Abstract
Evidence has been provided recently that shows that high concentrations of cytokines can fulfill functions previously attributed to stromal cells, such as promote the survival of, and led to a net increase in human primitive progenitors initiating long-term cultures in vitro (LTC-IC) or engrafting NOD-SCID (nonobese diabetic severe-combined immunodeficient) recipients in vivo. These data prompted us to re-evaluate whether stromal cells will further alter the properties of primitive progenitor cells exposed to cytokines. Single CD34+CD38low and CD38neg cells were incubated 10 days in serum-containing or serum-free medium in the presence or in the absence of murine marrow-derived stromal cells (MS-5). Recombinant human cytokines stem cell factor (SCF), pegylated-megakaryocyte growth and differentiation factor (PEG–MGDF), FLT3-L, Interleukin (IL)-3, IL-6, and granulocyte-macrophage colony-stimulating factor (GM–CSF) were systematically added at various concentrations (10 to 300 ng/mL). Cell proliferation and LTC-IC potential were evaluated in each clone after 10 days. A striking and consistent observation was the retention of a high LTC-IC potential in clones exposed to cytokines in the presence of stromal feeders, whereas clones exposed to cytokines alone in the absence of stromal feeders rapidly lost their LTC-IC potential as they proliferated. This was reflected both by the higher proportion of wells containing LTC-IC and by the high numbers of CFC produced after 5 weeks in clones grown with MS-5 during the first 10 days. We further showed by analyzing multiple replicates of a single clone at day 10 that MS-5 cells promoted a net increase in the LTC-IC compartment through self-renewal divisions. Interestingly, these primitive LTC-IC were equally distributed among small and large clones, as counted at day 10, indicating that active proliferation and loss of LTC-IC potential could be dissociated. These observations show that, in primitive cells, stromal cells counteract differentiation events triggered by cytokines and promoted self-renewal divisions. Furthermore, the almost identical distribution of the size of the clones with or without MS-5 suggests that proliferation and function of human primitive cells may be independently regulated by external signals, and that the former is primarily under the control of cytokines.
UNTIL RECENTLY, STROMAL cells were viewed as essential to the maintenance, in vitro, of stem cell properties such as the ability to reconstitute the hematopoietic system in vivo after transplantation1-3 or in vitro in long-term cultures.4,5 These properties are measured by the production of colony-forming cells (CFC) or cobblestone areas in long-term cultures that identifies long-term cultures in vitro (LTC-IC)6,7 and CAFC (cobblestone area forming cells).8 Surprisingly, in these long-term cultures, murine stromal feeder layers have proved to do as well as human marrow-derived adherent cells, if not better.9-14 Multiple mechanisms, often contradictory, have been proposed to explain the regulatory effect of stromal cells on stem cell functions: survival of quiescent cells,7,15,16 increased proliferation and differentiation,17 or, conversely, decreased cell proliferation mediated by contact with stromal elements.18-20 Accordingly, both stromal-derived negative as well as positive signals have been characterized.7,17,19,21-24 However, recently, the absolute requirement of stromal cells in vitro to maintain stem cell properties has been questioned because stromal-derived cytokines such as stem cell factor (SCF), FLT3-L,25 or MGDF (Megakaryocyte Growth and Differentiation Factor also known as thrombopoietin or MPL ligand) are, on their own, potent inducers of the survival and the proliferation of early stem cells.26-31 Moreover, when used in vitro at very high concentrations, these molecules led to a net increase in the number of various types of primitive progenitor cells, including human LTC-IC32 and NOD-SCID-competitive repopulating unit (CRU),33 and murine CRU.34 These studies showed two important observations: first, both the combination and concentrations of cytokines required to stimulate early (LTC-IC and CRU) versus late (CFC) progenitors were different,32,35 and second the retention or loss of a primitive function can be regulated independently from the number of cell divisions.32 However, controversy exists because experiments performed in vivo, as opposed to in vitro as above, yielded different conclusions and suggested that the administration of cytokines in murine transplantation models can lead to a loss of stem cells.36 37
Now that potent cytokines are available that act on stem cells, whether or not the addition of stromal cells can perturb the functions of the activated progenitors needs to be reevaluated. This reappraisal has obvious clinical relevance; first, to assess if stromal cells can counteract the potential damage induced by cytokines in stem cells, especially after drug exposure.38 Second, to design conditions to amplify stem cells ex vivo with compromising neither their quality, nor their ability to return to a quiescent state and survive for a long period.
In the present study, we examined if murine stromal cells MS-5, combined to cytokines during 10 days, will alter the LTC-IC function of human marrow-derived primitive CD34+CD38low/negcells. Experiments were performed at the single-cell level and both cell proliferation as well as LTC-IC function were separately evaluated for each clone. We show that the addition of murine stromal cells during the first 10 days of culture prevented the loss of both the number and the quality of LTC-IC, even when cells were stimulated by high concentrations of cytokines. Interestingly, stromal cells only slightly altered the number of nucleated cells per clone, thus showing that stem cell function and proliferation can be independently regulated.
MATERIALS AND METHODS
Collection and Fractionation of Human Bone Marrow Mononuclear Cells
Bone marrow samples were obtained with informed consent from patients undergoing hip surgery. Cells were extracted from the bone fragments as previously described.17 Low-density mononuclear cells were subjected to a standard CD34 immunomagnetic bead separation using the miniMACS system following the manufacturer’s guidelines (Miltenyi Biotec, CA). CD34+ cells were further purified by cell sorting either immediately or after overnight incubation at 4°C. Before sorting, CD34+ cells were incubated with a 1/5 dilution of a phycoerythrin-(PE)-Cy5-anti-CD34 monoclonal antibody (MoAb) and PE–anti-CD38 MoAb (both from Immunotech, Marseille, France). Sorting of CD34+CD38low and CD34+CD38neg fractions was performed using a FACSVantage (Becton-Dickinson) equiped with an argon ion laser (Innova 70-4-Coherent radiation, Palo Alto, Ca) tuned to 488 nm and operating at 500 mw. A morphological gate including all of the CD34+cells was defined on two parameter histograms side scatter (SSC) versus forward scatter (FSC). Limit for CD38neg cells was defined using control cells labeled with the PE-Cy5-CD34 and an irrelevant IgG1 MoAb. CD34+ cells were considered negative for the CD38 antigen (CD38neg ) if the MFI (mean fluoresence intensity) was half (or less) of that observed for control cells. CD38low cells included CD38neg cells and a proportion of cells expressing low levels of CD38. CD38negcells represented 1% to 2% and CD38low cells 10% to 15% of total CD34+ cells. Compensation was set up as described above. The automatic cloning design unit of the FACSVantage was used to initiate single-cell cultures into 96-well tissue culture plates filled with the appropriate cytokines and precoated or not with MS-5 cells as described.13 The presence of individual cells in wells was checked 10 to 24 hours later by microscopic examination of round-bottom 96-well plates. In some experiments, the viability of sorted cells was verified before sorting by labeling with 7-AAD (Sigma, St Quentin Fallavier, France).
