MORGAGNI IN HIS “Opera Omnia”-Epistola Anatomico-Medica XLI-1764, Sermo est de Urinae Suppressione,1 was the first to recognize the remarkable association between bleeding and renal dysfunction, which became a clinical problem in the early days of dialysis, when patients sometimes died from excessive bleeding from the gastrointestinal tract or abdominal organs.2 3
Modern dialysis techniques and the use of erythropoietin to correct anemia4 have reduced the frequency of uremic bleeding, which, however, still limits surgery and invasive procedures in these patients.
The cause of uremic bleeding has been the subject of a major debate in the last 30 years. The pathogenesis is considered multifactorial (Table 1); however, platelet-platelet and platelet-vessel wall interactions appear to be of crucial importance.5,6 Studies found that, in uremics, skin bleeding time (influenced by platelet and vascular function as well as by red blood cells), although far from ideal, is still a reliable predictor of clinical bleeding.2 7
Cause or Causes of Uremic Bleeding?
| Platelet abnormalities |
| Subnormal dense granule content |
| Reduction in intracellular ADP and serotonin |
| Impaired release of the platelet α-granule protein and β-thromboglobulin |
| Enhanced intracellular cAMP |
| Abnormal mobilization of platelet Ca2+ |
| Abnormal platelet arachidonic acid metabolism |
| Abnormal ex vivo platelet aggregation in response to different stimuli |
| Defective cyclooxygenase activity |
| Abnormality of the activation-dependent binding activity of GP IIb-IIIa |
| Uremic toxins, especially parathyroid hormone |
| Abnormal platelet-vessel wall interactions |
| Abnormal platelet adhesion |
| Increased formation of vascular PGI2 |
| Altered von Willebrand factor |
| Anemia |
| Altered blood rheology |
| Erythropoietin deficiency |
| Abnormal production of nitric oxide |
| Drug treatment |
| β-Lactam antibiotics |
| Third-generation cephalosporins |
| Nonsteroidal antiinflammatory drugs |
| Platelet abnormalities |
| Subnormal dense granule content |
| Reduction in intracellular ADP and serotonin |
| Impaired release of the platelet α-granule protein and β-thromboglobulin |
| Enhanced intracellular cAMP |
| Abnormal mobilization of platelet Ca2+ |
| Abnormal platelet arachidonic acid metabolism |
| Abnormal ex vivo platelet aggregation in response to different stimuli |
| Defective cyclooxygenase activity |
| Abnormality of the activation-dependent binding activity of GP IIb-IIIa |
| Uremic toxins, especially parathyroid hormone |
| Abnormal platelet-vessel wall interactions |
| Abnormal platelet adhesion |
| Increased formation of vascular PGI2 |
| Altered von Willebrand factor |
| Anemia |
| Altered blood rheology |
| Erythropoietin deficiency |
| Abnormal production of nitric oxide |
| Drug treatment |
| β-Lactam antibiotics |
| Third-generation cephalosporins |
| Nonsteroidal antiinflammatory drugs |
It has always been difficult to investigate the nature of platelet dysfunction in uremia, because dialysis has a complex influence on platelets and coagulation.8-10 Emblematic is the case of platelet function tests that may be worsened11,12 or improved12-14 at the end of an average dialysis. Circumstantial evidence exists that platelets are activated by the dialysis membrane, becoming refractory to further stimuli, which would explain the paradoxical effect of depuration on platelet function tests.15 In the search for the cause of uremic bleeding, abnormalities of platelet α-granules with lower than normal content of ADP and serotonin14,16 and defective arachidonate metabolism17 have been widely reported, but which of the above-noted actually triggered the clinical syndrome remained controversial.18 More recent evidence implicates acquired defects in specific receptors that impair platelet binding to fibrinogen and von Willebrand factor (vWF),13 although the role of vWF in uremic bleeding has been controversial for more than 20 years.19-22
EARLY STUDIES AND THE PROPOSED ROLE OF GUANIDINOSUCCINIC ACID
Because uremic platelets in normal plasma function normally, early studies18,23 suggested that molecules retained in uremic blood did induce platelet dysfunction. An extensive search for the toxin involved was frustrating until the late 1960s, when Horowitz et al24,25 first reported that uremic, but not normal plasma, inhibited platelet factor 3 activation, measured as prolongation of the Stypven time test,24,25 and ADP-induced aggregation.25 The finding, indicating an inhibitor of platelet function in uremic plasma, could not be reproduced with increasing concentrations of urea, creatinine, or guanidinoacetic acid. Rather consistently, however, guanidinosuccinic acid, when added to normal platelets, dose-dependently prolonged Stypven time and blocked ADP-induced aggregation.25
Guanidinosuccinic acid accumulates in uremics who need an alternative pathway for ammonia detoxification (Fig 1) to compensate for the reduced efficiency of urea cycle enzymes inhibited by excess products.26,27 Peritoneal dialysis, which removed guanidinosuccinic acid from uremic blood, also corrected the inhibitory action of uremic plasma on normal platelets,25 reinforcing the link between guanidinosuccinic acid and abnormal hemostasis, the toxin being defined as the “x factor in uremic bleeding.”28 This association was furthermore reinforced by another study.29 However, for 30 years thereafter, despite intensive research on uremic bleeding, the story of guanidinosuccinate was largely neglected.
An alternative pathway of L-arginine metabolism leading to the formation of guanidinosuccinic acid. The accumulation of urea in uremic plasma exerts an inhibitory effect on the enzymes of the urea cycle. This activates alternative metabolic pathways such as the transamidination of arginine leading to guanidinosuccinic acid formation.27 It has been proposed that guanidinosuccinic acid can also be formed by enzymatic cleavage of argininesuccinate, another product of the urea cycle.
An alternative pathway of L-arginine metabolism leading to the formation of guanidinosuccinic acid. The accumulation of urea in uremic plasma exerts an inhibitory effect on the enzymes of the urea cycle. This activates alternative metabolic pathways such as the transamidination of arginine leading to guanidinosuccinic acid formation.27 It has been proposed that guanidinosuccinic acid can also be formed by enzymatic cleavage of argininesuccinate, another product of the urea cycle.
FROM GUANIDINOSUCCINIC ACID TO NITRIC OXIDE (NO): IS THE LINK STRONG?
Accumulation of guanidinosuccinic acid in uremic blood depends on amidine being transferred to aspartic acid from L-arginine,26 a recognized intermediate of the urea cycle. Research on L-arginine and its metabolites has exploded in the last few years since L-arginine has been recognized as a major substrate of NO synthase.30-32 NO is a potent modulator of vascular tone that limits platelet adhesion to endothelium33 and platelet-platelet interaction increasing the formation of cell cyclic GMP.34-36
NO synthesis from L-arginine implies activation of specific synthase enzymes (2 constitutive and an inducible isoform).37 The role of NO in primary hemostasis rests on findings that the skin bleeding time is prolonged in healthy volunteers38 inhaling NO. In patients with adult respiratory distress syndrome,39NO-inhalation induced an increase in plasma cyclic GMP, prolongation of bleeding time, and inhibition of ex vivo platelet aggregation and P-selectin expression. Fibrinogen binding was also inhibited, which suggests that NO-dependent inhibition of platelet aggregation may be caused by a decrease in fibrinogen binding to the platelet GP IIb/IIIa receptor.39
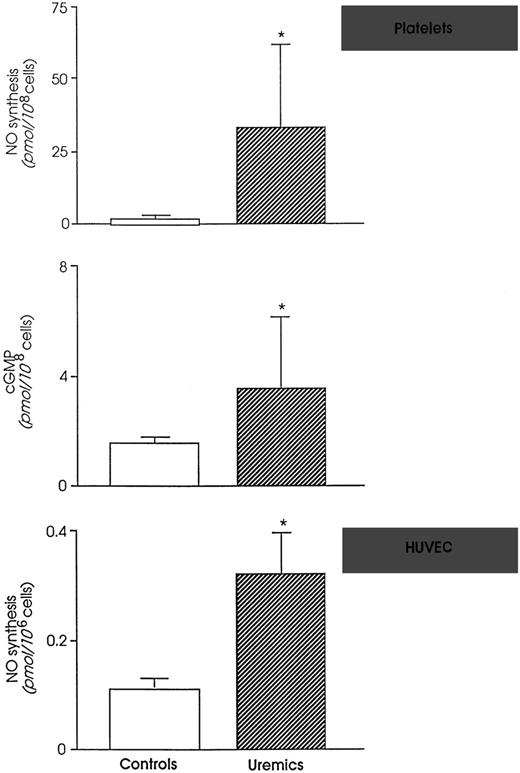
NO and NO metabolites are markedly enhanced in the circulation of rats with extensive surgical ablation of renal mass and progressive renal failure.40 This depends on upregulation of both inducible and endothelial constitutive NO synthase enzymes that occurs in uremia.40 Because monomethyl-L-arginine (L-NMMA), an NO inhibitor, normalized platelet dysfunction and bleeding time of uremic rats, a role of excessive NO was suspected in uremic bleeding tendency.41 Consistently, intravenous infusion of L-NMMA reduced in vivo NO production and significantly shortened bleeding time in healthy humans.42 The findings that the vascular expression of NO-forming enzymes and plasma concentrations of NO metabolites in uremic rats were normalized by conjugated estrogens43 (the best pharmacological tool for controlling bleeding in experimental44 and human45,46chronic renal insufficiency) are further evidence of the crucial importance of NO in uremic bleeding. Once more, enhancing NO availability by L-arginine fully abolished the beneficial effect of estrogens and reinduced bleeding47 in rats. Thus, simply limiting NO synthesis is enough to block excess bleeding and restore normal hemostasis in experimental uremia. Data are available in humans showing that platelets from uremic patients on hemodialysis generate more NO than healthy subjects48(Fig 2). In addition, uremics have higher levels of NO in the exhaled air49 and higher plasma levels of NO metabolites50-53 than normal humans.
(Top) NO formation and intracellular cyclic GMP in platelets from controls and uremic patients. (Bottom) NO formation by human endothelial cells (HUVEC) exposed to control or uremic plasma. *P < .01 versus controls. (Modified and reprinted with permission from Noris et al.48 57)
The biochemical nature of the findings listed above was addressed by studies showing that uremic plasma, unlike normal plasma, was a potent inducer of NO in umbilical48 or microvascular endothelial cells.54 This reflects accumulation in uremic plasma of substances that potently induce NO synthesis. Among putative candidates, at least 2 (tumor necrosis factor α and interleukin-1β) circulate in supranormal amounts in uremics, being released by monocytes activated on dialysis membranes48,55-57 and/or on challenge with endotoxin, which can cross the dialysis membrane and reach the blood, albeit in small amounts.58 If care is taken to reduce cytokine activation by the use of more biocompatible membranes, NO formation is controlled and its concentrations in blood may even decrease at the end of an average dialysis.49,50Also, heparin, which is used as anticoagulant during dialysis, might contribute to increased NO production in dialysis patients, as indicated by its capability to promote NO production by cultured human endothelial cells.59
Old data on the worsening or ameliorating effects of dialysis on bleeding may therefore be explained by the effect of different dialysis membranes and procedures on circulating cell activation and NO formation.
NO is mostly but not exclusively formed from L-arginine. Most of the endogenous guanidines, such as guanidino acetate and propionate, guanidine, and methylguanidine, which accumulates in uremic plasma in micromolar concentrations,25,29,60 had no effect on isolated rat aortas61,62 used as a biological indicator of NO formation. In contrast, guanidinosuccinic acid relaxed isolated rat aortas in a dose-dependent fashion62(Fig 3). The vasodilatory properties of guanidinosuccinic acid were dependent on an intact endothelium62 and implied increased cyclic GMP formation in vessels. Vasodilation and cyclic GMP formation were both attenuated by NO inhibitors such as monomethyl-L-arginine and hemoglobin,62 indicating that NO mediated guanidinosuccinic acid-induced aortic relaxation.
Dose-response curve showing the relaxation elicited by guanidinosuccinic acid in the rat aorta preparation. The NO-synthase inhibitor L-NMMA reverses the effect of guanidinosuccinic acid in the rat aorta. GSA, guanidinosuccinic acid. L-NMMA, N-monomethyl-L-arginine. (Modified and reprinted with permission from Thomas and Ramwell.62)
Dose-response curve showing the relaxation elicited by guanidinosuccinic acid in the rat aorta preparation. The NO-synthase inhibitor L-NMMA reverses the effect of guanidinosuccinic acid in the rat aorta. GSA, guanidinosuccinic acid. L-NMMA, N-monomethyl-L-arginine. (Modified and reprinted with permission from Thomas and Ramwell.62)
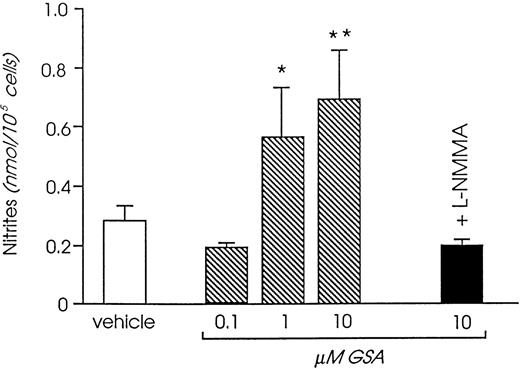
Challenging human cultured endothelium with increasing amounts of guanidinosuccinic acid promoted release of NO metabolites in the cell supernatant, an effect that was neutralized by specific NO synthase inhibitors.63 Concentrations of guanidinosuccinic acid maximally inducing NO release in vitro (Fig4) were remarkably close to the ones measured in uremic plasma before dialysis.63 Lower, although still significant stimulation was observed with concentrations close to those found in vivo in postdialysis blood,63 whereas the concentrations reached in normal plasma had no effect on NO synthesis.
Effect of guanidinosuccinic acid on NO synthesis (analyzed by measuring the concentration of the stable NO metabolites, nitrites). *P < .05, **P < .01 versus vehicle. L-NMMA, N-monomethyl-L-arginine. (Reprinted with permission from Todeschini et al.63)
Effect of guanidinosuccinic acid on NO synthesis (analyzed by measuring the concentration of the stable NO metabolites, nitrites). *P < .05, **P < .01 versus vehicle. L-NMMA, N-monomethyl-L-arginine. (Reprinted with permission from Todeschini et al.63)
That guanidine derivatives structurally related to L-arginine can form NO rests on recent data that many N-substituted arginines, such as benzoyl L-arginine, induce vascular relaxation and inhibit platelet function.61,64 On the other hand, guanidino compounds (guanidinoacetate, guanidinopropionate, guanidinosuccinate, and guanidine) have been found in micromolar concentrations in human vascular endothelium.61 Interestingly, guanidinosuccinate is the only guanidine derivative related to L-arginine that mimics perfectly the biological activities of NO ranging from vasodilation to antiproliferative effect on smooth muscle cells.61 Like NO, GSA also inhibits platelet function, as documented by platelet aggregation studies on human platelet-rich plasma stimulated with ADP (Horowitz et al25 and Fig 5). Pretreatment with the NO inhibitor Nω-nitro-L-arginine65completely restored ADP-induced irreversible platelet aggregation (Fig5), which would indicate that NO mediates the effect of GSA on platelet function. The in vivo relevance of these data is evidenced by data that GSA (1 mg/kg) prolonged the bleeding time of rats when injected intravenously (Macconi et al, abstract submitted to the Congress of the American Society of Nephrology, 1999). The bleeding time was significantly lengthened (P < .05) over basal 5 minutes after GSA injection (49% ± 11.9% increase over basal, n = 4) and reached maximal increase after 30 minutes (114% ± 30%, n = 4,P < .01 v basal). Administration of the specific NO inhibitor, Nω-nitro-L-arginine methyl ester (30 and 60 mg/kg),66 significantly attenuated the effect of GSA on bleeding time (30 minutes: 65% ± 8% increase over basal, n = 4, P < .05 v GSA alone).
Representative platelet aggregation tracings induced by ADP (1 μmol/L) in normal human platelet-rich plasma with the addition of buffer or GSA (500 μmol/L, 30 seconds of preincubation; A). GSA inhibited the second wave of platelet aggregation induced by 1 μmol/L ADP. Nω-nitro-L-arginine (1 to 2 mmol/L, 1 minute of preincubation) overcame GSA effect and restored ADP-induced irreversible platelet aggregation (B). As a positive control, L-arginine (30 seconds of preincubation) inhibited platelet aggregation when added at a concentration ≥1 mmol/L (C). (Macconi et al, abstract submitted to the Congress of the American Society of Nephrology, 1999).
Representative platelet aggregation tracings induced by ADP (1 μmol/L) in normal human platelet-rich plasma with the addition of buffer or GSA (500 μmol/L, 30 seconds of preincubation; A). GSA inhibited the second wave of platelet aggregation induced by 1 μmol/L ADP. Nω-nitro-L-arginine (1 to 2 mmol/L, 1 minute of preincubation) overcame GSA effect and restored ADP-induced irreversible platelet aggregation (B). As a positive control, L-arginine (30 seconds of preincubation) inhibited platelet aggregation when added at a concentration ≥1 mmol/L (C). (Macconi et al, abstract submitted to the Congress of the American Society of Nephrology, 1999).
Thirty years ago, those researchers were right: guanidinosuccinic acid, which accumulates in uremic patients, induced them to bleed. Data now accumulating tend to suggest that this was due to an exuberant formation of NO by uremic vessel, which here we find to be guanidinosuccinic acid-dependent.
ACKNOWLEDGMENT
Data and concepts presented in this report are derived from the contributions of the following persons in our laboratories to whom we are indebted: Carla Zoja, Ariela Benigni, Marta Todeschini, Daniela Corna, Daniela Macconi, Sistiana Aiello, Paola Boccardo, and Flavio Gaspari. The authors sincerely thank our colleagues Manuela Livio, Maria Benedetta Donati, and Giovanni De Gaetano, who, in 30 years of research in uremic bleeding, have contributed substantially by their stimulating discussion and exchange of information to the contents of this report.
Supported in part by a grant from Fondazione CARIPLO, Italy.
REFERENCES
Author notes
Address reprint requests to Marina Noris, Chem Pharm D, “Mario Negri” Institute for Pharmacological Research, Via Gavazzeni 11, 24125 Bergamo, Italy.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal