The mechanisms by which intravenously (IV)-administered hematopoietic cells home to the bone marrow (BM) are poorly defined. Although insightful information has been obtained in mice, our knowledge about homing of human cells is very limited. In the present study, we investigated the importance of very late activation antigen (VLA)-4 in the early phases of lodgment of human CD34+progenitors into the sheep hematopoietic compartment after in utero transplantation. We have found that preincubation of donor cells with anti–VLA-4 blocking antibodies resulted in a profound reduction of human cell lodgment in the fetal BM at 24 and 48 hours after transplantation, with a corresponding increase of human cells in the peripheral circulation. Furthermore, IV infusion of the anti–VLA-4 antibody at later times (posttransplantation days 21 to 24) resulted in redistribution or mobilization of human progenitors from the BM to the peripheral blood. In an attempt to positively modulate homing, we also pretreated human donor cells with an activating antibody to β1 integrins. This treatment resulted in increased lodgment of donor cells in the fetal liver, presumably for hemodynamic reasons, at the expense of the BM. Given previous involvement of the VLA-4/vascular cell adhesion molecule (VCAM)-1 adhesion pathway in homing and mobilization in the murine system, our present data suggest that cross-reacting ligands (likely VCAM-1) for human VLA-4 exist in sheep BM, thereby implicating conservation of molecular mechanisms of homing and mobilization across disparate species barriers. Thus, information from xenogeneic models of human hematopoiesis and specifically, the human/sheep model of in utero transplantation, may provide valuable insights into human hematopoietic transplantation biology.
HOMING AND ENGRAFTMENT of transplanted hematopoietic cells within the hematopoietic microenvironment are important biological processes for sustained long-term hematopoiesis. However, the molecular mechanisms that govern these processes are poorly understood. In many previous studies, observations on homing have not been dissociated from observations on engraftment. If homing is defined as a process by which parenterally administered hematopoietic cells lodge and firmly anchor themselves within the hematopoietic tissues, then parameters that influence this process need to be studied early posttransplantation, before cell proliferation or engraftment ensue. It is likely that the mechanism of homing is a multistep process consisting of adhesion to endothelial cells of the marrow sinusoids, followed by transmigration, and finally firm anchoring within the extravascular bone marrow (BM) spaces where proliferation and differentiation will occur. In contrast to the lymphocyte paradigm,1 however, where the steps and the molecules involved in lymphocyte homing and trafficking have been largely delineated, much less is known about the homing of hematopoietic cells.
Several attempts have been made in the past to modulate either the homing or the early engraftment of hematopoietic stem cells administered into irradiated recipients.2,3 Some of these studies have measured early engraftment and have made indirect inferences about homing, whereas other studies have drawn attention to the fact that initial BM lodgment can be modulated as a result of changes in lodgment in other nonhematopoietic tissues.4-6Thus, lodgment in BM can be altered either directly, or indirectly, by influencing early retention of hematopoietic cells in non-bone marrow sites. These and other experiments in the murine model7-10support the conclusion that transplanted hematopoietic cells do not selectively home to the BM, but are selectively retained within the BM once they lodge there. This applies not only to total nucleated BM cells, but to colony-forming unit-culture (CFU-C) and colony-forming unit-spleen (CFU-S) progenitor cells. Whether pluripotent long-term repopulating cells behave differently remains to be determined.
Recently xenogeneic models have surfaced as surrogate assays for long-term repopulating human hematopoietic stem cells.11-13The relevance of a xenogeneic assay system to events in human hematopoiesis is dependent on retention of fundamental aspects of hematopoietic biology across species barriers. Such information is crucial in evaluating subsequent engraftment and longevity of hematopoietic cells in these models and in determining whether these models can be used to develop clinical strategies to enhance engraftment of human hematopoietic cells in the future.
