoform evolution in a single patient. (A) Immunoblots after 1-dimensional electrophoresis of patient platelet lysate obtained at diagnosis in May 1997 and again in January 1999 were probed with the Mpl C-terminal antiserum. The immunoblot was reprobed with the glycoprotein IIb antiserum to verify equal protein loading. (B) Two-dimensional gel electrophoresis of the platelet lysates shown in (A) were probed with the C-terminal antiserum.
The observed pattern of Mpl isoform expression was independent of white blood cell or platelet count, concurrent therapy, previous therapy, splenectomy, or iron status. As controls for this study, we examined 5 patients with essential thrombocytosis, 5 with secondary erythrocytosis, 1 with sideroblastic anemia, 1 with chronic myelogenous leukemia (CML), 1 with thrombocytosis due to iron deficiency, and 6 patients with hemochromatosis undergoing regular phlebotomy. All 19 of these patients had normal Mpl isoform expression as assessed by 2-dimensional gel electrophoresis (data not shown).
DISCUSSION
Polycythemia vera is classified as a chronic myeloproliferative disorder together with IMF, essential thrombocytosis (ET), and CML because it involves a multipotent hematopoietic progenitor cell with overproduction of 1 or more of the formed elements of the blood. CML is unique among the chronic myeloproliferative disorders, as it alone has an invariant clonal marker, a reciprocal translocation of the distal portions of chromosomes 9 and 22. The major consequence of this translocation is a constitutively-active, nonreceptor tyrosine kinase fusion protein, bcr-abl. Experimentally, expression of bcr-abl in multipotent hematopoietic progenitor cells in vitro mimics many of the features of PV progenitor cells cultured in vitro such as growth factor hypersensitivity and resistance to apoptosis after growth factor deprivation.25 However, clinically CML differs greatly from PV, IMF, and ET. First, its duration is measured in years, while their durations are measured in decades. Second, malignant transformation is the inevitable consequence of untreated CML, while marrow failure or acute leukemia are far less common in PV, IMF, and ET, as long as mutagenic agents are not used in their treatment.19
PV erythroid progenitor cells cultured in vitro are characteristically hypersensitive to a variety of growth factors and are capable of surviving in the absence of erythropoietin. PV erythroid progenitor cells are hypersensitive to IGF-1, an antiapoptotic factor, and the IGF-1 receptor is constitutively phosphorylated in PV peripheral blood mononuclear cells.6,26 Because erythroid progenitor cells expressing a hypofunctional erythropoietin receptor are also hypersensitive to IGF-1,27 it is interesting to speculate that a similar situation might exist in PV, but involving a receptor more globally expressed than the erythropoietin receptor.10 12
Mpl was such a candidate given the fact that it can function as an oncogene by immortalizing multipotent hematopoietic progenitor cells and rendering them growth factor-independent.16 In contrast to CML platelets, there was no constitutive protein tyrosine phosphorylation in PV platelets.18,28 Indeed, even after exposure to TPO, there was no increase in protein tyrosine phosphorylation in PV platelets, although the machinery for this was intact. Lack of responsiveness to TPO with respect to tyrosine phosphorylation and enhancement of adenosine diphosphate (ADP)-induced platelet aggregation as well as the inability to cross-link TPO to PV platelets suggested an abnormality in Mpl.29 Using an antiserum raised against the extracytoplasmic domain of Mpl, we were not only unable to identify surface expression of Mpl in PV platelets by flow cytometry, but total platelet Mpl was severely diminished as well, as determined by immunoblotting.
Using a C-terminal Mpl antiserum, we now demonstrate that Mpl expression in PV platelets was not only reduced compared with normal platelets, but that its posttranslational processing was abnormal as well. Failure of the Mpl extracellular domain antiserum to recognize the in vitro translation product of Mpl (which lacks posttranslational carbohydrate modifications, 4 sites of which are present in the Mpl extracytoplasmic domain) suggested that this antiserum was conformation or epitope dependent. Recognition of the Mpl B form in PV platelets by our C-terminal antiserum, but not the Mpl extracytoplasmic domain antiserum, further localized the PV Mpl abnormality to the Mpl extracytoplasmic domain. With respect to specificity, the processing defect appeared to be limited to Mpl, as 2 other platelet membrane glycoproteins, glycoprotein IIb and multimerin, were processed normally, while the erythropoietin and stem cell factor receptors were demonstrated to be intact by others.2,8 Incomplete Mpl glycoslyation suggested that its transport from the Golgi apparatus was impaired and was consonant with our observation of reduced Mpl surface expression in PV platelets.29 Importantly, this defect was exaggerated with disease duration from the time of diagnosis and extent, suggesting that it may be a useful marker of disease activity.
A hematopoietic growth factor receptor abnormality is a natural candidate for oncogenic transformation due to the effects of these receptors on cell proliferation, differentiation, and apoptosis. Two observations illustrate how an acquired abnormality of a growth factor receptor might participate in human cancer. First, in many cases of the highly malignant brain tumor, glioblastoma, the epidermal growth factor receptor (EGFR) gene is amplified and undergoes intragenic deletions and rearrangements that result in the expression of a receptor protein lacking a portion of its extracellular domain.30,31 The mutant EGFR does not bind EGF and its tyrosine kinase domain is constitutively active. Interestingly, the altered EGFR has been shown to localize to the endoplasmic reticulum and to have a prolonged half-life compared with its normal counterpart, a property that may contribute to aberrant signaling and oncogenesis.32 Second, the development of acute myelogenous leukemia in patients with severe congenital neutropenia has been associated with acquired mutations in the granulocyte colony-stimulating factor receptor (G-CSFR).33 Recently, mutations of the G-CSFR that truncate its C-terminal domain have been shown to result in impaired ligand internalization, defective receptor downmodulation, enhanced cell proliferation, and impaired differentiation.34 35Additionally, such mutant receptors have been observed to have a dominant-negative effect on the function of the normal G-CSFR.
The observation that an essential multipotent hematopoietic growth factor receptor was hypofunctional in a chronic myeloproliferative disease whose phenotype is overproduction of hematopoietic cells was unexpected. An important feature of our observations is that the Mpl defect was unique to PV and a closely related disorder, IMF. In fact, so selective was its expression that the Mpl abnormality can be used to distinguish PV from other forms of erythrocytosis and thus avoid exposure of patients with a benign disorder to potentially mutagenic therapy.
Our results in ET patients are in direct opposition to the report of Horikawa et al,36 who found decreased platelet Mpl in some patients carrying this diagnosis. A difficulty in any study of ET is the identification of authentic ET patients, due to the lack of positive identifiers of this elusive disorder. Furthermore, isolated thrombocytosis may be the first manifestation of PV, IMF, or CML. We can only state that the patients we classify as ET have been extensively studied, followed on average for 8 years, and all other potential causes of an elevated platelet count have been excluded. Recently, Taskin et al37 also found no abnormalities of the Mpl gene or impaired responsiveness to thrombopoietin in 9 ET patients. Finally, it has recently been demonstrated that many patients considered to have ET actually have a nonclonal disorder, and in some families with inherited thrombocytosis, an abnormality of the TPO gene has been demonstrated.38 39
Impairment of Mpl glycosylation is the first molecular defect identified in PV. Because Mpl expression is impaired in PV megakaryocytes and there is no evidence that platelets can either synthesize or metabolize Mpl, PV platelets appear to represent a “fossil” record of an abnormality in progenitor cell Mpl expression. Thus, the progressive increase in expression of underglycosylated Mpl could represent either increasing severity of the molecular defect or the selection or evolution of clones with a more severe Mpl defect, possibly providing them with a survival advantage. To date, we have not identified gross structural abnormalities of the Mpl gene or its transcripts by Southern or Northern analysis. Although the mechanism and relevance to the pathogenesis of PV are still undefined, a unique molecular abnormality, defective Mpl glycosylation, is now available for use in the diagnosis, staging, and evaluation of specific therapies in PV patients.
ACKNOWLEDGMENT
The excellent technical assistance of Evelyn Connor and Mary Ann Isaacs is acknowledged with gratitude. We thank W.D. Hankins and Donna Williams for helpful discussions and our colleagues in the Division of Hematology, Drs William R. Bell, Paul F. Bray, Chi Van Dang, Lawrence B. Gardner, and Michael B. Streiff, and the physicians of the Johns Hopkins Oncology Center for referring patients for this study. We also acknowledge the unflagging support from the staff of the Johns Hopkins Hospital Blood Bank. We gratefully acknowledge contributions from the Myeloproliferative Disorders Support Group.
Supported by National Institutes of Health (NIH) Grant No. 58589 from the National Heart, Lung and Blood Institute (to J.L.S.) and a Doris Duke Clinical Scientist Award (to A.R.M.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jerry L. Spivak, MD, Traylor Building, Room 924, 720 Rutland Ave, Baltimore, MD 21205.