MS-5 cells39 were grown as previously described in minimal essential medium (αMEM) supplemented with 10% fetal calf serum (FCS) and passaged every 10 days. Cells from early passages (below 15) only were used as feeders to support human hematopoiesis.
Assessment of the Proliferation and LTC-IC Potentials of Single Cells Cultured With Cytokines and Stromal Cells
Experimental design.
Cells were cultured after a three-step procedure. Step 1, sorted single CD34+CD38low or CD38neg cells were first cultured during 10 days in αMEM (when serum was present) or in Iscove’s medium (IMDM) (when serum was omitted) with a cocktail of six growth factors in the presence or in the absence of a confluent layer of MS-5 cells. All cultures were performed at 37°C in air supplemented with 5% CO2. Step 2, at the end of this 10-day period, the total content of each well was transferred to standard LTC conditions on MS-5 feeder layers.6,10 Step 3, after 5 weeks in culture, the content of each well in CFC was assayed in standard semi-solid colony assays.10 In some experiments (detailed in the results section) the number of nucleated cells was counted in each well after 3 to 4 days to determine the onset of initial cell division, and again after 10 days to evaluate total cell proliferation. For each of these clones, two parameters were thus determined at day 10: the number of nucleated cells and the LTC-IC potential. The terminology used in this study is defined in Table1.
Step 1, short-term culture in cytokines with or without stromal cells.
During the first 10-day period, cells were cultured either in 10% prescreened FCS (Stem Cell Technologies, Vancouver, Canada) or in serum-free IMDM supplemented with 100 ng/mL insulin, iron-saturated human transferrin (300 μg/mL), 1% deionized serum albumin, and a mixture of sonicated lipids as previously described.40Cytokines were used either at low concentrations (low GF), or at high concentrations (high GF). Low GF included: 10 ng/mL of PEG-recombinant human (rhu)-MGDF (a kind gift from AMGEN, Thousand Oaks, CA), 100 IU/mL of rhu-Interleukin (IL)-6, 10 ng/mL of rhu-GM-CSF, 10 ng/mL of rhu-FLT3-ligand (FLT3-L, purchased from Diaclone, Besançon, France), 20 ng/mL of rhu-Stem Cell Factor (SCF) (a kind gift from AMGEN), and 2 ng/mL of rhu-IL-3 (a kind gift from Novartis, Basel, Switzerland). In high GF, rhu-SCF and rhu-FLT3-L were used at 100 to 300 ng/mL and rhu–IL-3 at 60 ng/mL. The concentration of the other cytokines was kept unchanged.
Cultures were initiated either by plating 5,000-10,000 CD34+CD38low cells in 24-well plates precoated or not with murine stromal cells MS-5 (bulk cultures), or by sorting single cells, both CD34+CD38low and CD34+ CD38neg , in flat-bottomed (with MS-5 cells) or round-bottomed (no MS-5 cells) 96-well plates. All cultures were kept at 37°C in an air atmosphere supplemented with 5% CO2 and saturated with humidity and fed once 6 to 7 days after initiation by a half-medium change (care was taken not to eliminate cells).
Steps 2 and 3, assessment of the LTC-IC potential.
At day 10, the supernatant of each well was carefully aspirated and the total cell content (including MS-5 cells) was transferred to a new 96-well plate precoated with MS-5 cells and incubated in standard LTC medium (ie, α-MEM with 12.5% horse serum, 12.5% FCS, 10−4 mol/L 2-β-mercaptoethanol, but with neither hydrocortisone nor cytokines).10 Plates were incubated at 33°C, in air atmosphere with 5% CO2, with a weekly half-medium change as described for the maintenance of LTC-IC. After 5 weeks in culture, wells were trypsinized and the total content of each well was plated in one mL of standard methylcellulose mixture (see below).
In some single-cell experiments, LTC-IC were precisely quantitated at day 10 by initiating 10 LTC replicates from the content of each clone. Replicate wells were then maintained as described above and the CFC content of each replicate assessed after 5 weeks. To measure the content of bulk cultures in LTC-IC, cultured CD34+ cells were sorted at day 10 and used to initiate limiting-dilution LTC-IC assays. The absolute number of LTC-IC was deduced as described.10
Quantitation of CFC at week 5 (step 3) was performed using standard methylcellulose colony-assays10 and the following cytokines: rhu-erythropoietin (Epo, 2 IU/mL, a gift from CILAG, Issi, Les-Moulineaux, France), rhu-SCF (50 ng/mL), rhu-granulocyte CSF (G-CSF) (10 ng/mL) and rhu–IL-3 at 2 ng/mL. Erythroid and granulomacrophagic colonies were scored at day 14 to 18 using previously described criteria.10 In single-cell experiments, the total cell content of each well was transferred in one mL of methylcellulose medium. In the case of bulk cultures, the number of colonies was evaluated by plating an aliquot (1,000 to 2,000 cells) and the absolute numbers of CFC produced per well (per input cell number) was calculated.