In the present studies, we have used the preimmune fetal sheep as a model to study the early lodgment of human hematopoietic cells and have shown a significant participation of the VLA-4–dependent pathway in the lodgment of human cells within the fetal sheep BM. Furthermore, administration of anti–VLA-4 at later days posttransplantation led to mobilization of human cells to circulation. This observation in combination with previous similar observations in the murine10 and primate14 model suggest that the importance of the VLA-4/VCAM axis in hematopoietic homing and mobilization is evolutionarily conserved. Furthermore, as the recipients in the present studies were not preconditioned, in contrast to previous murine studies, it is suggested that this pathway is operative both in the irradiated and nonirradiated setting. Our present data provide important background information about the homing of human cells in the human/sheep xenogeneic model, which may have relevance to hematopoietic homing during normal hematopoietic ontogeny, and to hematopoietic stem cell transplantation in man.
MATERIALS AND METHODS
Human/sheep model.
The human/sheep model of in utero hematopoietic stem cell transplantation has been previously described.l5 Briefly, time dated pregnant ewes were sedated with ketamine and placed under halothane general anesthesia. The uterine horns were exposed by maternal laparotomy and the preimmune fetal lambs directly visualized by the amniotic bubble technique.l6 Human cells were transplanted into the fetus by intraperitoneal injection and uterine and maternal incisions closed. In those animals receiving intravenous (IV) injections, IV catheters were placed by transmyometrial exposure of the segmental veins from the cotyledons, near their umbilical venous convergence. A venotomy in 1 of the segmental cotyledon veins was performed and a beveled 2.7 French (Fr.) silastic catheter was fed centrally into the umbilical vein. The catheter was secured at the venous insertion site with a ligature and to the myometrium at the closure site. It was then tunneled subcutaneously to the ewe’s neck and a port exteriorized for repeated venous access.
Preparation of human donor cells.
BM aspirations were obtained from the posterior iliac crest of healthy adult volunteers according to the guidelines established by the Institutional Review Boards for Human Research at the University of Nevada and the Department of Veterans Affairs Medical Centers, Reno, NV.
BM mononuclear cells (BMNC) were isolated by Ficoll-Hypaque density separation. Enriched CD34+ populations were isolated by passing anti-CD34–biotinylated monoclonal antibody (MoAb) labeled BMNC once or twice through an avidin immunoaffinity column (Cellpro, Bothell, WA) according to manufacturer’s instructions. Cells were resuspended to appropriate concentrations in Iscove’s modified Dulbecco’s medium (IMDM) with 2% fetal calf serum (FCS) and preincubated before transplantation in either the same medium or medium containing blocking concentrations of antibody.
Antibodies.
Endotoxin free HP1/2 murine MoAb that recognizes the α4 chain of human VLA-4 was used. This antibody blocks VLA-4–dependent adhesion in vitro and VLA-4–dependent function in vivo.17,18 Another antibody, B5G10, which does not block adhesion and that reacts with VLA-4 was also used as control.19 Both antibodies were generously provided by Dr Roy Lobb (Biogen, Cambridge, MA). Furthermore, an activating antibody to β1 integrin, 8A2, previously well characterized,20 has also been used, and it was generously provided by Dr John Harlan (University of Washington, Seattle, WA). The antibodies were used for treating cells or direct injection into the fetal circulation.
Analysis of human cell engraftment.
