CD16/CD30 bispecific monoclonal antibodies can induce remissions of Hodgkin’s disease refractory to chemo- and radiotherapy. However, the development of human antimouse immunoglobulin antibodies and allergic reactions precludes repeated applications of the antibody. Moreover, problems of producing and purifying sufficient amounts of material limit the clinical practicability of this novel treatment approach. To overcome these obstacles, we have constructed a bispecific antibody in a diabody form that only employs the variable domains of the CD16/CD30 hybrid hybridoma. The diabody compared favorably with the parent CD16/CD30 bispecific antibody in its ability to activate and target natural killer cells in vitro. Its administration to mice bearing xenografted Hodgkin’s lymphoma resulted in a marked regression of tumor growth, thus proving for the first time the capability of a diabody for immune recruitment in vivo. The CD16/CD30 diabody is a novel reagent that should considerably facilitate the immunotherapy of patients with refractory Hodgkin’s lymphoma.
NATURAL KILLER (NK) cells represent a potent subset of lymphocytes for targeting and lysing tumor cells. In contrast to T lymphocytes, they do not need to be preactivated in vitro because they constitutively express cytolytic functions against a number of different targets.1,2 Their inherent cytolytic activity can be stimulated via the FcγIIIA receptor (CD16), which is expressed on the surface of NK cells, macrophages, and activated monocytes.3 4 Bispecific antibodies binding to both CD16 and a tumor-associated antigen are therefore of great interest as potential reagents for cancer immunotherapy.
To target NK cells against Hodgkin’s disease (HD), a mouse hybrid hybridoma bispecific monoclonal antibody (biMoAb) was constructed with specificities for CD16 and CD30.5 CD30 is expressed on virtually all Hodgkin and Reed-Sternberg cells and on only a small proportion of activated lymphocytes.6 The antibody was able to induce the specific lysis of CD30+ tumor cells in vitro. Administration of the bispecific antibody in a severe-combined immunodeficiency (SCID) mouse model resulted in the complete remission of subcutaneously established tumors after one single injection.5 More recently, the biMoAb was used to treat 15 patients with refractory HD in a phase-I/II trial with promising results.7 However, human antimouse immunoglobulin (Ig) antibodies were found in a total of 9 patients and 4 patients developed an allergic reaction after attempted retreatment.
In addition to the problem of immunogenicity, hybrid hybridoma biMoAb are extremely difficult to purify as a homogenous molecule from the large number of similar molecules generated by the random L-H and H-H associations. We therefore decided to construct a bispecific antibody comprising only the variable domains of the hybrid hybridoma. Such bispecific molecules, for example, have been constructed by joining two single-chain Fv fragments (scFv) with a polypeptide linker.8,9 Alternatively, a structurally more compact bispecific heterodimer known as a diabody can be made by the noncovalent association of two fusion proteins comprising the VH domain of one antibody connected by a short linker to the VL domain of another antibody.10-13 Their relatively small size should facilitate a better penetration of tumor tissue. Crystallographic studies have shown that the two antigen-binding domains of diabodies are on opposite sides of the complex, such that they are able to bind two cells.14
We chose to construct an anti-CD30/anti-CD16 diabody rather than a single-chain bispecific scFv [(scFv)2], since in our experience, diabodies are produced in higher amounts in bacteria and appear to be functionally superior. This diabody was able to specifically induce the lysis of a CD30+ Hodgkin’s lymphoma–derived cell line by NK cells. Furthermore, after administration to mice bearing xenografted Hodgkin’s lymphoma, it induced a regression of tumor growth with an efficacy comparable to that of the parent hybrid hybridoma. To our knowledge, this is the first time that the immune-recruiting capacity of a bispecific diabody on tumor growth in an animal model system has been shown.
MATERIALS AND METHODS
Plasmids, antibodies, and cell lines.
The plasmid pHOG2115 was used for expressing scFvs and the plasmid pKID16 was used for expressing the diabody. DNA coding for the anti-CD30 scFv fragment was kindly provided by A. Hombach, (Medical Clinic I, University of Cologne, Germany).17 Hybridoma cell lines producing the anti-CD16 MoAb A9 (IgG1λ) and the anti-CD30 MoAb HRS-3 (IgG1κ) have been described previously.5,18,19 The bispecific monoclonal IgG1 antibody HRS-3/A9 had been previously produced and purified under good manufacturing practice (GMP) conditions by Biotest Pharma GmbH (Dreieich, Germany) and comprised at least 95% intact biMoAb. The CD30+ human Hodgkin’s disease–derived cell line L540CY has been described by von Kalle et al.20 The SW480 cell line (ATCC-No. CCL 228) was used as a control cell line in flow cytometric analyses and cytotoxicity assays. All hybridomas and cell lines were cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 μg/mL streptomycin, and 100 IU/mL penicillin.