RESULTS
Murine Stromal Cells Increase CFC and LTC-IC Progeny of CD34+CD38low Cells Exposed to Growth Factors
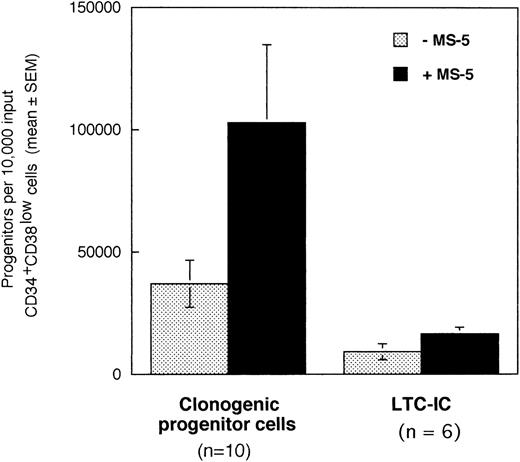
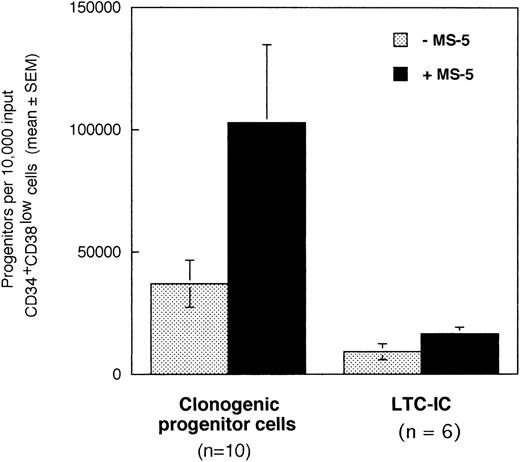
In a first series of 6 to 10 experiments, we tested if the addition of stromal cells to 10,000 CD34+CD38low marrow cells exposed to cytokines for 10 days will alter the output of CFC and LTC-IC. Cells were incubated in low GF (6 cytokines, 2 to 10 ng/mL each, see Materials and Methods for details) in the absence or in the presence of a monolayer of murine stromal cells MS-5. After 10 days, cultured cells were plated in standard-colony assays to determine the absolute numbers of CFC (n =10). Cultured CD34+ cells were sorted and plated at limiting dilution (10 to 100 cells/well) in LTC and the absolute number of day 10 LTC-IC (n = 6) was derived from the calculated frequency (Fig 1).10
Effect of the murine stromal cells MS-5 on the output of CFC and LTC-IC. In 6 to 10 experiments, 10,000 CD34+CD38low cells were grown in the presence of 10% FCS with low GF and in the presence (▪) or absence (░) of MS-5 cells. After 10 days, aliquots of cells were assessed in colony-assays and 5-week LTC-IC assays and the absolute numbers of CFC and LTC-IC derived from 10,000 input cells were calculated. Histograms represent the mean ± SEM of the absolute number of CFC and LTC-IC obtained from 6 to 10 independent experiments.
Effect of the murine stromal cells MS-5 on the output of CFC and LTC-IC. In 6 to 10 experiments, 10,000 CD34+CD38low cells were grown in the presence of 10% FCS with low GF and in the presence (▪) or absence (░) of MS-5 cells. After 10 days, aliquots of cells were assessed in colony-assays and 5-week LTC-IC assays and the absolute numbers of CFC and LTC-IC derived from 10,000 input cells were calculated. Histograms represent the mean ± SEM of the absolute number of CFC and LTC-IC obtained from 6 to 10 independent experiments.
Numbers of total nucleated cells were unaltered by the presence of stromal cells (data not shown). Conversely, absolute numbers of both CFC and LTC-IC were increased threefold in cultures maintained on MS-5 feeders during the first 10 days (paired data analysis,P < .001) (Fig 1). Thus, 10,000 CD34+CD38low cells produced on average 102,830 (±32,000) CFC and 16,620 (±2,660) LTC-IC at day 10 in the presence of MS-5 and 36,800 (±9,600) CFC and 9,170 (± 3,270) LTC-IC without MS-5. The average number of week-5 CFC per LTC-IC, calculated from limiting dilution experiments, was similar with (2.4 ± 0.9 CFC) and without (2.9 ± 1.5 CFC) MS-5 cells. Independently of the presence of stromal cells, the number of LTC-IC found after 10 days exceeded the input number of LTC-IC by a factor of 10 and 4 with and without MS-5 respectively (data not shown). In comparison, input numbers of CFC were expanded 100-fold in the presence of stromal cells, and 40-fold with cytokines alone (data not shown).
To understand if the increased number of LTC-IC in the presence of MS-5 feeders resulted from their recruitment, their increased survival, or self-renewal, we analyzed the proliferation and LTC-IC function of CD34+CD38low and CD34+CD38neg single cells exposed to cytokines with or without MS-5 feeder layers.
Proliferative Potential of Single CD34+CD38low and CD34+CD38neg Cells Grown With Cytokines and Murine Stromal Cells
Most CD34+CD38low/neg progenitors are classically in a quiescent G0/G1 stage but are induced to cycle within 72 hours when stimulated by adequate concentrations of cytokines despite marked heterogenity in the proliferative response.41 In our conditions also, most viable CD34+CD38low cells (84%) incubated with cytokines divided at least once within 72 hours, and 70% at least twice in high GF conditions. CD34+CD38neg cells were less immediately responsive to cytokines than CD34+CD38low cells because at day 3, 60% to 70% only of the wells contained two or more cells (>1 division).
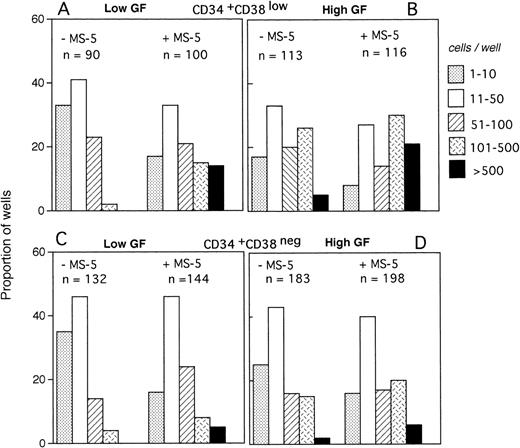
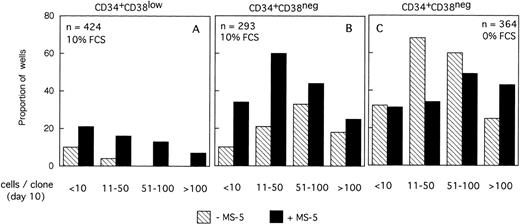
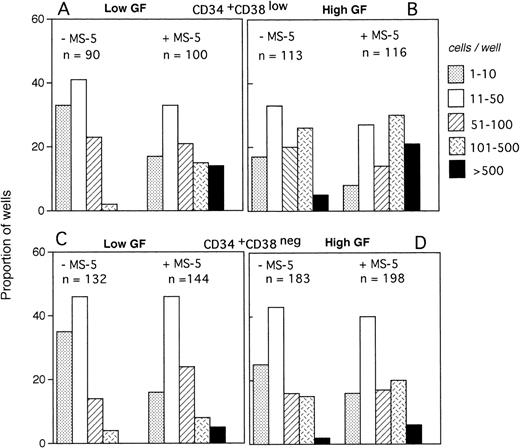
The size of the clones was also evaluated at day 10. In the absence of MS-5 cells, very few (4%) wells contained only one viable cell. The number of wells in which active cell proliferation was observed was identical with and without feeders, indicating that MS-5 cells did not act by recruiting additional cells into cycle. As shown in Fig 2A, the size of the clones was only slightly increased when MS-5 cells were present; 24% of clones grown without MS-5 cells, but 50% of those grown in the presence of MS-5 cells, contained more than 50 cells at day 10. As expected, in high GF (Fig 2B), these proportions raised to 50% and 83% without and with MS-5 feeders, respectively.
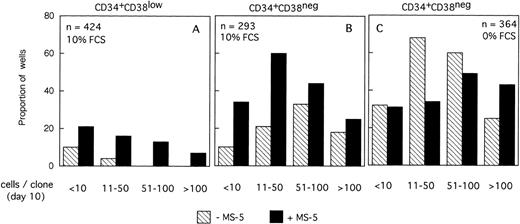
Distribution of the size of clones generated by single CD34+CD38low (3 experiments) and CD34+CD38neg (5 experiments) cells at day 10. Single-cell cultures were established from CD34+CD38low (A,B) or CD38neg(C,D) in the presence or the absence of MS-5 cells and in low GF (A,C) or high GF (B,D) as indicated. Nucleated cells were counted in each well at day 10 and the clones classified according to their size, as indicated. Each histogram represents the proportion of the (n) proliferating wells containing the indicated number of nucleated cells.
Distribution of the size of clones generated by single CD34+CD38low (3 experiments) and CD34+CD38neg (5 experiments) cells at day 10. Single-cell cultures were established from CD34+CD38low (A,B) or CD38neg(C,D) in the presence or the absence of MS-5 cells and in low GF (A,C) or high GF (B,D) as indicated. Nucleated cells were counted in each well at day 10 and the clones classified according to their size, as indicated. Each histogram represents the proportion of the (n) proliferating wells containing the indicated number of nucleated cells.
The distribution of the number of cells per clone initiated with CD34+CD38neg cells (n=5, 840 wells) (Fig 2C and D) was very similar, except that, in agreement with their delayed response to cytokines, a lower proportion of clones contained more than 50 cells. As noted for CD34+CD38low cells, MS-5 cells also increased by a factor of two the proportion of large clones (>100 cells).
These data indicate that MS-5 cells did not recruit input cells to proliferate, but acted in synergy with cytokines to increase cell proliferation, an effect that also occurs in colony assays as we have previously reported.17
Maintenance of a High LTC-IC Potential in Individual CD34+CD38neg/low Clones Grown in the Presence of MS-5 Cells
We next investigated if the addition of stromal cells to cytokines will alter the LTC-IC potential of input cells. Both the number of wells containing LTC-IC (ie, containing at least one CFC after 5 weeks in standard LTC) and the CFC output per well were measured. Results obtained with cells grown in the presence of 10% serum will be presented first and the last section will mention results obtained in serum-free conditions.
MS-5 cells increase the proportion of clones containing LTC-IC at day 10.
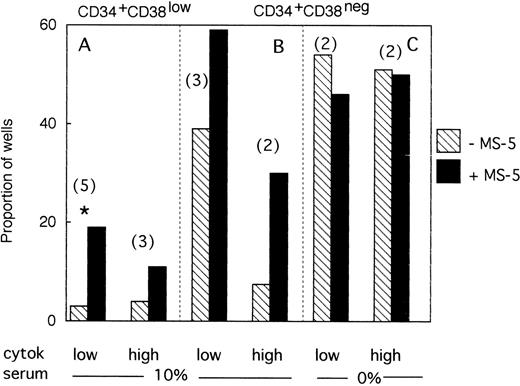
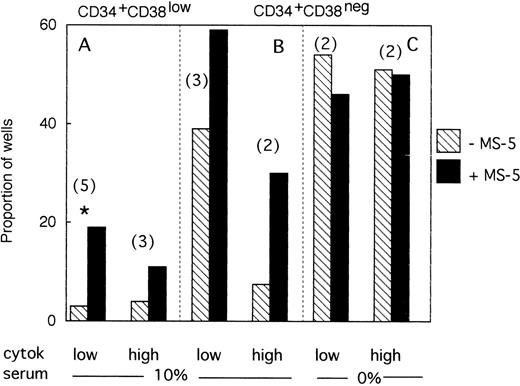
A total of 954 CD34+CD38low cells (5 different bone marrow samples) were cultured individually during 10 days, as described above, in low GF and 10% FCS either with (494 wells), or without (460 wells) MS-5 cells. Proliferating clones (>1 cell) at day 10 were transferred to standard LTC-IC conditions. Among the 460 clones cultured without MS-5, 140 were assessed for LTC-IC and 5 only (3%) were positive. Similar analysis of 140 of 494 clones grown with MS-5 cells yielded 27 positive clones (19%)(Fig3A). This difference in the proportion of LTC-IC–positive wells was observed in each of the five experiments and was statistically significant (paired data analysis, P < .001). This could have been explained if MS-5 cells supplied additional amounts of FLT3-L and/or SCF, because in these initial experiments concentrations of exogeneously added cytokines were below those reported to amplify primitive cells.28 To test this hypothesis, additional wells (540 in three separate experiments) were seeded with CD38low in high GF and 229 analyzed for LTC-IC content. In the presence of MS-5, 13 of 116 (11%) contained LTC-IC at day 10 but only 5 of 113 (4.5%) without MS-5 cells (Fig 3A).
Effect of MS-5 cells on the proportion of CD34+CD38low or CD38neg clones that contain LTC-IC at day 10. Single CD34+CD38low (A) cells and CD34+ CD38neg (B,C) cells were seeded in the presence (▪) or absence () of MS-5 either in 10% serum (A,B) or in serum-free condition (C) with low and high GF. At day 10, each proliferating well (containing >1 cell) was transferred in standard LTC-IC conditions in wells precoated with MS-5. After 5 weeks, each well was assessed for its content in CFC and a well was scored as LTC-IC positive if it contained at least one CFC. Each histogram represents the proportion of LTC-IC–positive wells at day 10 among proliferating clones. In brackets is indicated the number of experiments. * paired t-test was statistically significant. Paired t-test calculated for pooled A + B data was also significant.
Effect of MS-5 cells on the proportion of CD34+CD38low or CD38neg clones that contain LTC-IC at day 10. Single CD34+CD38low (A) cells and CD34+ CD38neg (B,C) cells were seeded in the presence (▪) or absence () of MS-5 either in 10% serum (A,B) or in serum-free condition (C) with low and high GF. At day 10, each proliferating well (containing >1 cell) was transferred in standard LTC-IC conditions in wells precoated with MS-5. After 5 weeks, each well was assessed for its content in CFC and a well was scored as LTC-IC positive if it contained at least one CFC. Each histogram represents the proportion of LTC-IC–positive wells at day 10 among proliferating clones. In brackets is indicated the number of experiments. * paired t-test was statistically significant. Paired t-test calculated for pooled A + B data was also significant.
We obtained similar results in three experiments initiated with CD34+CD38neg cells. At day 10, 18 of 45 wells without a layer of MS-5 cells read out as LTC-IC–positive (39%) and 41 of 68 (60%) with MS-5 (Fig 3B). As described above, increasing the concentration of GF did not abolish this effect of MS-5 cells because 7 of 94 wells (7.5%) cultured without stromal cells and 30 of 102 (30%) with MS-5 cells were scored as LTC-IC–positive. Although the number of experiments in each condition was too low to yield statistical significance, calculations, analysis of pooled data (paired data analysis) from all five experiments, yield a statistically significant difference.
Therefore, MS-5 cells increased two- to fivefold the proportion of LTC-IC–positive wells seeded with CD38low and CD38neg populations and this effect was not abolished by a 30-fold increase in the concentration of FLT-3L, SCF, and IL-3.
In some wells precoated with MS-5, we did not detect hematopoietic cells at day 10. To rule out that these will be erroneously considered as LTC-IC–negative, we replated individually 414 of these empty wells in LTC. Twenty-eight (6%) yielded CFC at week 5 (geometric mean of 13 CFC/positive well). A similar analysis of 377 clones without MS-5 with ≤1 cell yielded 12 positive clones (3%) with a mean of 8 CFC per positive well.
MS-5 cells had a major impact on the number of CFC produced by day-10 LTC-IC.
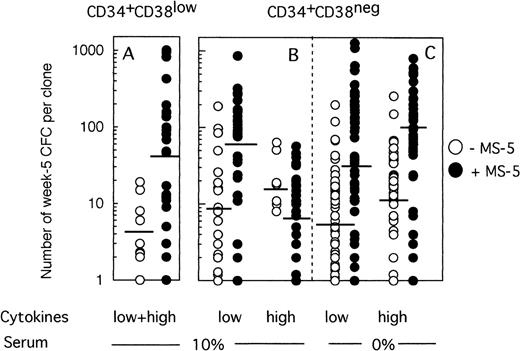
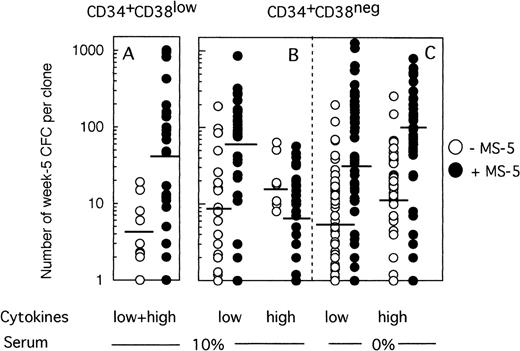
Because any cell that generates at least one CFC at week 5 is defined as an LTC-IC, the LTC-IC compartment is very heterogeneous and includes cells producing high as well as low numbers of CFC at week 5. To determine if culture conditions selected for high-producing or low-producing LTC-IC, we analyzed the number and type of CFC produced at week 5 from each clone. Two striking observations emerged from data illustrated on Fig 4. First, the heterogeneity in CFC output per clone was very large, ranging from 1 CFC to over 1,000 CFC per clone, independently of the culture conditions. Second, strikingly, more CFC were produced at week 5 from wells precoated with MS-5 cells. Thus, in two experiments initiated with CD34+CD38low cells (Fig 4A), 4 CFC (geometric mean, range 1 to 19, 10 wells) were produced without MS-5 and 42 (range 1 to 1,013, 27 wells) with MS-5 cells (P < .001). This difference was highly significant and reproduced in both experiments. Ten wells with feeder cells, but none without MS-5, yielded more than 100 CFC and 4 over 400 CFC. Thirty percent of positive wells contained both CFU-GM and immature burst-forming unit (BFU)-E.
Effect of MS-5 cells on the number of CFC produced in each clone at week 5. Single cells were cultured as described in the legend of Fig 3, either in the presence (•) or the absence (○) of stromal cells. After 10 days, each clone was replated in standard LTC-IC assays and the number of LTC-IC–derived CFC assessed at week 5. Each point represents the CFC output per individual clone seeded either with (•) or without (○) MS-5 during the first 10 days of culture. For each condition, the geometric mean of the numbers of CFC produced is indicated by the horizontal bar.
Effect of MS-5 cells on the number of CFC produced in each clone at week 5. Single cells were cultured as described in the legend of Fig 3, either in the presence (•) or the absence (○) of stromal cells. After 10 days, each clone was replated in standard LTC-IC assays and the number of LTC-IC–derived CFC assessed at week 5. Each point represents the CFC output per individual clone seeded either with (•) or without (○) MS-5 during the first 10 days of culture. For each condition, the geometric mean of the numbers of CFC produced is indicated by the horizontal bar.
MS-5 cells similarly affected the number of CFC produced by CD34+CD38neg LTC-IC at day 10 in low GF conditions (Fig 4B). Thus, 9 CFC (range 1 to 190, 18 wells) on average were produced without MS-5 and 54 (range 1 to 864, 30 wells) with MS-5 (P < .001) (Fig 4B). Interestingly, in the presence of high concentrations of cytokines, the number of CFC per well was unexpectedly low in the presence of MS-5 (7 CFC, range 1 to 57, 30 wells). This loss of potential likely resulted from cell differentiation, as confirmed by the high number of nucleated cells present at week 5 in wells that no longer contained CFC.
Influence of MS-5 Cells on the LTC-IC Potential of CD34+CD38neg Cells Grown in Serum-Free Medium
All experiments described above were performed in 10% FCS. To rule out that serum components will interfere with the above-described effect of stromal cells, in two experiments we seeded and analyzed 364 individual CD34+CD38neg cells in serum-free conditions (Fig 3C and 4C) and examined the proportion of LTC-IC–positive clones and the output of CFC. Interestingly, the proportion of LTC-IC–positive wells was identical with and without MS-5 (Fig 3C), and this result was independent of the concentrations of cytokines used.
Nevertheless, the number of CFC produced per clone grown with MS-5 cells was still much higher (geometric mean: 28 [1 to 1,272, 47 wells] in low GF and 77 [2 to 800, 40 wells] in high GF) than without a stromal support (geometric mean: 6.5 [1 to 198, 54 wells] in low GF and 10 [1 to 259, 53 wells] in high GF) (Fig 4C). These differences were highly significant in both experiments.
Therefore, a major and consistent effect of MS-5 cells was to retain at day 10 a very high LTC-IC potential (reflected by the CFC output) in individual clones, even in the presence of high concentrations of cytokines. In contrast, stromal cells only marginally affected cell proliferation, and recruited LTC-IC only in the presence of serum.
MS-5 cells increased the LTC-IC potential of individual cells by promoting self-renewal divisions.
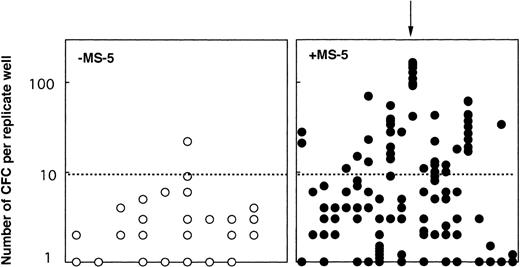
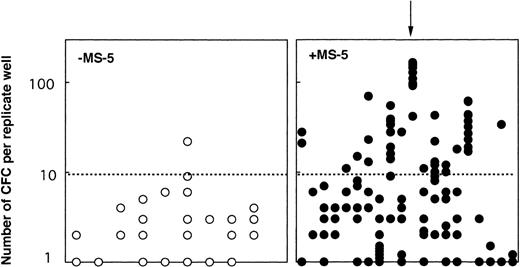
One possibility for primitive cells to retain their LTC-IC potential while proliferating through self-renewal divisions. We tested this hypothesis in two experiments: 103 CD34+CD38neg clones grown 10 days with (65) or without (38) MS-5 were each divided at day 10 into 10 replicate LTC-IC assays. Two conclusions can be drawn from these experiments (Table2). First, 60% (38 of 65) of the clones initially grown on MS-5, but only 53% (19 of 38) of the clones grown in cytokines alone yielded at least one positive replicate. However, there was a major difference in the number of positive replicates; 17 of the 38 positive clones grown on MS-5 yielded greater than 5 positive replicates and in 5 clones, all 10 replicates generated CFC at week 5 (Table 2). In contrast, 9 of 19 positive clones grown without MS-5 generated two or more positive replicates, but only one gave 6 positive replicates. Second, the number of CFC per replicate well was also dramatically higher if MS-5 cells were present initially (Fig 5). Moreover, the numbers of CFC detected in each of the replicates from a same clone were very similar indicating that the potential of each daughter LTC-IC was very similar (see arrow on Fig 5). These experiments show that multiple LTC-IC were present in clones grown 10 days in the presence of MS-5 cells, and that these were produced by self-renewing divisions of LTC-IC (or the differentiation of a more primitive ancestor). Interestingly, these results were in agreement with our observations derived from bulk culture experiments (Fig 1).
Number of week-5 CFC produced in replicate LTC-IC assays initiated at day 10 with cells from individual clones. At day 10, 38 clones grown without MS-5 cells and 65 clones grown with MS-5 were subdivided into 10 replicate wells further kept in standard LTC-IC conditions. CFC were measured in each replicate after 5 weeks. Results for 20 clones with MS-5 cells (right panel, •) and 9 clones without MS-5 (left panel, ○) are shown. Each point represents the number of CFC in one replicate and results for all replicates from one clone are lined on the vertical axis (as indicated by the arrow). Data presented are from one experiment (out of two) initiated with CD34+CD38neg cells.
Number of week-5 CFC produced in replicate LTC-IC assays initiated at day 10 with cells from individual clones. At day 10, 38 clones grown without MS-5 cells and 65 clones grown with MS-5 were subdivided into 10 replicate wells further kept in standard LTC-IC conditions. CFC were measured in each replicate after 5 weeks. Results for 20 clones with MS-5 cells (right panel, •) and 9 clones without MS-5 (left panel, ○) are shown. Each point represents the number of CFC in one replicate and results for all replicates from one clone are lined on the vertical axis (as indicated by the arrow). Data presented are from one experiment (out of two) initiated with CD34+CD38neg cells.
Extensive cell proliferation did not result in the loss of LTC-IC potential.
We have shown previously that MS-5 cells did not dramatically change the numbers of cells/clone at day 10. However, because the size of the clones varied from 10 to over 500 (Fig 2), it was possible that high-potential LTC-IC would have been found preferentially among low-size clones, as expected if there is concomittant loss of potential as the cell divides.
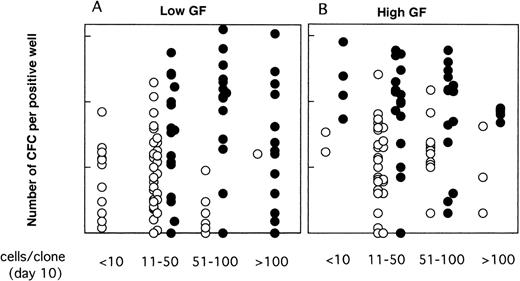
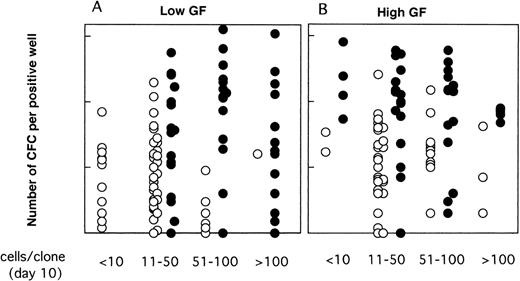
As illustrated, this was the case neither for the proportion of LTC-IC–positive wells (Fig 6) nor for their CFC content (Fig 7). Thus, CD34+CD38low clones with greater than 50 cells contained LTC-IC only if MS-5 cells were present during the first 10 days. Results were somewhat different for CD34+CD38neg clones (Fig 6B and C); very small clones (<10 cells) usually did not contain LTC-IC, as opposed to 10 to 100 cell clones, suggesting that parental cells (which probably correspond to extended LTC-IC7) have to undergo a minimal number of divisions before they can be assayed as LTC-IC in a 5-week assay (as opposed to a 12-week assay7). The decrease in the proportion of LTC-IC–positive wells with increasing clone size was more rapid in the absence of MS-5 cells, and this was particularly noticed in serum-free conditions (Fig 6C).
Proportions of LTC-IC–positive clones as a function of the size of the clones at day 10. Data are from the same experiments as for Fig 4. Single CD34+CD38low (A) and CD38neg (B to C) cells were grown with (A,B) or without (C) serum, in the presence (▪) or in the absence () of MS-5 cells. Results obtained in low GF and high GF have been pooled. Each bar represents the proportion of LTC-IC–positive wells at day 10 according to the size of the clone as indicated on the horizontal axis.
Proportions of LTC-IC–positive clones as a function of the size of the clones at day 10. Data are from the same experiments as for Fig 4. Single CD34+CD38low (A) and CD38neg (B to C) cells were grown with (A,B) or without (C) serum, in the presence (▪) or in the absence () of MS-5 cells. Results obtained in low GF and high GF have been pooled. Each bar represents the proportion of LTC-IC–positive wells at day 10 according to the size of the clone as indicated on the horizontal axis.
Moreover, as shown in Fig 7, in the presence of MS-5 cells, there was no decrease in CFC output per clone with increasing clone size; thus, clones with 11 to 50, 51 to 100, and greater than 100 cells contained on average 29, 54, and 21 CFC (geometric mean) in low GF and 78, 62, and 66 CFC in high GF. In contrast, in clones grown without MS-5 cells, the mean number of CFC decreased with increasing clone size and was below that observed with MS-5 (Fig 7). In keeping with these results, we should also mention that 7 of 17 clones that yielded more than 5 replicates in Table 1contained 200 cells or more at day 10.
Effect of MS-5 cells on the number of week-5 CFC as a function of the size of the clone at day 10. Single CD34+CD38neg cells were cultured in serum-free conditions, in low GF (A) or high GF (B) either in the presence (•) or the absence (○) of MS-5 cells. After 10 days, each clone was replated in standard LTC-IC assays and the number of LTC-IC–derived CFC assessed at week 5. Each point represents the number of CFC produced by an individual clone.
Effect of MS-5 cells on the number of week-5 CFC as a function of the size of the clone at day 10. Single CD34+CD38neg cells were cultured in serum-free conditions, in low GF (A) or high GF (B) either in the presence (•) or the absence (○) of MS-5 cells. After 10 days, each clone was replated in standard LTC-IC assays and the number of LTC-IC–derived CFC assessed at week 5. Each point represents the number of CFC produced by an individual clone.
DISCUSSION
We have shown in this study that MS-5 cells could rescue the loss of LTC-IC potential induced in single CD34+CD38lowor CD38neg marrow cells by high concentrations of soluble cytokines. We further showed that a net expansion in the number of LTC-IC generated in 10 days was responsible for this effect. Interestingly, even though stromal cells dramatically altered the LTC-IC potential of cultured cells, they did not significantly modify cell proliferation, which implies that cell proliferation and function can be independently controlled by external signals.
The retention of LTC-IC potential by MS-5 cells was reflected in the increased proportion of wells scored as LTC-IC–positive at day 10, but primarily in the higher output of CFC per clone at week 5, as compared with wells initiated in the absence of stromal cells. Thus, 40% to 50% of the clones grown 10 days with MS-5, but only less than 10% of those cells cultured without, produced 100 CFC or more at week 5, and 25% of these wells contained both immature BFU-E and CFU-GM. As shown by initiating multiple replicate assays from individual clone at day 10, multiple LTC-IC were present at day 10 in wells seeded on MS-5 feeders, indicating that self renewal has occurred.
As previously mentioned by others,42,43 there was a wide variation in the number of CFC produced at week 5 by individual clones (from 1 to 1,000 CFC), but no inverse correlation was observed between these CFC numbers and that of nucleated cells per well as could have been expected (as illustrated in Fig 7). This first confirms the heterogeneity in LTC-IC function of cells that initially share the same phenotype (CD34+CD38neg) and cell cycle status (G0/G1).44 Whether this diversity reflects intrinsic differences between input cells or a stochastic response to external regulators is unknown.45 Importantly, this also highlights the need to work at single-cell level, and to rely on function rather than phenotype to assess the primitiveness of both fresh46,47 and cultured cells.48 49
A second finding was that, in contrast to their striking effect on the amplification of high potential LTC-IC, MS-5 cells only marginally affected the size of the clones. Cell proliferation was controlled primarily by cytokines and MS-5 acted in synergy with those to enhance, although slightly, the size of the clones at day 10.17Consequently, the extent of cell proliferation at day 10 was not a reliable indicator of the loss/gain of cells with a primitive function. This was illustrated by the fact that a significant number of high-proliferative LTC-IC (which generated high numbers of CFC at week 5) was still detected in large clones.
The net increase in the number of LTC-IC at day 10 observed in this study excludes that MS-5 cells acted either by maintaining primitive cells in a quiescent state16,50 or by delivering signals that inhibit cell proliferation as shown by others in different settings.19,20,51 However, because at day 10 most individual clones contained both LTC-IC and CFC (our unpublished observations) it remains possible that within a clone, LTC-IC proliferated slower than more mature CFC and precursor cells, or/and that asymetric divisions occurred.52 This will be tested by monitoring the decay of fluoresence dyes in functionally different cell populations.53
Our data suggest that stem cell functions can be, at least in part, under the control of external signals, an issue that is still controversial.54 It has been shown that soluble cytokines, if used in adequate combinations and concentrations in vitro, induce a net increase in both human LTC-IC,55 and NOD-SCID-RC/CRU,33 and that LTC-IC function could be modulated independently of cell cycle progression.32Whether or not stromal cells will further alter the response of stem cells exposed to cytokines has rarely been directly investigated. Our data strongly suggest that MS-5 counteracted differentiation events triggered by cytokines and thus allow cells to retain primitive properties, at least if the selected endpoint is the ability to generate CFC after 5 weeks, defined as the LTC-IC function. Other murine and porcine stromal cells have also been shown recently by two other groups to rescue the proliferation and functional defect of cells exposed to cytokines alone.9,24 56 Nevertheless, this effect is transitory and loss of LTC-IC potential will eventually occur despite the continuous presence of MS-5 cells. This was particularly illustrated in experiments initiated with CD34+CD38low because, when stimulated, these mature LTC-IC rapidly loose their function as compared with CD34+CD38neg cells. More than 50% of CD34+ CD38neg cells are immature LTC-IC, which are very homogeneous in their initial response to cytokines, explaining why the proportions of LTC-IC–positive wells among those seeded with and without MS-5 were very similar (Fig 3). It will take several divisions before these cells loose the ability to produce CFC in 5 weeks, (Fig 4) which facilitates the uncovering of MS5 effect. In contrast, CD34+ CD38low cells contained a majority of mature LTC-IC and CFC that differentiated very rapidly in response to cytokines, and the effect of MS-5 cells could not be translated anymore in changes in the LTC-IC compartment.
Apart from the cell phenotype, the presence of serum also affected the readout. First, in 10% serum and without MS-5 cells, entry into mitosis was delayed, the proportion of LTC-IC–positive clones and the CFC output in proliferating clones were lower than in serum-free conditions. Both effects are reminiscent of the action of transforming growth factor (TGF)-β1 on murine stem cells,27,57,58 and it is conceivable that in the presence of MS-5 cells, TGF-β1 can be neutralized by proteoglycans secreted in the vicinity of stromal cells.59 However, stromal cells did not significantly increase cell proliferation as would be expected by TGF-β neutralization, and this discrepancy suggested a more complex balance of positive and negative regulators. In particular, our data seem to indicate that serum can accelerate differentiation in high GF conditions (Fig 5).
The persistent effect of MS-5 cells even in experiments initiated in the presence of high concentrations of FLT3-ligand and SCF probably ruled out a major contribution of MS-5–derived cytokines.60 However, we cannot exclude that MS-5 cells or its extracellular matrix provided a solid substrate that binds cytokines and enhanced their biological activity.61-63Although we have not yet determined stringently that physical contact was required for the action of MS-5 cells, this was shown by Breems.9 Candidate molecules for the control of LTC-IC function include adhesion ligands and receptors, which have been shown to synergize with cytokines64 and the Notch-Jagged pathway. The latter hypothesis is appealing because stromal cells express Notch ligands and CD34+ cells the Notch receptors, and because it has been shown that ligand-receptor interaction prevents differentiation with little change in proliferation.65 66
Finally, our observations may have some clinical relevance, because they suggest that there might be a period of time during which stem cells can be amplified with minimal loss of their potential. It will therefore be crucial to document whether stromal cells will similarily maintain additional stem cell functions, such as the ability to engraft NOD-SCID mice,33,67 or full differentiative potential of totipotent lymphomyeloid cells that we have recently identified in cord blood CD34+ cells.68
ACKNOWLEDGMENT
We thank surgeons and nurses who helped us to collect bone marrow samples. We are indebted to AMGEN (Thousand Oaks, CA) and AMGEN (France) for providing rhu-SCF and rhu-Peg-MGDF, Novartis for rhu-IL-3, CILAG for erythropoietin (Epo), and K. Mori for the MS-5 cell line. We also thank P. Rameau and A. Katz for helping us with the cell sorting, and F. Louache for participating in the initiation of this study.
Supported by grants from INSERM, Electricité de France, Association pour la Recherche contre le Cancer (6532 to LC), Institut Gustave Roussy. CT was funded by a fellowship from Fondation de France (fondation contre la leucémie).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.