Recipients were examined for donor cell engraftment/expression at intervals posttransplant as detailed below. Blood, BM, liver, and spleen of recipients were analyzed for the presence of human cells by analysis of human CD45 by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) as previously described.21 Peripheral blood was collected by cardiac puncture, and the liver, spleen, and bones harvested aseptically and placed in sterile saline. The 8 long bones collected were then flushed with IMDM using an 18 gauge needle. After washing and weighing, single cell suspensions were prepared from thymus, liver, and spleen using a glass tissue homogenizer followed by filtration through a 70-μm nylon strainer (Becton Dickinson, Franklin Lanes, NJ). BM and peripheral blood from all recipients was examined for the presence of human hematopoietic progenitors as reported.13,22 Briefly BMNC (0.4 to 2 × 105 cells/mL) were assayed for hematopoietic progenitor cells in methylcellulose assays. All cultures were established with IMDM and erythropoietin (2 IU/mL). For optimal growth of sheep CFU-Mix, CFU-granulocyte macrophage (GM), and burst-forming unit-erythroid (BFU-E), the cultures were supplemented (5% vol/vol) with a preparation of phytohemagglutinin (PHA)-stimulated leukocyte-conditioned medium (LCM) produced from a mixture of fetal sheep spleen, thymus, liver, and BM cells in IMDM with 2% fetal sheep serum. In these cultures, maximal numbers of sheep colonies develop by day 9 of incubation. Optimal growth of human hematopoietic progenitors was achieved with the addition of 5 ng/mL each of human interleukin-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF) in the absence of sheep PHA-LCM with maximal colony growth at day 19. Colonies were enumerated by type on days 9 and 19 of incubation. On day 19, individual colonies were removed from the culture plates and processed for karyotyping as previously described.13 22
Statistics.
A Student’s two-tailed t-test was used to determine significance of differences in paired results with significance determined as P < .05.
RESULTS
Preincubation with anti–VLA-4 antibody decreases homing of human CD34+ cells to the sheep fetal BM and prolongs their circulation in peripheral blood.
To test whether preincubation of donor human cells with anti–VLA-4 changes the homing patterns in the sheep model, we purified BM mononuclear cells through CellPro Columns (see Materials and Methods). Ninety-one percent of the purified cells were CD34+ and 0.3% were CD3+. The purified cells were divided in 3 aliquots of 2.05 × 106 each. Aliquot no. 1 was incubated at 4°C for 30 minutes with control medium (IMDM with 2% of FCS). Aliquot no. 2 was incubated with 100 ng/mL of nonblocking anti–VLA-4 antibody B5G1019 of the same isotype as the blocking anti-α4 antibody HP1/2. Aliquot no. 3 was incubated in the presence of 100 ng/mL anti–VLA-4 blocking antibody, HP1/2.17 Cells from each aliquot were transplanted into 3 separate groups of fetuses of ≈70 days gestation: group 1A received aliquot no. 1, group 1B received aliquot no. 2, and group 1C received aliquot no. 3. An equivalent of 3.4 × 105 CD34+ cells from each aliquot was transplanted into each fetus, and each group was comprised of 6 fetuses. One fetus from each group was killed 3 hours posttransplantation. Two fetuses were killed at 24 hours after transplantation and the remaining 3 fetuses from each of the 3 groups were killed at 48 hours after transplantation. In each animal BM, liver, spleen, and peripheral blood were sampled and analyzed by flow cytometry for the presence of human CD45+ cells, by karyotype analysis for human cells after PHA stimulation, and for the presence of human progenitors with karyotype analysis of individual colonies. If no human cells were detected by flow cytometry, no progenitor assessment was performed.