Cell preparations.
CD16+ granulocytes were freshly prepared from the blood of healthy donors by centrifugation on a discontinuous density gradient of Histopaque-1077 and Histopaque-1119 (Sigma Diagnostics, St Louis, MO) at 700g for 30 minutes at room temperature without braking. The second opaque layer containing the granulocytes was aspirated and washed three times with phosphate-buffered saline (PBS). Peripheral blood lymphocytes (PBL) containing about 10% NK cells were isolated from healthy donors (Bloodbank, Saarland University, Homburg, Germany) by Ficoll Hypaque gradient centrifugation followed by plastic adherence to remove the monocytes. To determine the percentage of NK cells, the PBL were stained with a phycoerythrin-labeled anti-CD3 MoAb and with fluorescein isothiocyanate (FITC)-labeled anti-CD56 and anti-CD16 MoAb and analyzed by flow cytometry.
Construction of the anti-CD16 anti-CD30 diabody.
The complementary DNA (cDNA) sequence of the heavy- and light-chain variable domains of the anti-CD16 MoAb A9 were amplified by polymerase chain reaction (PCR) with individually designed 5′ consensus primers and 3′ primers annealing to the Cλ region of the light chain and CH1 region of the heavy chain, respectively. The following oligonucleotides containing appropriate restriction sites for directional cloning were used: VH5′, 5′ CAGCCGGCCATGGCGCAGGTC(G)CAGCTGCAGC(G)AG3′ (NcoI); VH3′, 5′ CCAGGGGCCAGTGGATAGACAAGCTTGGGTGTTGTTTT3′ (HindIII); VL5′, 5′ AGAGACGCGTACAGGCTGTTGTGACTCAGG3′ (MluI); VL3′, 5′ GACTGCGGCCGCAGACTTGGGCTGGCC3′ (NotI). The restriction sites are underlined and named in brackets. The PCR was performed as follows: 1 cycle, 5-minute denaturation at 94°C, 3-minute hybridization at 58°C, and 2-minute extension at 72°C, followed by 30 cycle, 80 seconds at 94°C, 80 seconds at 58°C, 2 minutes at 72°C, and 10-minute extension in the final circle. The PCR products were gel purified and ligated into the pCR-Script SK(+) vector (Stratagene, La Jolla, CA) for didesoxy-sequencing.21 For soluble scFv expression, the heavy chain was digested with NcoI/HindIII and the light chain was digested with MluI/NotI and successively ligated to the linearized vector pHOG 21 15.
The heavy and the light chain of the anti CD30 scFv fragment were extended at the ends of their framework 4 regions by PCR using primers encoding the first five amino acids of the CH1-domain and the Cκ-domain, respectively, and vector-compatible restriction sites. The PCR primers used for the heavy chain were 5′ATGACCATGATTACGCCAAGC3′ and 5′ AGACAAGCTTGGGTGTTGTTTTGGCTGAGGAGACGG3′ (HindIII); for the light-chain 5′ GGCGGATATCGAGCTCACTCAGTCTCC3′ (EcoRV) and 5′ TATAGCGGCCGCAGCATCAGCCCGTTTGATTTCC3′ (NotI). The restriction sites are underlined and named in brackets. The heavy- and the light-chain variable domains of the anti-CD30 scFv fragment and anti-CD16 scFv fragment were cloned into the diabody expression plasmid pKID for cosecretion of the fusionproteins VH16-VL30 and VH30-VL16 as previously described for the construction of an anti-CD19/anti-CD3 diabody.16 Their sequences were verified by the didesoxynucleotide method.21
Expression and purification of scFv fragments and diabody.
The Escherichia coli K12 strain XL1-Blue (Stratagene) transformed with the scFv expression plasmid pHOG21 or the diabody expression plasmid pKID16-30 were grown overnight in 2YT medium (270 mmol/L NaCl, 1% Yeast Extract, 0.5% Tryptone, pH 7.0) containing 100 μg/mL ampicillin and 100 mmol/L glucose (2YTGA) at 37°C. The overnight cultures were diluted 1:20 in 2YTGAand grown as flask cultures at 37°C with shaking at 280 rpm. After reaching OD600 = 0.8 the bacteria were pelleted by centrifugation at 1500g for 10 minutes and 20°C and resuspended in the same volume of fresh 2YT medium containing 100 μg/mL ampicillin, 0.4 mol/L sucrose and 0.2 mol/L IPTG. Induction was performed at 21°C for 18 to 20 hours. Soluble scFv fragments and the diabody were prepared as previously described.22Briefly, for isolation of the scFv fragments, the culture supernatant and the soluble periplasmatic extract were combined and concentrated using Amicon YM10 membranes with a 10 kD cutoff (Amicon, Witten, Germany) followed by thorough dialysis against 50 mmol/L Tris-HCl, 1 mol/L NaCl, pH 7.0. The diabody was concentrated by ammonium sulfate precipitation with a final 70% concentration of saturation. The protein precipitate was collected by centrifugation (30,000g, 4°C, 45 minutes) and dissolved in 1/10 of the initial volume of 50 mmol/L Tris-HCl, 1 mol/L NaCl, pH 7.0. Both, the scFv fragments and the diabody were purified by immobilized metal affinity chromatography (IMAC) as previously described.23 The purified antibody preparations were dialyzed against PBS.
Flow cytometry.
Flow cytometric analyses using a FACScan (Becton Dickinson, Heidelberg, Germany) to confirm the binding ability of anti-CD16 and anti-CD30 scFv and the anti-CD16/anti-CD30 Diabody to CD16-positive granulocytes and CD30-positive L540Cy cells were performed as follows: 1 × 106 target cells were washed twice in ice-cold PBS-N (PBS, 0.05 % NaN3) and incubated with 100 μL of a sample containing the scFv fragment or the diabody for 45 minutes at 4°C. Cells were pelleted at 1200 rpm at 4°C for 5 minutes and washed with 2 mL PBS-N. For detection the cells were resuspended in 100 μL PBS-N containing the 9E10 antibody (10 μg/mL; ICI Chemikalien, Ismaning, Germany) that binds to the c-myc tag and incubated for 30 minutes at 4°C. Cells were pelleted and washed as above. Finally, the cells were resuspended with fluorescein-labeled goat antimouse IgG (GIBCO BRL, Gaithersburg, MD) 1:100 in PBS-N and incubated for 30 minutes at 4°C in the dark. After washing again with PBS-N, the cells were prepared for analysis with PBS-N containing 1 μg/mL propidium iodide (Sigma, Deisenhofen, Germany) to exclude dead cells. Background fluorescence was determined by using target cells incubated with 9E10 antibody and FITC-labeled goat antimouse antibody under the same conditions.
Cytotoxicity assays.
The cytotoxicity assay was performed according to the JAM-Test method described by Matzinger.24 The assay measures DNA fragmentation and is performed similarly to a standard 51Cr release assay. Target cells were labeled with3H thymidine to a final concentration of 2.5 to 5 μCi/mL for 4 to 6 hours. They were then pelleted, washed once with culture medium, and distributed in triplicates (104 cells per well) into 96-well round-bottomed microwell plates. After adding effector cells in serial dilutions, the plates were incubated in a humidified atmosphere at 5% CO2 for 4 hours. The cells and medium were than aspirated onto fiber glass filters using a cell harvester (Betaplate; Amersham Pharmacia Biotech Europe GmBH, Freiburg, Germany). After washing and drying the filters, they were placed in vials containing liquid scintillation fluid and counted using a liquid scintillation counter (LKB, Wallach, Freiburg, Germany). The radioactivity measured corresponds to intact DNA because DNA from dead cells is degraded into small fragments that pass through the filter. For calculating % specific killing, the standard formula for the JAM test is: % specific killing = (S-E)/S 100, with E = experimentally retained DNA in the presence of effector cells (in cpm) and S = retained DNA in the absence of effector cells (spontaneous).
Animals.
Pathogen-free female mice with SCID (C.B-17 lcr scid/scid) were obtained from Charles River (Sulzfeld, Germany). The SCID mice were maintained under pathogen-free conditions and fed autoclaved standard chow and water. Their functional lymphocyte deficiency was tested by quantifiying the serum immunglobulin levels with the enzyme-linked immunosorbent assay (ELISA) technique using the protocol of IFFA Credo (L’Arbresle, France). Briefly, the sera of the mice were diluted 1:100 with PBS-TB (PBS, 0.05% Tween20, 1% BSA) and incubated at 37°C for 90 minutes on ELISA plates precoated with rabbit antimouse antibody (DAKO, Glostrup, Denmark). A standard curve was constructed using a purified mouse IgG (ICN, Eschwege, Germany) serial diluted in PBS-TB. In addition, the serum of a BALB/c mouse (1:40,000 with PBS-TB) was taken as a positive control. After extensive washing with PBS-T (PBS, 0.05% Tween) murine antibodies were detected with goat antimouse IgG+A+M antibodies conjugated to alkaline phosphatase (1:1,500; Zymed, San Francisco, CA). Animals with antibody titers exceeding 0.05 mg/mL were excluded from the experiment. To eradicate residual NK cells, 0.1 mL of antiasialo-GM1 antibody solution (Wako, Osaka, Japan) was administered intraperitoneally starting 3 days before tumor-cell grafting and every 5 days until day 17 after tumor-cell injection.
Treatment of tumor-bearing SCID mice.