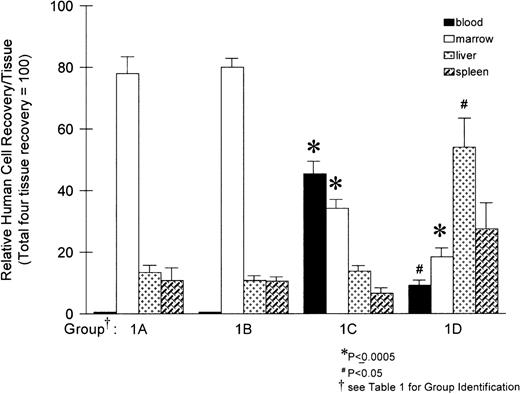
At 3 hours posttransplantation, we could not detect human cells in the hematopoietic sites sampled from all 3 fetuses (1 from each group). Thus, no further analyses were performed on these animals. At 24 and 48 hours posttransplantation, the distribution of human cells in the recipient hematopoietic sites is shown in Fig 1, whereas the proportion of cells are listed in Table 1. At both 24 and 48 hours, the highest proportion of human cells was detected in BM in control animals (group 1A), and these proportions were not different between 24 and 48 hours. The group that received cells incubated with nonblocking antibody (group 1B) had distributions similar to the control group receiving cells incubated with medium only. No human cells were detectable in the peripheral circulation in either group of animals (groups 1A and 1B, Table 1 and Fig 2). This is in agreement with our previous studies on engraftment in the sheep model at this gestational age.23 In contrast to groups 1A and 1B, group 1C animals, which received cells preincubated with anti–VLA-4 antibody (group 1C, Fig 1, Table 1, and Fig 2), had a reduced proportion of human cells in the BM, from ≈80% to 30% (P = .0005), with a corresponding increase of human cells in circulation from undetectable levels (in groups 1A and 1B) to approximately 45% of the total human cell distribution in the 4 tested sites (P = .0003). The total number of cells recovered in BM (= all long bones), liver, spleen, and blood were considered as 100 in Fig1. No significant effect on the distribution of human donor cells lodged to the fetal liver or spleen was observed with this treatment.
Distribution of human (CD45+) cells in sheep hematopoietic tissues at 24 and 48 hours after transplantation. Values are expressed as the mean percentage of human cells in each tissue taken from 5 animals per group ± 1 standard error (SE), with the total number of human cells detected in the 4 tissues taken as 100%. Significance values are relative to group 1A (medium control). Values from group 1B (nonblocking anti–VLA-4 antibody) were not significantly different than group 1A.
Distribution of human (CD45+) cells in sheep hematopoietic tissues at 24 and 48 hours after transplantation. Values are expressed as the mean percentage of human cells in each tissue taken from 5 animals per group ± 1 standard error (SE), with the total number of human cells detected in the 4 tissues taken as 100%. Significance values are relative to group 1A (medium control). Values from group 1B (nonblocking anti–VLA-4 antibody) were not significantly different than group 1A.
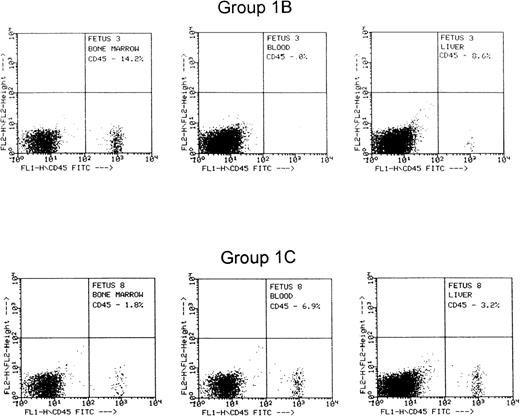
FACS histogram from 2 representative fetuses from group 1B that received cells treated with control nonfunction blocking anti-CD49d, and group 1C that received cells treated with function-blocking anti-CD49d.
FACS histogram from 2 representative fetuses from group 1B that received cells treated with control nonfunction blocking anti-CD49d, and group 1C that received cells treated with function-blocking anti-CD49d.
Preincubation of donor cells with an activating anti-β1 integrin antibody reduces lodgment of donor cells in BM and increases lodgment in the fetal liver.
In an attempt to enhance the homing pattern of human cells into the hematopoietic sites of the sheep fetus, we incubated donor cells before transplantation (at 4°C for 30 minutes) with an activating anti-β1 integrin antibody, 8A2, which increases the avidity of expressed integrins by locking them in the active conformational state.20 Thus, treatments in vitro increase adhesion of treated cells to stroma by 50% or more.20 A total of 6 fetuses were transplanted with 8A2 preincubated donor cells (group 1D). As this transplantation experiment was performed concurrently with groups 1A, 1B, and 1C, described above, groups 1A and 1B also served as controls for this group of animals.
The results obtained from sampling the fetuses in this group differed strikingly from the controls and from the anti–VLA-4 group (group 1C, Table 1). An enhancement from 2-fold to 7-fold in the recovery of cells from liver was found (Table 1). In the 2 control groups (groups 1A and 1B), lodgment in the liver was 11% to 13%, whereas in the 8A2-treated group (group 1D), it was 54% (P = .02). At the same time, there was a marked reduction in donor cell lodgment in the BM. Whereas control groups had 78% and 80%, the proportion of human cells in the BM of fetuses of group 1D was ≈18% (P = .001).
Anti–VLA-4 antibody administration mobilizes human progenitors into the peripheral circulation from the fetal BM.
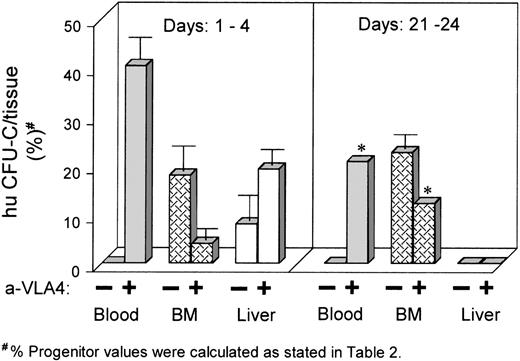
In a separate set of experiments, 4 subsequent groups of animals were transplanted with donor human cells, but in contrast to groups 1A through 1D above, they were infused with anti-α4 antibody at different times after transplantation. In this set of experiments, 20 fetuses at 64 days gestation were fitted with catheters for IV injection at the time of the initial intraperitoneal transplant. Human BMNCs were CD34 enriched by a single passage through a Cellpro column yielding a population of donor cells, which was 77% CD34+. There were 4 experimental groups. Group 2A (6 fetuses): each fetus received an intraperitoneal injection of 1.5 × 105CD34+ cells preincubated in IMDM/2% FCS for 30 minutes, followed by IV administration of 0.5 mL of IMDM/2% FCS daily for 4 days. Group 2B (8 fetuses): each fetus of this group received an intraperitoneal injection of 1.5 × 105CD34+ cells preincubated in IMDM/2% FCS containing 100 ng of anti–VLA-4 for 30 minutes, followed by IV administration of 10 μg of anti–VLA-4 (1 mg/kg estimated fetal weight) in 0.5 mL of IMDM/2% FCS daily for 4 days. Group 2C (3 fetuses): each fetus received an intraperitoneal injection of 1 × 105CD34+ cells and at day 21 posttransplant received IV 60 μg of anti–VLA-4 in 1 mL of IMDM/2% FCS daily for 4 days (ie, days 21 to 25). Group 2D (3 fetuses): each fetus received an intraperitoneal injection of 1 × 105 CD34+cells followed by IV administration of 1 mL of IMDM/2% FCS daily for 4 days beginning on posttransplant day 21, like group 2C. Groups 2A and 2B were killed on posttransplant day 16 (ie, 12 days after the last IV injection) and groups 2C and 2D were killed on posttransplant day 28 (ie, 4 days after the last IV injection). After killing, each fetus from every group had all 4 long bones harvested as well as fetal liver and peripheral blood. Analysis was by flow cytometry for human CD45+ cells and by karyotype analysis of PHA-stimulated cells and colonies grown from liver, BM, and peripheral blood. In the last 2 groups of fetuses (groups 2C and 2D), we wanted to test whether IV administration of antibody late after transplantation (at a time when most of the transplanted cells are found in BM) could mobilize these cells to the peripheral blood. For groups 2A and 2B, we wanted to test how the continuous administration of anti-α4 during the first 4 days after transplantation impacted subsequent engraftment. Results from these experiments are shown in Table 2and Fig 3. Six fetuses receiving only medium (group 2A) showed at day 21 the expected distribution, ie, most of the detected cells were in the BM with less than 1% detection in the liver and no detection in the peripheral blood. By contrast, the group that had received anti–VLA-4–treated cells, followed by 4 days of IV administration of anti–VLA-4 (group 2B), showed a significant proportion of human cells circulating in the blood in all 8 animals treated this way, with a significant reduction of cells detected within the BM compared with group 2A. There was also an increased proportion of cells detected in the liver compared with controls, but this did not reach statistical significance. Thus, although these fetuses were tested 12 days after the last administration of antibody, they showed a sustained redistribution of human cells and progenitors similar to the group of fetuses (group 1C) tested 24 and 48 hours after transplantation of anti-α4–treated cells (Table 1). This would suggest that human cells continued to circulate in the peripheral blood as they were unable to lodge in BM, if they did not lodge for the first 2 to 4 days. Unfortunately, it was not tested whether these cells continued to display anti–VLA-4 in their surface. As the antibody used was a humanized version of anti–VLA-4 with a very long half-life (T.P., unpublished, 1994), this was not an unlikely possibility. If, however, we assume that no antibody was present on the cell surface, the data would indicate that these cells have lost their ability to lodge to the BM if they did not do so for the first 24 hours or for the period of the first 4 days.
Tissue distribution of human CFU-C transplanted into sheep fetuses (vertical axis reflects the mean CFU-C recovered/tissue as percent of CFU-C recovered in the 3 tissues tested). Left panel shows mean data from animals that received anti–VLA-4 for the first 4 days posttransplantation. Note the persistence of human cells in circulation and the low levels in BM. Higher levels in liver likely reflect the increased blood volume of this tissue; right panel shows data from fetuses that received anti–VLA-4 later, between days 21 to 24. Note the release of human cells from BM to circulation (*data are from 1 fetus; see also Table 2).
Tissue distribution of human CFU-C transplanted into sheep fetuses (vertical axis reflects the mean CFU-C recovered/tissue as percent of CFU-C recovered in the 3 tissues tested). Left panel shows mean data from animals that received anti–VLA-4 for the first 4 days posttransplantation. Note the persistence of human cells in circulation and the low levels in BM. Higher levels in liver likely reflect the increased blood volume of this tissue; right panel shows data from fetuses that received anti–VLA-4 later, between days 21 to 24. Note the release of human cells from BM to circulation (*data are from 1 fetus; see also Table 2).
Results from the remaining 2 groups (2C and 2D) are also shown in Table2 and Fig 3. The group 2D not receiving anti–VLA-4 showed that BM had the highest proportion of human cells (≈6.5%) with only 0.1% in fetal liver at day 25 of transplantation. This number represents the proportion of human cells detected by fluorescence-activated cell sorting (FACS) within BM or fetal liver single cell samples. The group treated with anti–VLA-4 (group 2C) showed that a significant proportion of human cells, by all methods of analyses, was present in the blood, whereas none were detected in control animals. It is of interest that in addition to detection of a significant proportion of cells in the blood, there was a reduction of cells detected within the BM in this group, at least in 1 animal that showed the highest number in peripheral blood. These data were compatible with mobilization of human cells from the BM to the peripheral blood as a result of anti–VLA-4 treatment. Although the animals were tested 4 days after the cessation of IV administration of anti–VLA-4 antibody, the antibody used was the long-lasting humanized anti–VLA-4, as indicated above.
DISCUSSION
In vitro assays are currently not available for human long-term repopulating cells, and transplantation studies in xenogeneic models, either the immune-deficient murine models11,12 or the human/sheep model,13,15 have shown great promise as in vivo assays of long-term repopulating cells. Validation of these models requires an understanding of their biology and confirmation of the conservation of basic aspects of transplant biology in xenogeneic environments. Most of the preliminary data presented with these models relate to engraftment of human cells several weeks after transplantation.21 24-26 Data on early distribution of hematopoietic cells in these models have not been published. We studied early events, ie, lodgment of human cells in the human/sheep model, to determine whether known adhesion interactions were conserved across species barriers and whether this model would be of use in more detailed studies of the homing properties and the stable engraftment of human cells hematopoietic in the microenvironment of the sheep.
Whether principles derived from the murine model about the early trafficking of transplanted cells and their distribution apply to larger animal models and specifically to human cells has not been clear thus far. The data presented herein provide for the first time information on the initial, early distribution of human cells transplanted into sheep fetuses and our attempts to modulate such a distribution. In contrast to the mouse model,10 at 3 hours posttransplantation, human cells were not detected in any of the tissues studied or in blood. This may not be surprising as the cells were given by intraperitoneal injection rather than intravenously and not in high inocula. By 24 hours, there is a significant proportion of human cells detected in BM and in other tissues, but not in blood, implying that by this time cells have been largely cleared from the blood. The fact that no significant differences in tissue distribution of human CD34+ cells were noted between 24 and 48 hours would also suggest that no significant proliferation has taken place between 24 and 48 hours, or that no net population increase has occurred assuming concurrent cell losses. The pattern of tissue distribution appears to be similar to the one observed in the murine model with the exception of the more active lodgment in the spleen of the mouse.10 Given that only ≈40% of the injected cells can be recovered within the tissues sampled (liver, BM, blood, spleen; Table 1) in the present or prior studies (data not shown), we assume that the missing cells are either distributed in other tissues in addition to the ones sampled, or are lost shortly after transplant. In either case, the present data are consistent with the notion that the majority of transplanted human CD34+ cells, like their murine counterparts, are not taken up preferentially by the BM, but are retained selectively once landed there, because of specific adhesive interactions and/or because they are able to survive and proliferate within the BM environment.
In sharp contrast to these background data, when cells are transplanted after they have been preincubated with an anti–VLA-4 MoAb, which blocks VLA-4–dependent adhesion in vitro, there is a significant proportion of cells detected in the blood at 24 and 48 hours with concomitant reduction of those settled in BM, suggesting inability of cells to settle in the BM. Detection in other tissues did not vary significantly. In other words, the data indicate that antibody-treated cells could no longer firmly adhere to BM sinusoidal cells, implicating the VLA-4 integrin as a significant determinant of the early lodgment of human hematopoietic cells into the fetal sheep BM, similar to data obtained in mice.10,27 Preincubation with another anti–VLA-4 antibody, B5G10, that does not block adhesion,19 resulted in a distribution of human cells similar to control data. Thus, only the antifunctional VLA-4 antibody limits the interaction of human cells with ligands on the sheep BM endothelial cells and/or stroma. The most likely ligand for this interaction is sheep VCAM-1, although VLA-4/fibronectin interactions cannot be excluded in this setting. As anti-human VCAM-1 antibody (4B9) appeared to cross-react with sheep VCAM-1 (E.D. Zanjani, unpublished results, 1996), this interaction appears likely. Within the same context, it was recently observed that VLA4 (+) porcine cells adhere to human endothelial monolayers in vitro,28 showing the presence of similarly cross-reacting ligands in this different xenogeneic setting.
Relative to the data in the murine model, the spleen did not appear to avidly capture circulating human CD34+ cells. While our data do not address the mechanism of splenic lodgment or its importance in fetal hematopoiesis, it is worth emphasizing that the murine studies were performed in preconditioned, irradiated, recipients, which has the potential to enhance splenic lodgment.29
The fact that we can influence the BM lodgment in the nonirradiated setting would imply that a functional VCAM-1 is likely expressed by the nonirradiated sheep BM vascular bed, as previously reported for murine BM.30 Furthermore, the effects in vivo of both anti–VCAM-1 and anti–VLA-4 antibodies in progenitor mobilization in normal mice10 and the mobilizing effect of anti-human VLA-4 in the sheep model can be cited to support the conjecture that the VLA-4/VCAM axis plays a pivotal role in normal trafficking of hematopoietic cells in a physiologic setting. It is of note that in the present experiments, for the first time, a significant reduction of human cells in BM accompanied the mobilization process (Table 2). This finding provides direct evidence that anti–VLA-4 causes a release of progenitors from BM, rather than inhibiting their reentry.
Having established patterns of interactions of human cells with the sheep microenvironmental cells, one could use this model then to manipulate homing patterns and test its impact on the level and durability of engraftment of human hematopoietic cells. For example, cytokines have been shown previously to influence the level of engraftment.31-33 In this model, one can also study whether the level of homing is altered with such treatments, or whether the increase in engraftment was solely due to enhanced proliferation. Finally, improvement of BM homing may prove to be of equal importance to the ex vivo expansion of hematopoietic cells for promoting engraftment. As an attempt to approach this issue, we treated the donor BM cells with an activating antibody to β1 integrin. Unfortunately, results of this treatment showed that much of the lodgment posttreatment is observed in the fetal liver with a concomitant reduction in BM lodgment. Such a preferential lodgment to fetal liver was observed previously in ontogenetically earlier transplants, before the BM is adequately developed for hematopoiesis.23Subsequently, donor cells predominantly engraft only in the fetal BM. Homing in fetal liver, like in BM, is also inhibited by anti–VLA-4 treatment of donor cells in prenatal transplants.34Furthermore, treatment of pregnant mice with anti–VLA-4 significantly reduced erythropoietic activity in the liver,35 and homing of fetal liver cells to adult BM in mice with platelet (P) and endothelial (E) selectin ablation was also inhibited by anti–VCAM-1.36 Taken together, the above observations could support the concept that the VLA-4/VCAM-1 axis plays a prominent role in the establishment of hematopoiesis in the fetal liver, but additional pathways are likely to participate. To explain the results with anti-β1 treatment, we do not believe that changes within the fetal liver environment have occurred and are responsible for the preferential lodgment of cells in fetal liver. Instead, we believe that activation of VLA-4 after anti-β1 treatments leads to enhanced adherence of donor cells in the endothelial vascular bed of many tissues, including the organ with the largest blood volume, ie, the liver. Thus, the higher retention of cells within the liver is explained, at least in part, by the liver-specific vascular anatomy and hemodynamics. Nevertheless, it was somewhat surprising to see higher levels in circulation than in controls. These persistently circulating cells may represent either loosely attached cells to larger vessels, or they may reflect a state of integrin unresponsiveness that follows the initial stimulation by 8A2.20 Additional experiments are necessary to clarify some of these issues.
In summary, in the present studies, we have described the patterns of homing of human cells in the fetal sheep and its modulation by activating and inhibiting antibodies of VLA-4–dependent adhesion. We have noted that aspects of homing and mobilization of human cells in the sheep BM microenvironment are similar to those found for murine cells. Although several adhesion pathways may play a role in homing, either concurrently or sequentially, the VLA-4–dependent adhesion pathway assumes a dominant role in hematopoietic homing, which is conserved between widely disparate species. Therefore, our model offers the potential to gain further insights into the normal biologic behavior of human hematopoietic cells of different ontogenetic stages, as well as their behavior following prenatal or postnatal transplantation.
ACKNOWLEDGMENT
The assistance of Betty Nakamoto in certain aspects of this work and the secretarial help of Margaret Oppenheimer are gratefully acknowledged.
Supported by Grants No. HL46556, HL46557, HL49042, HL52955, HL96020, and DK51427 from the National Institutes of Health and by the Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Thalia Papayannopoulou, MD, DrSci, Division of Hematology, University of Washington, Box 357710, Seattle, WA 98195-7710; e-mail: thalp@u.washington.edu.