Solid L540CY Hodgkin’s-derived tumors were established in SCID mice as previously described.5,25 Briefly, exponentially growing L540CY cells (1.5 × 107) resuspended in 200 μL PBS were injected subcutaneously into the right flank with a 26-gauge needle. Tumor development was measured with vernier calipers every 3 days. Animals with established tumors of 4 to 6 mm in diameter were divided randomly into different groups and received various antibody preparations and 1 × 107 monocyte-depleted human peripheral blood lymphocytes in 200 μL PBS intravenously through the tail vein. The tumor volume was calculated as follows: Volume = d2 × D × π /6, with d as the smaller and D as the larger diameter.19 Animals were killed when the tumor size exceeded 1 cm in diameter, corresponding to a tumor volume of approximately 0.4 to 0.6 cm3. The data on tumor volumes and survival times was evaluated using several widely-used statistical methods. The result of a statistical test assumed to be statistically significant if the P value was smaller or equal to 5%.
RESULTS
Cloning and expression of a functional anti-CD16 scFv.
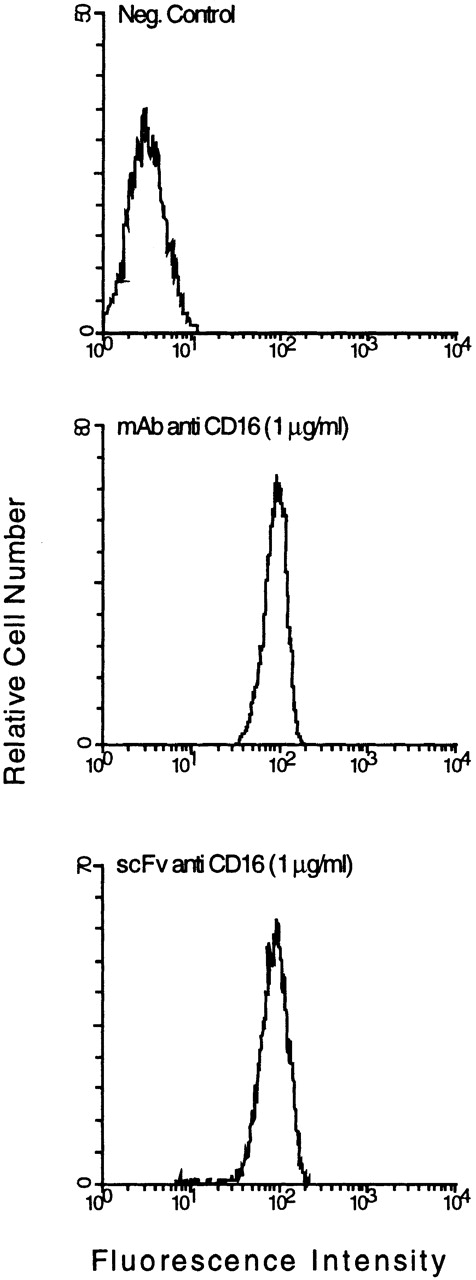
A FACScan analysis of the cloned anti-CD16 scFv produced in E. coli showed minimal binding on CD16+ granulocytes (data not shown). We therefore looked for possible errors by comparing the sequence of the heavy- and light-chain variable domains with sequences of other antibodies within the same subgroup. One marked difference was the presence of a glutamic acid residue at position 6 of the VH domain, which is invariably glutamine in the corresponding VHIIB subgroup. We therefore exchanged this residue for glutamine by site-directed mutagenesis. After expression inE. coli and purification of the soluble scFv from the periplasmic fraction by metal-chelate chromatography, a FACScan analysis showed very little difference in binding of the scFv to CD16+ granulocytes compared with the parental MoAb (Fig1). The cloned anti-CD30 scFv was produced and purified in a similar fashion to that of the anti-CD16 scFv. It bound well to CD30+ cell lines K562 and L540CY in FACScan analysis (data not shown).
FACS analysis of anti-CD16 scFv binding to CD16+ granulocytes. The granulocytes were incubated with 1 μg of parental MoAb A9 or 1 μg purified anti-CD16 scFv. The scFv binding was detected with the anti–c-myc MoAb 9E10 and fluorescein-conjugated goat antimouse IgG. A CD16− cell line (SW 480) was used as a negative control.
FACS analysis of anti-CD16 scFv binding to CD16+ granulocytes. The granulocytes were incubated with 1 μg of parental MoAb A9 or 1 μg purified anti-CD16 scFv. The scFv binding was detected with the anti–c-myc MoAb 9E10 and fluorescein-conjugated goat antimouse IgG. A CD16− cell line (SW 480) was used as a negative control.
Expression and purification of a CD16/CD30 diabody.
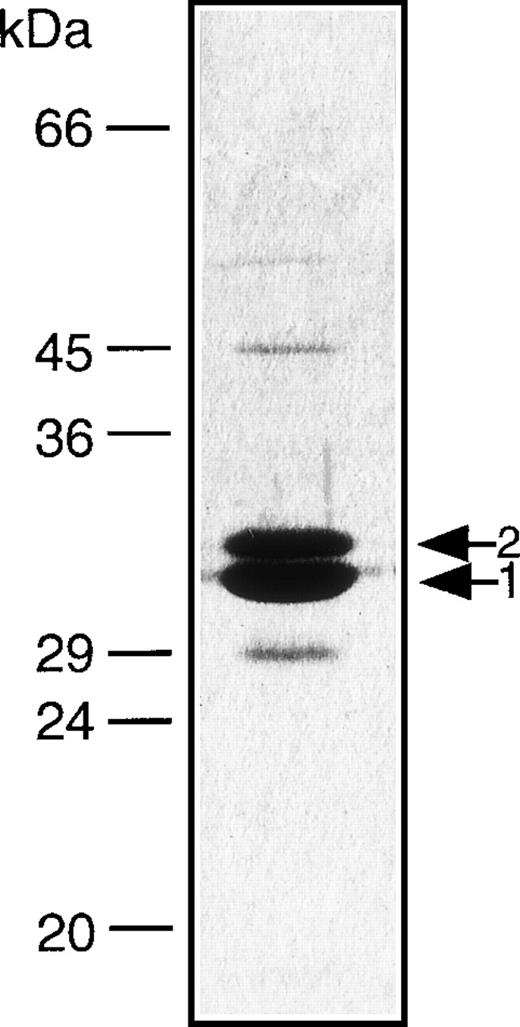
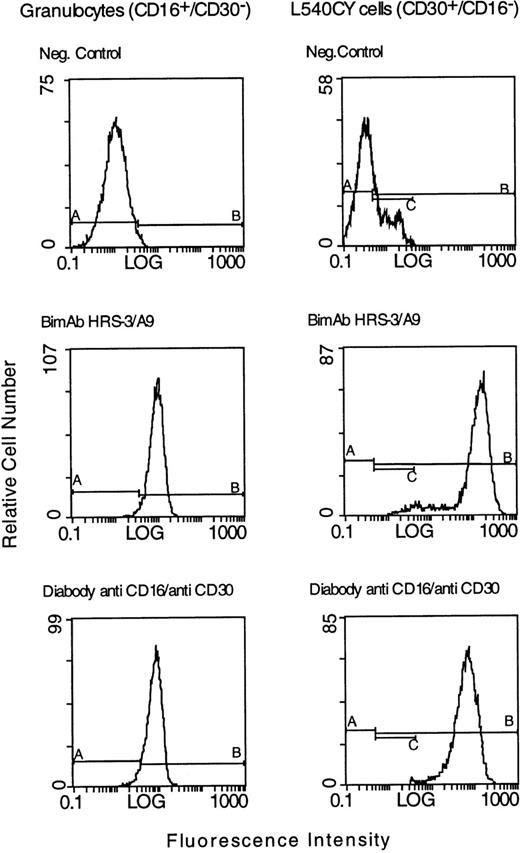
DNA coding for each of the variable domains of the anti-CD16 and anti-CD30 scFv was inserted into a vector that we previously used to express an anti-CD19/anti-CD3 diabody.16 The cloned diabody was expressed in E. coli and isolated according to the procedure we previously described.23 Approximately 3 mg/L of soluble diabody was isolated from the periplasmic fraction of E. coli. After purification by metal-chelate chromatography and gel-exclusion chromatography yields of 300 μg to 500 μg of functional diabody were obtained. It appeared to be fairly pure and contained only minor impurities as shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 12% gels (Fig 2). To test its binding properties, we made a FACScan analysis after incubation of the diabody with CD16+ granulocytes and the HD-derived tumor cell line L540CY. Its binding to both cell types was comparable to that of the parental biMoAb (Fig 3). No binding was observed to the irrelevant human colorectal cell line SW480 (data not shown).
Gel electrophoresis of the CD16/CD30 diabody. The diabody was expressed in E. coli, purified by IMAC and analyzed on 12% SDS-polyacrylamide gels. The protein bands were stained with Coomassie Blue. Band 1: VH16-VL30; Band 2: VH30-VL16.
Gel electrophoresis of the CD16/CD30 diabody. The diabody was expressed in E. coli, purified by IMAC and analyzed on 12% SDS-polyacrylamide gels. The protein bands were stained with Coomassie Blue. Band 1: VH16-VL30; Band 2: VH30-VL16.
FACS analysis of bispecific antibodies binding to the CD30+ L540CY Hodgkin cell line and to CD16+granulocytes. The tumor cells and the granulocytes were incubated with 20 μg of the parental biMoAb HRS-3/A9 or with 20 μg of the anti-CD16/anti-CD30 diabody. The binding of the anti-CD16/anti-CD30 diabody to L540CY cells and granulocytes was detected with the anti–c-myc immunotag antibody 9E10 and fluorescein-conjugated goat antimouse IgG. Bound BiMoAb HRS-3/A9 was detected with fluorescein-conjugated goat antimouse IgG. As a negative control, target cells were incubated with 9E10 and FITC-labeled goat antimouse IgG alone.
FACS analysis of bispecific antibodies binding to the CD30+ L540CY Hodgkin cell line and to CD16+granulocytes. The tumor cells and the granulocytes were incubated with 20 μg of the parental biMoAb HRS-3/A9 or with 20 μg of the anti-CD16/anti-CD30 diabody. The binding of the anti-CD16/anti-CD30 diabody to L540CY cells and granulocytes was detected with the anti–c-myc immunotag antibody 9E10 and fluorescein-conjugated goat antimouse IgG. Bound BiMoAb HRS-3/A9 was detected with fluorescein-conjugated goat antimouse IgG. As a negative control, target cells were incubated with 9E10 and FITC-labeled goat antimouse IgG alone.
Cytoxicity to L540CY cells in vitro.
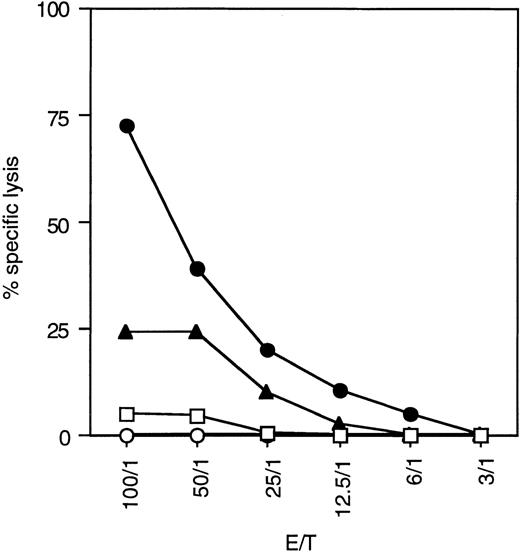
A JAM-test assay measuring the amount of NK cell–mediated DNA fragmentation was performed to determine the relative cytoxicity of the diabody compared to the biMoAb and the parental antibodies (Fig4). A strong cytolytic activity was induced by the diabody against CD30+ L540CY cells in the presence of nonstimulated freshly prepared PBL from healthy donors. Its cytotoxic effect in vitro was clearly better than that of the biMoAb, even though the parental antibody was added in approximately 1.5 × greater amounts on a molar basis. A mixture of the parental antibodies, each at 2 μg/mL, had no effect on cytolysis (data not shown). This superior potency of the diabody was apparent at any effector to target ratio. No lysis of the CD30+ cells was observed using the diabody without PBL, and PBL without antibodies did not induce any spontaneous lysis. Similar assays with the irrelevant CD30− cell line SW480 showed no significant amounts of NK cell–mediated cytotoxicity (data not shown).
Cytolytic activity of resting PBL containing approximately 10% NK cells against the CD30+ cell line L540CY at different effector:target ratios in a 5-hour JAM-test assay. The diabody (•) was used at concentration of 1 μg/mL and the biMoAb (▴) at a concentration of 4 μg/mL. Thus, approximately 1.5× more biMoAb than diabody was used when calculated on a molar basis. The diabody (1 μg/mL) without PBL (○) and PBL alone (□) were used as negative controls.
Cytolytic activity of resting PBL containing approximately 10% NK cells against the CD30+ cell line L540CY at different effector:target ratios in a 5-hour JAM-test assay. The diabody (•) was used at concentration of 1 μg/mL and the biMoAb (▴) at a concentration of 4 μg/mL. Thus, approximately 1.5× more biMoAb than diabody was used when calculated on a molar basis. The diabody (1 μg/mL) without PBL (○) and PBL alone (□) were used as negative controls.
Effect of diabody on xenotransplanted Hodkgin’s lymphoma.
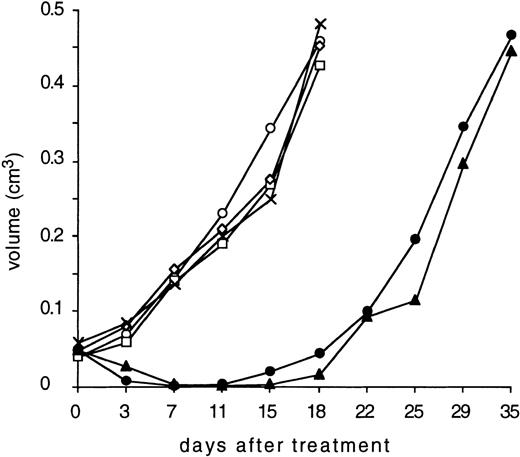
To assess the efficacy of the diabody in preclinical trials, we compared it with the original biMoAb, which has already been successfully administered to patients in a phase-I/II clinical trial.7 Subcutaneous tumors were established in SCID mice with a single injection of 1.5 × 107 L540CY cells into the right flank. The mice were treated with the diabody or the biMoAb when the tumors reached a size of 4 to 6 mm in diameter. Groups of 10 tumor-bearing animals received a dose of 100 μg of the anti-CD16/anti-CD30 diabody or the biMoAb per mouse together with 107 nonstimulated human PBL administered as a single tail-vein injection. Control groups included 5 tumor-bearing mice that were treated with the monospecific parental MoAbs and 107human PBL, 100 μg of the diabody per mouse without PBL, human PBL in PBS, and PBS alone.
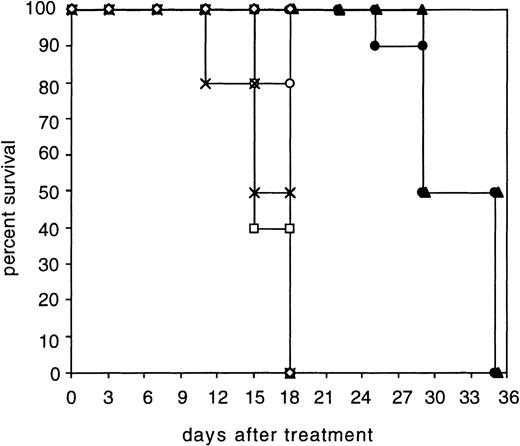
All the animals in the control groups developed tumors larger than 1 cm in diameter (0.4 to 0.6 cm3) within 18 days. In contrast, the diabody and the biMoAb caused a tumor regression until day 11 (Fig5). After this period of time, tumors were able to be detected once again in both antibody-treated groups and a critical tumor growth occurred within 35 days. A statistical analysis of the tumor volumes at day 11 and day 15 using the Mann-Whitney test and t-test, respectively, showed a highly significant diference (P < .001) between the control groups and the group treated with diabody. The graphical representation of the survival times was plotted according to the method of Kaplan and Meier26 (Fig6). A comparison of survival times using the logrank test (Mantel-Haenszel test),27 showed that the survival time of mice treated with the diabody was significantly prolonged compared with control animals (P < .0001). Comparison of the diabody-treated group with the biMoAb-treated group using the logrank test resulted in a P value of .143 indicating that no statistically significant difference exists between the two therapeutic regimes.
Treatment of SCID mice bearing human Hodgkin’s lymphoma xenografts with bispecific antibodies. The mice were treated on day 0 by intravenous injection with 200 μL PBS (x); 1 × 107human peripheral blood lymphocytes (PBL) in 200 μL PBS (□); a mixture of 100 μg parental MoAb HRS-3 and A9 together with human PBL (◊); 100 μg purified anti-CD16/anti-CD30 diabody with human PBL (◍) or without human PBL (○); 100 μg purified biMoAb HRS-3/A9 together with human PBL (▴). Tumor diameters were recorded twice a week and tumor volume was calculated as follows: volume = d2 × D × π/6 with d as the smaller and D as the larger tumor diameter.
Treatment of SCID mice bearing human Hodgkin’s lymphoma xenografts with bispecific antibodies. The mice were treated on day 0 by intravenous injection with 200 μL PBS (x); 1 × 107human peripheral blood lymphocytes (PBL) in 200 μL PBS (□); a mixture of 100 μg parental MoAb HRS-3 and A9 together with human PBL (◊); 100 μg purified anti-CD16/anti-CD30 diabody with human PBL (◍) or without human PBL (○); 100 μg purified biMoAb HRS-3/A9 together with human PBL (▴). Tumor diameters were recorded twice a week and tumor volume was calculated as follows: volume = d2 × D × π/6 with d as the smaller and D as the larger tumor diameter.
Survival of SCID mice bearing xenotransplantated Hodgkin’s tumors. The survival of the groups treated with the diabody (•) or biMoAb HRS-3/A9 (▴) was significantly prolonged (P< .0001, logrank test) compared to the following control groups: PBS alone (x); PBL in PBS (□); mixture of parental MoAb HRS-3 and A9 with PBL (◊); diabody without PBL (○). Animals were removed from the experiment when the tumor size exceeded 1 cm in diameter (approximatly 0.4 to 0.6 cm3).
Survival of SCID mice bearing xenotransplantated Hodgkin’s tumors. The survival of the groups treated with the diabody (•) or biMoAb HRS-3/A9 (▴) was significantly prolonged (P< .0001, logrank test) compared to the following control groups: PBS alone (x); PBL in PBS (□); mixture of parental MoAb HRS-3 and A9 with PBL (◊); diabody without PBL (○). Animals were removed from the experiment when the tumor size exceeded 1 cm in diameter (approximatly 0.4 to 0.6 cm3).
DISCUSSION
The parental biMoAb of the CD30/CD16 diabody has been used for treating refractory HD in a clinical phase-I/II trial with encouraging results.7 One complete remission, 1 partial remission, 3 minor responses, and 1 mixed response were observed in a group of 15 HD patients. These responses were achieved with a single cycle of antibody application over a period of 2 weeks. The treatment was well tolerated and the maximum tolerated dose (MTD) was not reached at an application of 64 mg/m2. Further cycles of treatment and an estimate of the MTD were not possible because of the limited amount of material available. This was mainly due to the prohibitive cost of producing and purifying the bispecific antibody from a hybrid hybridoma cell line, a procedure requiring the separation of the bispecific antibody from 9 other similar molecules comprising all of the possible combinations of heavy and light chains. In addition to the problem of obtaining enough material, a more-extensive treatment of the patients is limited by the development of a human antimouse antibody (HAMA) response. For example, 9 of the patients developed antimouse Ig antibodies, and 4 of these patients developed an allergic reaction after attempted retreatment.
The diabody described in the present investigation was designed to overcome these problems. It consists only of the variable domains of the parental bispecific antibody, thus considerably reducing the immunogenic potential. Recombinant dimeric antibodies have also been produced in relatively large quantities in E. coli13,28 and are therefore expected to be much easier to produce and purify. Previous attempts to produce (scFv)2molecules of another specificity as recommended by Gruber et al8 were unsatisfactory in our hands because of the small yields of functional bispecific molecules made in E. coli. We therefore favored the more-rigid crossover scFv dimer (diabody), which has been shown to have two antigen-binding sites at opposite ends of the molecule separated by 65Å, a distance sufficient to span the distance between two cells.11,14 The anti-CD16 domains of the diabody could only bind well after exchanging glutamic acid at position 6 for glutamine. A similar restoration of the binding activity and improved secretion upon substituting an amino acid at this position with glutamine was observed for an anti-CD3 scFv.22
The results of in vitro cytoxicity assays indicated that the diabody was more effective than the parent bispecific antibody in recruiting NK cells for lysing the target CD30+ L540CY cell line. A similar phenomenon was observed with a BCL-1 idiotype/CD3 diabody that was able to induce the lysis of target cells with activated T cells more efficiently than its parent bispecific antibody.11 The greater potency of diabodies may be due to the closer proximity of the target and effector cells, because the antigen binding sites are only separated by 65 Å. For comparison, the antigen-binding sites in an IgG molecule can span a distance of up to 150 Å.14 Another possibility is that the Fc domains of the biMoAb may reduce the efficiency of lysis by competing for binding to the FcγIII receptor.
In animal model experiments, the CD30/CD16 diabody was able to induce a marked regression of xenotransplanted human Hodgkin’s tumors in SCID mice similar to that obtained after administration of the parental bispecific antibody. In a previous study using exactly the same conditions, it was shown that just one application of the biMoAb was able to cure nearly half of the animals.5 The fact that no permanent cures were achieved with the biMoAb or the diabody in the current series of experiments and that the growth rate of the Hodgkin cell line had increased indicate the evolution of a more-aggressive tumor cell line in the interval between these two series of experiments. This in vivo performance of the diabody is quite impressive considering that it was administered as a single dose and is probably cleared much faster than the biMoAb. For example, single-chain antibodies (29 kD) were found to have a t1/2β of approximately 3.5 hours, 29 a diabody (50 kD) had a t1/2β of 6.42 hours,30 and an IgG1 (150 kD) had a t1/2β of 107 hours.31 The bispecific CD16/CD30 diabody has a molecular weight of 59 kD. In addition to the greater efficacy of the diabody for cell lysis shown in in vitro tests, the small size of the diabody probably facilitates a more-extensive tumor penetration as already shown for single-chain antibodies.32 This will be investigated in future pharmacokinetic studies.
These results, to our knowledge the first describing an in vivo application of bispecific diabodies for treating experimental tumors, suggest that the diabody can be used to replace the bispecific MoAb for treating refractory HD. The possibility of producing and purifying the diabody at relatively low cost and its lower immunogenicity should facilitate larger clinical trials with more-extensive cycles of treatment.
ACKNOWLEDGMENT
We thank Dr A. Hombach (Klinik I für Innere Medizin, Labor Tumorgenetik, Universität zu Köln, Köln, Germany) for kindly providing the anti-CD 30 scFv fragment and A. Benner (Biostatistics Group, German Cancer Research Center [DKF2], Heidelberg, Germany) for statistical analyses of the animal experiments.
M.A.E.A. and J.K. contributed equally to this work.
Supported by a grant from Deutsche Krebshilfe/Dr Mildred-Scheel-Stiftung, No. W5/93/PF 3.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal