Erythropoietin (EPO) and its receptor (EPOR) are required for the development of mature erythrocytes. After binding of ligand, the EPOR activates a variety of signaling pathways that ultimately control cellular proliferation, survival, and specific gene expression. Although erythroid progenitors appear to be the principal EPO-responsive cell type in vivo due to the restricted expression of the EPOR, many growth factor–dependent cell lines expressing the EPOR can respond to EPO by activating many or all of these pathways. In the present study, we have identified a cellular context (the interleukin-2 [IL-2]–dependent HT-2 line) in which the EPO stimulation of the EPOR fails to support cellular proliferation, STAT-5 induction, or MAPK activation, despite efficient phosphorylation of the EPOR and JAK2 and inhibition of apoptosis after withdrawal of IL-2. Interestingly, when we fused HT-2 cells expressing the EPOR with Ba/F3 cells in a complementation assay, the resulting hybridomas proliferated and potently activated STAT-5 and MAPK in response to EPO. These data indicate that an unidentified cellular factor is needed to mediate signaling by the EPOR. Moreover, Ba/F3 cells apparently express this factor(s) and somatic fusions can, therefore, confer EPO-responsiveness to HT-2 cells that lack this factor.
MAMMALIAN BLOOD CELL differentiation depends on cellular proliferation and crucial cell-fate decisions that are coordinated by a family of hematopoietic cytokines, particularly erythropoietin (EPO). EPO, a 34-kD cytokine, is produced by the kidney under conditions of low oxygen tension and acts on erythroid progenitors via a specific erythropoietin receptor (EPOR).1EPO plays a critical role in mammalian hematopoiesis, because it is required for the development of mature erythrocytes, and animals deficient in this cytokine or its receptor die early in fetal development due to severe anemia.2,3 In addition, EPO is used extensively in the clinic to treat anemia associated with EPO deficiency without serious side effects. Numerous signaling molecules and pathways have been linked to the EPOR, the combined effects of which generally include cellular survival and/or proliferation, differentiation, and specific gene expression, ultimately leading to erythroid maturation and expansion of the red blood cell compartment.4
The EPOR belongs to the cytokine receptor superfamily, members of which are characterized by conserved structural motifs in the extracellular domain including four conserved cysteine residues and a membrane-proximal WSXWS motif.5 Like other members of this family, the cytoplasmic tail of the EPOR contains no known enzymatic activity, but instead associates with various second messenger molecules to effect signal transduction. EPO stimulation is thought to trigger homodimerization of EPOR subunits, initiating a cascade of signal transduction events. Interestingly, a mutation within the EPOR extracellular region of cysteine for arginine-129 results in constitutive dimerization through a disulfide bridge between receptor subunits that causes ligand-independent signaling.6-9 In addition, synthetic peptide dimers that cause dimerization of the EPOR function similarly to EPO, and crystal structures of the EPOR co-complexed with these peptides show that the receptor adapts a symmetrical twofold dimer configuration.10 Homodimerization of the receptor results in the tyrosine phosphorylation of a variety of intracellular substrates, including the EPOR itself.11-13Tyrosine phosphorylation of the receptor and many of its associated signaling molecules is mediated by the Janus kinase JAK2, which associates constitutively with EPOR monomeric subunits and is activated upon ligand binding by autophosphorylation.14 One consequence of JAK2 activation is the recruitment of the SH2-domain–containing transcription factors STAT-5A and STAT-5B15,16 This recruitment leads to tyrosine phosphorylation of STAT-5A/5B and dimerization via reciprocal SH2-phosphotyrosyl interactions, followed by rapid nuclear import of STAT-5 dimers, resulting in transcription of EPO-responsive genes such as oncostatin-M17 and the signaling inhibitor CIS.18 Studies using dominant negative STAT-5 mutants or EPOR mutants defective in STAT-5-association domains have linked the activation of STAT-5 to cellular proliferation and/or differentiation in certain cultured cell lines.19-22 However, mice deficient in STAT-5A, STAT-5B, or both exhibit normal erythropoiesis,23-25 indicating that STAT-5 is not essential for EPOR function in vivo. Tyrosine phosphorylation of the receptor also leads to the recruitment of other SH2-containing proteins such as Shc, PI3K, SHIP, Vav, and PLCγ, and the tyrosine phosphatases SHP-1 and SHP-2.4 26 Therefore, the EPOR activates a striking array of signaling pathways that are characteristic of the cytokine receptor superfamily and act in concert to coordinate the physiological effects of this cytokine.
The study of cytokine signaling pathways has been aided greatly by the availability of cytokine-dependent cell lines. For example, in the interleukin-3 (IL-3)–dependent cell lines 32D (pro-myeloid) and Ba/F3 (pro-B), ectopic expression of the EPOR allows growth in EPO in place of IL-3. Similarly, transfection of the IL-2Rβ chain into these cell lines renders them responsive to IL-2.27 These cell lines have been valuable in molecular mapping studies of the EPOR, allowing specific signaling events to be linked to particular subdomains of the EPOR cytoplasmic tail. In addition, such studies suggest that there is tremendous overlap in the array of signals used by various cytokine receptors. For example, in the 32D and Ba/F3 cell lines, the EPOR, IL-2R, and IL-3R complexes all activate JAKs, STAT-5A/B, the MAPK cascade, c-fos gene expression/DNA binding, bcl-2 andbcl-xL gene expression, and inhibit apoptosis. The present report describes a cell line in which signaling through the EPOR is markedly but selectively impaired, while signaling through the IL-2R is intact. Although the EPOR can activate JAK2 and exert a potent anti-apoptotic effect in this cellular background, it fails to activate STAT-5 or MAPK, or to support EPO-dependent proliferation, despite the ability of the IL-2R to engage these pathways efficiently. Genetic complementation by somatic fusion to permissive cells rescues signaling, indicating that a missing factor(s) can be provided intrans to restore full EPOR function. Thus, this cell line provides a valuable system in which to identify previously unrecognized components of the EPOR signaling pathway.
MATERIALS AND METHODS
Cell culture, preparation of cell lines, and cytokine stimulations.
HT-2 cells (American Type Culture Collection [ATCC], Rockville, MD) were maintained in RPMI 1640 medium (Life Technologies, Gaithersburg, MD), 10% fetal bovine serum (FBS), 2 mmol/L glutamine, 0.05 mmol/L 2-mercaptoethanol and 1 nmol/L recombinant IL-2 (generously supplied by Chiron Corp, Emeryville, CA). HT-2.EPOR.3 cells were maintained in this media with 1 mg/mL G418 (Life Technologies). HT-2EPOβγ cells were grown in this media with EPO (5 U/mL, a gift of Ortho Biotech, Raritan, NJ) in place of IL-2. HT-2 transfectant cell lines were prepared as described.28,29 Briefly, cells were electroporated at 300 V/950 μF and cultured without selective media for 24 hours. They were transferred to selective media and monoclonal lines obtained by limiting dilution. 32D and Ba/F3 cells were cultured in RPMI 1640, 10% FBS, 2 mmol/L glutamine, 0.05 mmol/L 2-mercaptoethanol, and 10% WEHI-3B conditioned medium (CM) as a source of IL-3. 371.2 cells are 32D cells that stably express a transgene encoding IRS-1,30and were also maintained in this media. HCD57 cells were cultured in Iscove’s medium (Life Technologies), 20% FBS, 2 mmol/L glutamine, and 1 U/mL EPO. For cytokine stimulations, cells were washed twice in phosphate-buffered saline (PBS), stripped with a 30-second incubation in 10 mmol/L sodium citrate/140 mmol/L NaCl, and incubated in serum– and growth factor–free RPMI 1640 medium with 1% bovine serum albumin (BSA fraction V; Sigma, St Louis, MO) for 2 to 4 hours (immunoprecipitations and nuclear extracts) or in growth factor–free medium for 12 to 15 hours (mRNA preparations) or 40 hours (apoptosis assays). Stimulations were for indicated time periods with recombinant human IL-2 (10 nmol/L), EPO (0.01 to 100 U/mL), or IL-3 (10% WEHI-3B-CM).
Proliferation assays.
Conventional 24- to 48-hour [3H]thymidine incorporation assays and transient proliferation assays were performed as described previously.31 For the transient proliferation assay, 107 cells were electroporated with 20 μg DNA at 300 V/950 μF and incubated in 1 nmol/L IL-2 for 24 hours. Cells were washed twice with PBS and incubated in 96-well plates with IL-2 (10 nmol/L), EPO (50 U/mL), or no cytokine. [3H]thymidine incorporation was measured between days 6 and 16. Data are presented as averages of triplicate samples expressed as a percentage of [3H]thymidine incorporation of cells treated with 10 nmol/L IL-2 (HT-2 cells) or 10%WEHI-3B-CM (32D and 371.2 cells).
Apoptosis assays.
Cells, 2 to 5 × 106, were incubated for 40 hours in medium without cytokines, or stimulated with EPO (50 U/mL) or IL-2 (10 nmol/L). Cells were harvested, washed with PBS/2.5 mmol/L CaCl/2% FBS (Wash Buffer), and incubated with a 1:1,000 dilution of Annexin-GFP (generously provided by Dr Joel Ernst, University of California, San Francisco32) on ice for 10 minutes. Cells were washed twice with Wash Buffer and resuspended in 1 mL Wash Buffer and 1 μL of 50 μg/mL Propidium Iodide (Pharmingen, San Diego, CA). Samples were analyzed on a Becton Dickinson FACScan using the Cellquest computer program.
MAPK assay.
Cells, 10 to 20 × 106, were rested for 6 hours and stimulated with no cytokines, IL-2 (10 nmol/L), EPO (50 U/mL), or murine IL-4 (10 ng/mL). In vitro MAPK assays were performed using an MAPK assay kit (New England Biolabs, Beverly, MA). In short, cells were lysed after stimulation with indicated cytokines. Erk1/Erk2 MAPK was immunoprecipitated using an antiphospho-MAPK antibody. Kinase assays were performed using an Elk1-glutathione S-transferase fusion protein as a substrate with subsequent immunoblotting for phosphorylated Elk1.
Immunoprecipitations, nuclear extracts, and electrophoretic mobility shift assays (EMSAs).
For immunoprecipitations of JAK kinases, EPOR, and STAT-5, 20 to 60 × 106 cells were rested as described above and lysed in 1% Nonidet P-40 (Calbiochem, La Jolla, CA), 20 nmol/L Tris-HCl pH 8.0, 150 mmol/L NaCl, 50 mmol/L NaF, 100 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg aprotinin, 1 μg/mL pepstatin A. Immunoprecipitations were performed with 5 μg of the indicated JAK antibodies (Upstate Biotechnology Inc, Lake Placid, NY), 3 μL of a C-terminal, polyclonal rabbit anti-EPOR antisera,33 or 4 μL of anti-STAT-5A and STAT-5B antisera34 and protein A-Sepharose (Boehringer Mannheim, Indianapolis, IN). Immunoprecipitations were separated on 8.75% (JAK and STAT-5) or 10% (EPOR) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. Membranes were probed with 1:1,000 dilution of anti-phosphotyrosine 4G10 antibodies (Upstate Biotechnology Inc). Membranes were stripped per manufacturer’s instructions and reprobed with anti-JAK1, -JAK2, and -JAK3 antisera (data not shown) or with anti–STAT-5A or anti-EPOR antisera (not shown) to verify equivalent loading. For nuclear extractions, 30 × 106 cells were rested in serum-free medium containing 1% BSA for 2 to 4 hours and stimulated with indicated cytokines for 15 minutes. Cells were lysed and nuclear extractions were prepared. DNA binding assays were performed using 1 × 105 cpm of32P-radiolabeled FcγRI oligonucleotide encoding an STAT response element and 10 μg of nuclear extract/reaction as described previously.35 36
RNA preparation and Northern blot analysis.
Cytoplasmic RNA was prepared from 1 to 2 × 107 cells using an RNeasy kit (Qiagen, Valencia, CA) and quantified by UV spectrophotometry. Denaturing 1.4% agarose gels were run with 10 to 15 μg RNA/lane, blotted to Zeta Probe membranes (BioRad, Hercules, CA), and hybridized with 32P-labeled c-fos, bcl-2, and GAPD probes as described previously.34,37 The 435-nucleotide bcl-x probe (derived from the 5′ end of the bcl-x cDNA that recognizes both bcl-xS and bcl-xL transcripts) was from ATCC.38
Somatic fusions.
A protocol to create fusion cell lines was adapted from Goldsmith et al.39 1.5 × 106 HT-2.EPOR.3 and 1.5 × 106 Ba/F3 cells were washed in RPMI 1640, mixed in a 1:1 ratio (or 3 × 106 of either cell type alone) in flat-bottomed 12-well plates, centrifuged for 10 minutes at 1,200 rpm in an RT6000B table top centrifuge (Sorvall, Newtown, CT), and depleted of media by gentle aspiration. 0.3 mL of RPMI 1640 prewarmed to 37°C containing various concentrations (40% to 65%) of polyethylene glycol (PEG) 4000 (ATCC) diluted in RPMI 1640 was overlaid slowly onto the cells at room temperature. After 3 minutes, 1.5 mL RPMI 1640 was slowly added to wells and removed by gentle aspiration. Wells were washed four to five times in RPMI 1640 and resuspended in complete media containing 50 U/mL EPO, transferred to fresh 6-well plates, and cultured at 37°C.
125I-EPO binding assays.
Cells were washed in RPMI 1640 supplemented with 10% FBS, resuspended at a concentration of 6 × 107 cells/mL, and incubated at room temperature for 30 minutes and pelleted. Aliquots of 3 × 106 cells in triplicate were incubated with a range of concentrations of iodinated EPO at 4°C overnight in binding buffer (RPMI/10% FBS/50 mmol/L HEPES, pH 7.2) in the absence or presence of 100-fold excess unlabeled EPO. After overnight incubation, cells were determined to be greater than 90% viable by trypan blue exclusion analysis. Free EPO was separated from cell-bound EPO by centrifugation through 100% FBS cushion and the cell pellets were counted in a γ counter. The data were plotted according to the method of Scatchard. Dissociation constants (kd) and cell-surface receptor numbers were then determined through Scatchard analyses.
RESULTS
The EPOR inhibits apoptosis in HT-2 cells.
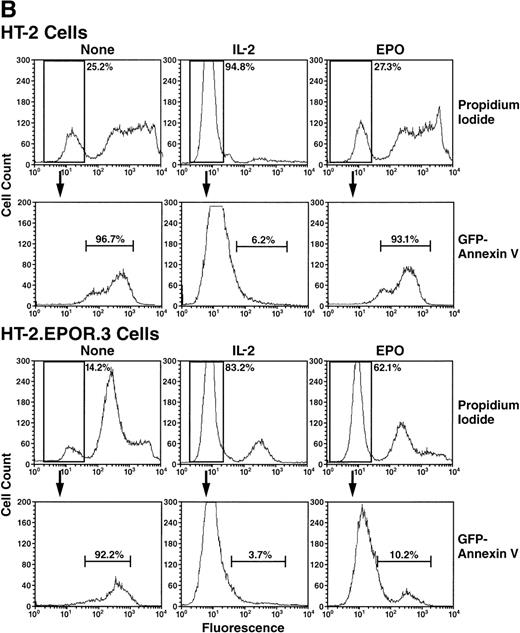
To examine the function of the EPOR in an IL-2–responsive cell, stable HT-2 cell lines were generated that expressed the EPOR (designated HT-2.EPOR.329), and mRNA expression was confirmed by Northern blotting (Fig 1A). It has been shown that the EPOR serves to inhibit apoptosis in erythroid progenitors,40,41 and several cytokines inhibit apoptosis in HT-2 cells.42 Therefore, we examined the ability of the EPOR to inhibit apoptosis induced by IL-2 withdrawal by incubating cells for 40 hours without cytokine, with IL-2 or with EPO. Cells were then stained with propidium iodide (PI) and Annexin V coupled to green fluorescent protein (Annexin-GFP)32 and analyzed by flow cytometry. First, PI staining was used to measure cell death. Incubation of HT-2 or HT-2.EPOR.3 cells without cytokines resulted in significant cell death, because only 25.2% or 14.2% of HT-2 or HT-2.EPOR.3 cells excluded PI, respectively, in a typical experiment (Fig 1B). The slight differences in cell death among cell lines represent experimental variation, because they were not consistently observed over multiple experiments. However, signaling through the EPOR dramatically and reproducibly protected HT-2.EPOR.3 cells from death in the presence of EPO, as 62.1% of cells excluded PI, compared with 27.3% of the parental HT-2 cells lacking the EPOR. Finally, IL-2 signaling prevented cell death in both cell lines (94.8% of HT-2 and 83.2% of HT-2.EPOR.3 cells were PI-negative). Thus, stimulation by EPO conferred cell survival on EPOR-expressing HT-2 cells, although the protection was not as complete as that observed when cells were incubated with IL-2.
(A) HT-2.EPOR cells express EPOR mRNA. Ten micrograms of cytoplasmic RNA from HT-2.EPOR.3 and HT-2 cells was separated by denaturing agarose gel electrophoresis, transferred to Zeta Probe membranes and probed with a 32P-labeled 1.2-kb DNA fragment corresponding to the EPOR extracellular domain. (B and C) The EPOR protects HT-2 cells from cell death and apoptosis. HT-2 and HT-2.EPOR.3 cells were incubated for 40 hours with no cytokine (None), 20 U/mL or indicated doses of EPO (Epo), or 10 nmol/L IL-2 (IL-2). Cells were costained with PI and Annexin-GFP and analyzed by flow cytometry.
(A) HT-2.EPOR cells express EPOR mRNA. Ten micrograms of cytoplasmic RNA from HT-2.EPOR.3 and HT-2 cells was separated by denaturing agarose gel electrophoresis, transferred to Zeta Probe membranes and probed with a 32P-labeled 1.2-kb DNA fragment corresponding to the EPOR extracellular domain. (B and C) The EPOR protects HT-2 cells from cell death and apoptosis. HT-2 and HT-2.EPOR.3 cells were incubated for 40 hours with no cytokine (None), 20 U/mL or indicated doses of EPO (Epo), or 10 nmol/L IL-2 (IL-2). Cells were costained with PI and Annexin-GFP and analyzed by flow cytometry.
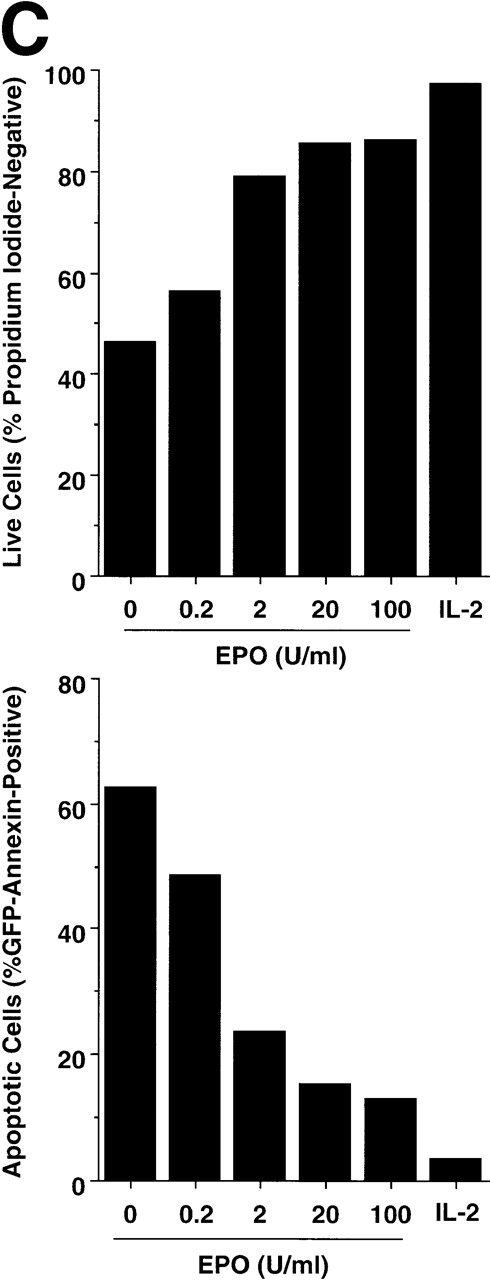
To determine whether the EPOR was inhibiting apoptosis per se, the PI-negative populations were examined for staining by Annexin-GFP, which binds to phosphatidylserine and serves as a marker of apoptosis43 44 (Fig 1B). Virtually all HT-2 and HT-2.EPOR.3 cells incubated without cytokine that were still alive (ie, PI-negative) bound to Annexin-GFP (96.7% of HT-2 and 92.2% of HT-2.EPOR.3 cells), indicating that they were apoptotic. Similarly, 93.1% of PI-negative HT-2 cells (that did not express the EPOR) were Annexin-GFP–positive in the presence of EPO, indicating that the entire culture was undergoing apoptosis. In contrast, HT-2.EPOR.3 cells showed significant, EPO-dependent protection from apoptosis, because only 10.2% of the cells stained positively with Annexin-GFP in the presence of EPO. As expected, IL-2 treatment strongly protected both cell lines from apoptosis (6.2% of HT-2 and 3.7% of HT-2.EPOR.3 cells were Annexin-GFP–positive). In addition, the anti-apoptotic effect of EPO occurred in a dose-responsive fashion, with detectable inhibition of apoptosis and a corresponding increase in percentage of live cells evident at concentrations of EPO as low as 0.2 U/mL (Fig 1C). These data show that the EPOR prevents HT-2.EPOR.3 cell death upon cytokine withdrawal by delivering a potent anti-apoptotic signal.
EPOR alone fails to support proliferation in HT-2 cells.
The ability of the EPOR to mediate growth signaling in the HT-2 cell background was evaluated by measuring incorporation of [3H]thymidine in cells incubated in either IL-2 or EPO. As a positive control, HT-2 cells expressing chimeric receptors in which the cytoplasmic domain of the EPOR was replaced with the cytoplasmic tails of either the IL-2Rβ or γc chains (EPOβ and EPOγ) responded vigorously to EPO after 24 hours of stimulation (Fig 2A28,29). In contrast, parental HT-2 cells failed to incorporate significant levels of [3H]thymidine in response to EPO. Strikingly, HT-2.EPOR.3 cells did not incorporate high levels of [3H]thymidine, although there was a small but reproducible signal that peaked at approximately 8% of IL-2 levels in very high doses of EPO (Fig 2A). However, EPO failed entirely to sustain growth of HT-2.EPOR.3 cells in the absence of IL-2 (data not shown). Thus, EPO triggers a minimal but transient proliferative response in HT-2 cells that is greatly reduced compared to that induced by IL-2. To confirm that the EPOR cDNA used in these experiments encodes a functional receptor that supports cell growth in an permissive cellular background, the EPOR was expressed stably in the IL-3–dependent myeloid cell line, 32D.45 In contrast to HT-2 cells, 32D.EPOR cells exhibited a vigorous proliferative dose response to EPO, while the parental 32D line lacking the EPOR failed to proliferate (Fig 2B). Together with the anti-apoptotic effect described earlier, these data show that inhibition of apoptosis and induction of proliferation are separable signaling events.
(A) The EPOR fails to support proliferation signaling in HT-2 cells. HT-2 ⊡), HT-2.EPOR.3 (⧫), and HT-2EPOβγ () cells were incubated with no cytokine, various doses of EPO, or 10 nmol/L IL-2 and incorporation of [3H]thymidine was measured at 48 hours. Data are presented as percent of [3H]thymidine incorporation relative to IL-2. (B) The EPOR supports proliferation signaling in 32D cells. 32D (⊡) and 32D.EPOR (⧫) cells were incubated with no cytokine, various doses of EPO, or 10%WEHI-CM (IL-3). Data are presented as percent of [3H]thymidine incorporation relative to IL-3. (C) The EPOR can pair with EPOβ to support EPO-dependent proliferation signaling in HT-2 cells. HT-2.EPOR.3 cells were transfected with 20 μg of pCMV4 vector with no insert (control, ⧫) or pCMV4.EPOβ (EPOβ, ⊡) and [3H]thymidine incorporation measured on days 6-16, as indicated.
(A) The EPOR fails to support proliferation signaling in HT-2 cells. HT-2 ⊡), HT-2.EPOR.3 (⧫), and HT-2EPOβγ () cells were incubated with no cytokine, various doses of EPO, or 10 nmol/L IL-2 and incorporation of [3H]thymidine was measured at 48 hours. Data are presented as percent of [3H]thymidine incorporation relative to IL-2. (B) The EPOR supports proliferation signaling in 32D cells. 32D (⊡) and 32D.EPOR (⧫) cells were incubated with no cytokine, various doses of EPO, or 10%WEHI-CM (IL-3). Data are presented as percent of [3H]thymidine incorporation relative to IL-3. (C) The EPOR can pair with EPOβ to support EPO-dependent proliferation signaling in HT-2 cells. HT-2.EPOR.3 cells were transfected with 20 μg of pCMV4 vector with no insert (control, ⧫) or pCMV4.EPOβ (EPOβ, ⊡) and [3H]thymidine incorporation measured on days 6-16, as indicated.
Finally, to verify that the HT-2.EPOR.3 cells express potentially functional EPO receptors, the EPOβ chimeric receptor was transiently transfected into HT-2.EPOR.3 cells, and [3H]thymidine incorporation was measured over a 16-day period. We have previously reported that, although the EPOβ receptor alone is not sufficient to support growth signaling, it can signal proliferation when paired with either the EPOγ chimera or with a truncated EPOR retaining the JAK2-binding domain [EPOR(1-321)29]. EPOβ also exhibited a characteristically potent proliferative response to EPO when paired with the full-length EPOR in HT-2.EPOR.3 cells, whereas a control vector alone failed to induce [3H]thymidine incorporation (Fig 2C). Thus, these experiments show that the HT-2 cell line is restricted in its ability to respond to cytokines: while the IL-2R (or EPOβ/EPOγ chimeras) delivers effective growth signals to these cells, the EPOR is insufficient to promote proliferation or long-term survival in EPO.
The EPOR fails to activate STAT factors in HT-2 cells.
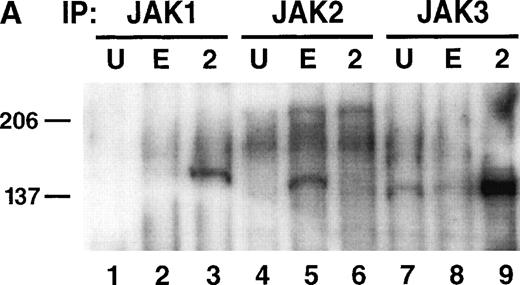
A dominant pathway activated by cytokine receptors is the JAK-STAT pathway, and EPOR-derived proliferation signaling has been linked to JAK2 and STAT-5 activation in some settings.14,15,20 21Therefore, to determine the basis of the signaling block in HT-2.EPOR.3 cells, the ability of EPO to activate JAK2 and STAT-5 was examined. HT-2.EPOR.3 cells were starved of IL-2 and incubated for 10 minutes with no cytokine, EPO, or IL-2 (in these and subsequent experiments, supra-physiological doses of EPO were used to achieve maximal stimulation of the EPO receptors expressed on target cells). Cellular lysates were prepared and immunoprecipitated with antibodies to JAK1, JAK2, or JAK3. Immunoprecipitates were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies (Fig 3A). As expected, when HT-2.EPOR.3 cells were stimulated with IL-2, JAK1 and JAK3, but not JAK2, became phosphorylated on tyrosine. In contrast, when the same cells were stimulated with EPO, JAK2 alone was found to be phosphorylated (Fig 3A, lane 5). Therefore, JAK2 activation is clearly not the block to EPO signaling in this context.
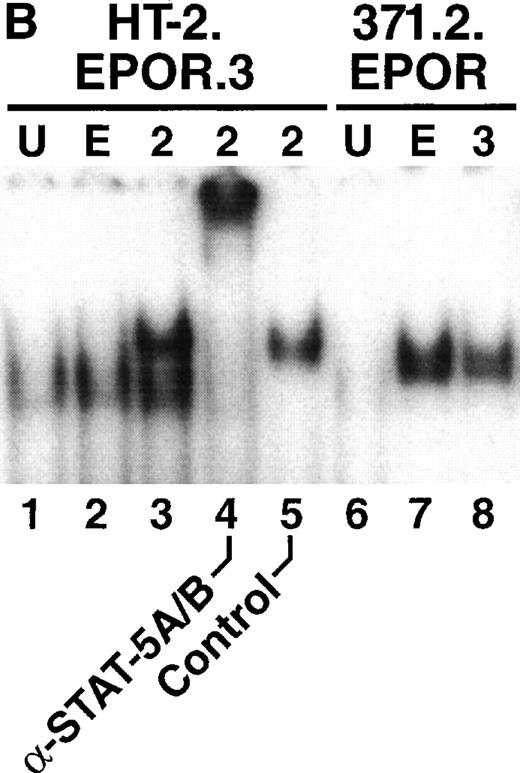
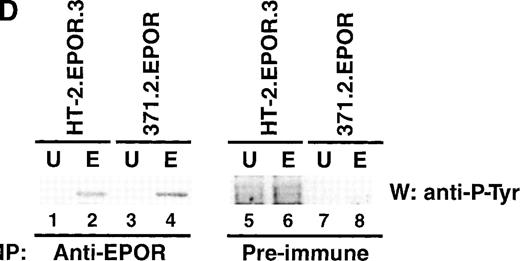
EPO signaling mediates phosphorylation of JAK2 and the EPOR but not STAT-5 in HT-2 cells. (A) HT-2.EPOR.3 cells were rested and stimulated with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2) and lysates were immunoprecipitated with 5 μg of antibodies to JAK1 (lanes 1 through 3), JAK2 (lanes 4 through 6), or JAK3 (lanes 7 through 9). Immunoprecipitates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies. Migration of molecular-weight markers is indicated. The presence of a small amount of phosphorylated JAK3 in lanes 7 and 8 is probably background phosphorylation resulting from incomplete IL-2 deprivation of the cells before restimulation with the indicated cytokines. (B) HT-2.EPOR.3 and 371.2 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), 10 nmol/L IL-2 (2), or 10% WEHI-CM (IL-3) (3) and nuclear extracts were prepared. EMSAs were performed with 10 μg of nuclear extracts and a32P-labeled oligonucleotide corresponding to the FcγRI STAT-response element.46 Supershifts were performed by preincubating nuclear extracts with anti–STAT-5A and -5B antisera (lane 4) or preimmune rabbit sera (lane 5) for 45 minutes before the EMSA reaction. (C) HT-2.EPOR.3 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates were immunoprecipitated with antibodies to STAT-5A and -5B. Immunoprecipitates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. The membrane was stripped and reprobed with anti–STAT-5A and -5B antibodies. (D) 5 × 106 HT-2.EPOR.3 cells/sample or 2 × 106371.2.EPOR cells/sample were rested and incubated with no cytokine (U) or 50 U/mL EPO (E) and lysates were immunoprecipitated with anti-EPOR antibodies. Immunoprecipitates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies.
EPO signaling mediates phosphorylation of JAK2 and the EPOR but not STAT-5 in HT-2 cells. (A) HT-2.EPOR.3 cells were rested and stimulated with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2) and lysates were immunoprecipitated with 5 μg of antibodies to JAK1 (lanes 1 through 3), JAK2 (lanes 4 through 6), or JAK3 (lanes 7 through 9). Immunoprecipitates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies. Migration of molecular-weight markers is indicated. The presence of a small amount of phosphorylated JAK3 in lanes 7 and 8 is probably background phosphorylation resulting from incomplete IL-2 deprivation of the cells before restimulation with the indicated cytokines. (B) HT-2.EPOR.3 and 371.2 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), 10 nmol/L IL-2 (2), or 10% WEHI-CM (IL-3) (3) and nuclear extracts were prepared. EMSAs were performed with 10 μg of nuclear extracts and a32P-labeled oligonucleotide corresponding to the FcγRI STAT-response element.46 Supershifts were performed by preincubating nuclear extracts with anti–STAT-5A and -5B antisera (lane 4) or preimmune rabbit sera (lane 5) for 45 minutes before the EMSA reaction. (C) HT-2.EPOR.3 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates were immunoprecipitated with antibodies to STAT-5A and -5B. Immunoprecipitates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. The membrane was stripped and reprobed with anti–STAT-5A and -5B antibodies. (D) 5 × 106 HT-2.EPOR.3 cells/sample or 2 × 106371.2.EPOR cells/sample were rested and incubated with no cytokine (U) or 50 U/mL EPO (E) and lysates were immunoprecipitated with anti-EPOR antibodies. Immunoprecipitates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies.
We next examined the ability of EPO to activate STAT-5 in HT-2.EPOR.3 cells. Cells were starved of cytokine and stimulated for 15 minutes with EPO, and nuclear lysates were prepared and subjected to EMSA using a generic STAT-response element derived from the FcγRI gene that recognizes a variety of STAT factors.46 As previously reported,36 IL-2 stimulation of HT-2.EPOR.3 cells resulted in the appearance of a specific retarded nucleoprotein complex (Fig 3B, lane 3) that could be supershifted with antibodies to STAT-5A and STAT-5B (Fig 3B, lane 4). In contrast, EPO stimulation of HT-2.EPOR.3 cells failed to cause significant retardation of the probe (Fig 3B, lane 2). Notably, the STAT-response element used in this experiment can also bind STAT-1 and STAT-3,42 which have been linked to the EPOR47; therefore, the EPOR fails to induce any known STAT factors in this cellular context. Furthermore, EPO stimulation of 371.2.EPOR cells (derived from 32D cells30) induced STAT-5, verifying that the EPOR cDNA is fully functional in permissive cells (Fig 3B, lane 7).
Because STAT-5 DNA binding activity was not induced by the EPOR, we next examined whether STAT-5 was inducibly phosphorylated on tyrosine after receptor stimulation. HT-2.EPOR cells were starved of IL-2 and incubated with no cytokine, EPO, or IL-2 for 15 minutes. Cell lysates were immunoprecipitated with antibodies to STAT-5A and -5B, separated by SDS-PAGE, transferred to nitrocellulose and probed with antiphosphotyrosine antibodies (Fig 3C). Although IL-2 induced strong phosphorylation of STAT-5A and -5B in HT-2 cells, EPO failed to induce detectable phosphorylation. Thus, the initial step of STAT-5 activation by phosphorylation on tyrosine fails to occur in HT-2.EPOR.3 cells.
This lack of STAT-5 activation could be explained if the EPOR itself were not phosphorylated after receptor engagement and JAK2 activation, because it would then be unable to provide docking sites for signaling intermediates such as STAT-5. To determine whether the EPOR was phosphorylated in HT-2.EPOR.3 cells, cells were stimulated with EPO for 25 minutes, and cell lysates were immunoprecipitated with anti-EPOR antibodies.33 Lysates were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antiphosphotyrosine antibodies (Fig 3D). In both HT-2EPOR.3 cells and control 371.2.EPOR cells, EPO stimulation resulted in tyrosine phosphorylation of the EPOR (Fig 3D, lanes 2 and 4), indicating that the defect in signaling is not attributable to impairment of this proximal step.
The EPOR fails to activate the MAPK cascade or specific gene expression in HT-2 cells.
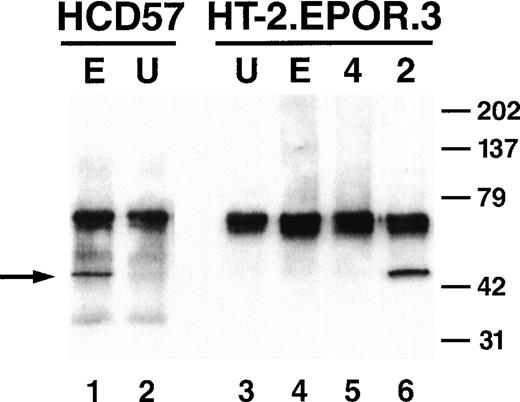
To characterize other signaling pathways known to be induced by the EPOR in permissive cells, the ability of EPO to induce phosphorylation of MAPK on serine residues was examined. Cells were starved and stimulated with cytokine, and lysates were prepared and immunoprecipitated with antibodies to phosphorylated MAPK. The immunoprecipitates were then subjected to an in vitro kinase reaction, separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies to a phosphorylated substrate, Elk1. EPO stimulation of a positive control cell line HCD57 (a murine, EPO-dependent erythroleukemia cell line) induced MAPK phosphorylation of its substrate (Fig 4, lane 1). However, in HT-2.EPOR.3 cells, EPO stimulation failed to activate MAPK, while IL-2 stimulation resulted in efficient MAPK induction (Fig 4, lanes 4 and 6).
Absence of MAPK induction by the EPOR in HT-2 cells. HCD57 cells (lanes 1 and 2) or HT-2.EPOR.3 cells (lanes 3 through 6) were starved and stimulated with no cytokine (U), 50 U/mL EPO (E), 10 ng/mL murine IL-4 (4), or 10 nmol/L IL-2 (2). Total cell lysates were prepared and immunoprecipitated with anti-phosphoMAPK antibody. Immunoprecipitates were subjected to in vitro kinase assays with an Elk1-glutathione S-transferase fusion protein, separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti–phospho-Elk1 antibodies. Arrow indicates phosphorylated Elk1.
Absence of MAPK induction by the EPOR in HT-2 cells. HCD57 cells (lanes 1 and 2) or HT-2.EPOR.3 cells (lanes 3 through 6) were starved and stimulated with no cytokine (U), 50 U/mL EPO (E), 10 ng/mL murine IL-4 (4), or 10 nmol/L IL-2 (2). Total cell lysates were prepared and immunoprecipitated with anti-phosphoMAPK antibody. Immunoprecipitates were subjected to in vitro kinase assays with an Elk1-glutathione S-transferase fusion protein, separated by 10% SDS-PAGE, transferred to nitrocellulose, and probed with anti–phospho-Elk1 antibodies. Arrow indicates phosphorylated Elk1.
Another common consequence of MAPK activation is the induction of specific gene expression, including the proto-oncogenec-fos.48 To assay c-fos gene expression, cells were starved of cytokine and stimulated with EPO or IL-2 for the indicated time periods. Cytoplasmic RNA was prepared from these cells, separated on denaturing agarose gels, transferred to membranes, and probed for c-fos or GAPD(Fig 5A). Parental HT-2 cells lacking the EPOR failed to induce c-fos gene expression (Fig 5A, lanes 1 through 4), while IL-2 stimulation resulted in marked c-fosmRNA induction which peaked at 30 to 60 minutes, as described previously (Fig 5A, lanes 5 through 834). As expected, EPO stimulation of HT-2EPOβγ cells also induced c-fos mRNA (Fig5A, lanes 14 through 17), but EPO stimulation of HT-2.EPOR.3 cells failed to induce detectable c-fos gene expression (Fig 5A, lanes 9 through 13). As c-fos gene expression is considered typically to be a consequence of MAPK signaling, this result is consistent with the lack of MAPK activation by EPO in HT-2.EPOR.3 cells (Fig 4).
(A) The EPOR fails to induce the c-fos orbcl-2 proto-oncogene mRNA in HT-2 cells. HT-2, HT-2.EPOR.3, and HT-2EPOβγ cells were incubated in media without growth factors for 15 hours and stimulated with no cytokine (U, lanes 1, 5, and 14) 50 U/mL EPO (lanes 2 through 4, 9 through 13, 15 through 17) or 10 nmol/L IL-2 (lanes 6 through 8) for the indicated time periods. Total cellular RNA was prepared, separated on a denaturing agarose/formaldehyde gel, and probed with 32P-labeled c-fos, bcl-2, orGAPD cDNA probes. (B) The EPOR fails to induce bcl-xproto-oncogene mRNA in HT-2 cells. HT-2.EPOR.3 and 371.2.EPOR cells were starved as described in (A) and stimulated with no cytokine (lane 1), EPO (lanes 2 through 4), or IL-2 (lanes 5 through 7) for the indicated time periods. RNA from 371.2.EPOR cells serves as a positive control (lane 8). RNA was prepared and blotted as in (A) and probed with a 32P-labeled bcl-x cDNA probe derived from the 5′ end of the gene.
(A) The EPOR fails to induce the c-fos orbcl-2 proto-oncogene mRNA in HT-2 cells. HT-2, HT-2.EPOR.3, and HT-2EPOβγ cells were incubated in media without growth factors for 15 hours and stimulated with no cytokine (U, lanes 1, 5, and 14) 50 U/mL EPO (lanes 2 through 4, 9 through 13, 15 through 17) or 10 nmol/L IL-2 (lanes 6 through 8) for the indicated time periods. Total cellular RNA was prepared, separated on a denaturing agarose/formaldehyde gel, and probed with 32P-labeled c-fos, bcl-2, orGAPD cDNA probes. (B) The EPOR fails to induce bcl-xproto-oncogene mRNA in HT-2 cells. HT-2.EPOR.3 and 371.2.EPOR cells were starved as described in (A) and stimulated with no cytokine (lane 1), EPO (lanes 2 through 4), or IL-2 (lanes 5 through 7) for the indicated time periods. RNA from 371.2.EPOR cells serves as a positive control (lane 8). RNA was prepared and blotted as in (A) and probed with a 32P-labeled bcl-x cDNA probe derived from the 5′ end of the gene.
We likewise examined whether the anti-apoptotic gene bcl-2 is induced by EPO in HT-2.EPOR.3 cells. However, despite the potent anti-apoptotic effect of EPOR seen in Fig 1, bcl-2 mRNA was only weakly induced upon EPO stimulation (Fig 5A, lane 13), although IL-2 induced a strong upregulation of bcl-2 transcripts in HT-2 cells (Fig 5A, lane 8) and EPO induced bcl-2 mRNA in HT-2.EPOβγ cells (Fig 5A, lane 17). This experiment suggests that the anti-apoptotic effect of EPOR probably occurs via a pathway independent of Bcl-2. Recent evidence has also implicated the Bcl-2 family member Bcl-xL in anti-apoptotic effects mediated by the EPOR.49 50 However, we found by analysis of RNA that HT-2.EPOR.3 cells do not express detectable levels ofbcl-xL or bcl-xS mRNA, nor isbcl-xL mRNA induced by either IL-2 or EPO (Fig 5B).
Somatic hybrids between HT-2 and Ba/F3 cells rescue EPO signaling.
To determine whether the defect in EPOR signaling in HT-2 cells could be overcome by genetic complementation, we generated a series of stable hybridoma cell lines between HT-2.EPOR.3 and Ba/F3, a cell line lacking EPOR expression. Cells were cocultured, fused with various concentrations of PEG, and selected for growth in 50 U/mL EPO. Notably, when the fusion protocol was performed with either HT-2.EPOR.3 or Ba/F3 cells alone, no cell lines whatsoever survived selection in EPO. However, EPO-dependent fusion lines were readily generated when HT-2.EPOR.3 and Ba/F3 cells were mixed in the fusion reaction. Two polyclonal fusion lines were chosen for further analysis, designated HB40 and HB55 (for HT-2-Ba/F3 fused at 40% or 55% PEG). Both HB40 and HB55 cells proliferated vigorously in response to EPO, while Ba/F3 and HT-2.EPOR.3 cells showed little or no response (Fig 6A). The abilities of HB40 and HB55 to proliferate were directly dependent on the dose of EPO, and these cells responded to physiological doses of EPO (Fig 6B).
Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.
Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.
Moreover, 125I-EPO binding analyses showed that the fusion cell lines expressed approximately the same number of surface EPO receptors with similar affinities as the parental HT-2.EPOR.3 cells (Table 1). Both the HT-2.EPOR.3 cells and the HB40 and HB55 cells expressed 200 to 250 cell-surface EPO receptors, with affinities in the range of 1,500 to 1,800 pmol/L, whereas the parental HT-2 cells did not express detectable receptors for EPO (Table 1). These results indicate that restoration of signaling in the fusion cell lines is not simply caused by an increase in receptor number.
We next examined STAT-5 activation by the fusion cell lines. In contrast to HT-2.EPOR.3 cells, HB40 and HB55 cells activated STAT-5 DNA binding activity vigorously in response to EPO (Fig 6C). In addition, phosphorylation of STAT-5 proteins in response to EPO was also restored in the fusion lines (Fig 6D). Thus, Ba/F3 cells are able to complement the EPOR signaling defect of HT-2 cells. Furthermore, rescuing the cells’ ability to grow in EPO correlates with concurrent restoration of STAT-5 activation, which suggests that the missing component(s) functions upstream of both STAT-5 activation and proliferation.
Finally, to determine whether the MAPK/c-Fos pathway is likewise rescued in the fusion cell lines, we measured induction ofc-fos mRNA. Cells were starved of cytokines, stimulated for various times with EPO, and mRNA and Northern blots were prepared and probed with 32P-labeled c-fos and GAPD probes. Induction of c-fos mRNA by EPO was indeed evident in these cell lines (Fig 6E, lanes 7 and 11). Thus, all aspects of EPOR signaling measured were reconstituted in the fusion cell lines HB40 and HB55.
DISCUSSION
This study shows that the EPOR fails to mediate STAT-5 and MAPK activation or to deliver proliferative signals in the IL-2–dependent T-cell line, HT-2, despite the presence of potentially functional cell-surface EPO receptors that can mediate JAK2 and EPOR phosphorylation and inhibit apoptosis. The signaling defect(s) can be complemented by somatic hybridization between HT-2.EPOR cells and IL-3–dependent Ba/F3 cells, suggesting that a missing factor required for signaling in HT-2 cells can be provided in trans by Ba/F3 cells. These results delineate a dichotomy between the signaling processes that drive anti-apoptosis and those that induce proliferation. In addition, they provide a valuable platform for identifying new components of the EPOR signaling cascade, which may also yield further insights into signaling mechanisms by other cytokine receptors.
Cytokines regulate a myriad of physiological processes within their target cells, including proliferation, survival, and differentiation. Other studies have suggested that the mechanisms that promote cell survival by inhibiting apoptosis are distinct from those that trigger cellular proliferation.51-54 The HT-2.EPOR.3 cell line described here supports this separation of function, as the EPOR delivers a strong anti-apoptotic signal while failing to induce proliferation. The immature erythroid cell line J2E-NR exhibits similar characteristics with respect to proliferation; while the EPOR fails to induce growth signaling or differentiation in these cells, it does serve to enhance cell viability.55 However, in many other cytokine-dependent cells, the EPOR supports proliferative signaling (eg, 32D cells, Fig 2B). These results may indicate that certain cell types need only an anti-apoptotic signal to transit the cell cycle and proliferate, while HT-2 cells require an additional impetus to proliferation that apparently can be provided by the IL-2R but not by diverse cytokine receptors such as the EPOR (this study), IL-7R,56 IL-9R,42 IL-4R,57 growth hormone receptor (GHR), thrombopoietin receptor (c-mpl), or granulocyte colony-stimulating factor receptor (G-CSFR) (data not shown). Moreover, HT-2 cells provide a valuable system to define regions of the EPOR that deliver anti-apoptotic signals versus those that mediate proliferation. Although there is evidence that STAT-5 may regulate the anti-apoptotic signals of the EPOR in some cell backgrounds,58 the EPOR fails to activate any known STAT factors in HT-2 cells, thus arguing that STAT-5 is not involved in this particular anti-apoptotic pathway. Likewise, despite evidence that induction of the AP1 transcription factor (which includes the c-Fos proto-oncogene) by the MAPK pathway has been linked to protection from apoptosis,59 it is clear that MAPK activation is not required for inhibition of apoptosis in the HT-2 cell background, because this pathway is not engaged by the EPOR. In 32D cells, the EPOR is known to induce Bcl-2 and Bcl-xL to effect apoptosis inhibition.60 However, neither of these genes is significantly induced by the EPOR in HT-2 cells (Fig 5). Therefore, the molecular mechanism underlying EPOR-mediated apoptotic inhibition in this context is distinct from recognized pathways and remains to be elucidated.
In vivo, some developmental events appear to require cytokines mainly to prevent apoptosis, while others appear to need additional proliferative signals.61 Although the EPOR does exert anti-apoptotic effects 40,41 (Fig 1), prevention of apoptosis in erythroid progenitors is probably not sufficient to eliminate their dependence on EPO for maturation.62Specifically, in mice expressing a bcl-2 transgene under the control of an erythroid promoter, growth of erythroid progenitors in vitro is impaired in the absence of EPO. While apoptosis of colony forming units-erythroid (CFU-E) is delayed in these transgenic mice, no spontaneously differentiating colonies are found in the absence of EPO.62 It will be interesting to see the results of crossing these mice with EPO−/− or EPOR−/− mice2,3 to determine whether differentiation in vivo is equally affected. However, using abcl-2 transgene to inhibit apoptosis in erythroid progenitors may not mimic native anti-apoptotic signaling by the EPOR, because our work shows that the EPOR is able to prevent apoptosis without inducingbcl-2 mRNA significantly (Figs 1 and 5A), and because Bcl-2 is apparently not expressed in erythroid progenitors.63
Another in vivo system illustrating the importance of apoptotic inhibition is mice lacking the IL-7Rα chain (or its partner, γc).64-66 IL-7Rα−/− mice are characterized by profound immunodeficiency, lacking mature B and T cells.67 When these mice were crossed with animals that overexpress the human bcl-2 gene, T-cell development was rescued, but not B-cell development.64-66 Thus, the IL-7–derived signal necessary for T-cell maturation can be replaced solely by inhibiting apoptosis, but B-cell differentiation requires additional signals from the IL-7R. Similarly, the EPO-EPOR system appears to require such additional signals for erythroid development.
The dominant paradigm of the JAK-STAT pathway indicates that STAT factors are recruited to the receptor complex following phosphorylation of receptor tyrosine residues. In the case of the EPOR, this model is supported by the severe diminution in STAT-5 activation by EPO receptor mutants that lack cytoplasmic tyrosine residues.20,68 69However, the block in JAK-STAT signaling in this study shows that there is likely to be an additional component of this pathway that has not been previously recognized. Although very proximal signals are intact in this pathway (eg, phosphorylation of JAK2 and the EPOR, Figs 3A and3D), phosphorylation of STAT-5 is impaired (Fig 3C) despite the presence of potential phosphotyrosine docking sites on the EPOR in these cells. It is interesting to note that the DNA binding complexes induced by EPO in 371.2 cells migrate slightly faster than does the IL-2–induced STAT-5 complex in HT-2 cells or the EPO-induced STAT-5 complex in HB40 and HB55 cells. We have not yet determined the biochemical basis for this difference.
There are two opposing hypotheses to explain the lack of EPOR signaling in the HT-2 cell line. The most likely explanation is that the cells are missing a factor(s) necessary to mediate signaling. Supporting evidence comes from the somatic fusions with Ba/F3 cells, which can complement the lack of function in HT-2.EPOR.3 cells. The resulting fusion cell lines rescue not only proliferation signaling, but also STAT-5 activation and proto-oncogene induction. Although the nature of this factor(s) can only be determined by isolating a gene from an EPO-responsive cell line that can rescue the proliferative defect, some possibilities include an additional EPOR subunit or an adapter molecule that might couple STAT-5 to JAK2 or to the EPOR. Indeed, there have been hints that the EPOR complex might contain an additional subunit70-73; in this scenario, the missing receptor would be expressed in most cell lines but would be absent in HT-2 cells. We are confident that the missing factor is not the adapter Shc, because IL-2 signaling through Shc that leads to MAPK and c-fos mRNA induction proceeds normally in this cell line (Figs 4 and 5, and refs34, 74, and 75). On the other hand, it is formally possible that HT-2 cells express an overabundance of a suppressor factor that inhibits the EPOR. Although it is known that the tyrosine phosphatases SHP-1 and SHP-2 are involved in EPOR signaling,76-78 mRNA levels of SHP-1 and SHP-2 in HT-2 cells are comparable to those in EPO-responsive cell lines such as HCD57 and Ba/F3 (data not shown). Furthermore, it is unlikely that the cytokine inhibitors of the SOCS/CIS family79 are responsible, because: (1) the STAT downregulation that occurs upon SOCS activation is linked to a decrease in JAK activity, while JAK2 activation by the EPOR is retained in HT-2 cells (Fig 3); (2) none of the SOCS family members identified so far are specific to the EPOR,80,81 and IL-2, IL-7, and IL-9 can activate STAT-5 vigorously in HT-2 cells36,42,57; and (3) the EPOR can pair with EPOβ to activate JAK2 and STAT-529and is thus unlikely to carry any other dominant suppressor molecule(s) that inhibits proximal signaling within this hybrid receptor complex. Therefore, the available evidence supports the hypothesis that HT-2 cells are missing a necessary signaling component that links the EPOR to STAT-5, MAPK, and proliferation signaling.
An issue of considerable interest is whether cytokine signaling differs depending on cell context. Although there are dozens of cytokines, each with a specific receptor, the downstream events that follow receptor ligation are remarkably similar among members of the cytokine receptor superfamily; eg, both the IL-2R and EPOR activate STAT-5, c-Fos, Bcl-2, MAPK, IRS-2, and PI3K.27 Do the target cells of different cytokines “interpret” these signals differently, or is the basis for this dichotomy simply their anatomical location? To address this question in the context of homodimeric receptors such as the EPOR, recent work using an in vivo differentiation system showed that the cytoplasmic tail of the EPOR can be substituted with the cytoplasmic domains of diverse cytokine receptors (G-CSFR, c-mpl, and GHR) yet exhibit similar signaling outcomes with regard to erythropoiesis, thus favoring the notion that cytokine signals are generally replaceable.82 Similarly, prolactin and its receptor can substitute for the EPOR in driving differentiation of erythrocytes in vitro.83 In an analogous study, the cytoplasmic tail of the IL-2Rβ chain was found to substitute functionally for the IL-7Rα chain in driving proliferation of B cells in an ex vivo culture system.84 However, the data presented here suggest that the EPOR cannot replace the IL-2R, despite the apparent similarity in the intracellular signaling molecules engaged by these receptors.
Although the restoration of STAT-5 activation in the HT-2-Ba/F3 hybridomas implies a link between STAT-5 function and proliferation, STAT-5 activation is clearly not sufficient to drive proliferation in the HT-2 cell background. First, it has been previously shown that IL-7R signaling fails to support proliferation in HT-2 cells, despite a vigorous induction of STAT-5 DNA binding.56 Second, the IL-9R activates STAT-5 (and STAT-1 and STAT-3) quite well in HT-2 cells and also mediates an anti-apoptotic response, but does not permit proliferation.42 Finally, the IL-4R also induces JAK-STAT activation (in this case, STAT-6) without driving proliferation of HT-2 cells.57 At present, it is unclear precisely how IL-2 signaling differs from that of the other γc-utilizing cytokine receptors and the EPOR to induce proliferation in HT-2 cells.
The failure of EPOR to signal in a T-cell background has also been described for sublines of the IL-2–dependent T-cell line CTLL transfected with the EPOR.85 In this case, the cells’ nonresponsiveness was attributed to the failure of JAK2 to associate with the EPOR. This mechanism contrasts with our results in HT-2.EPOR.3 cells in which JAK2 is potently activated in response to EPO, while STAT-5 activation is impaired. Because IL-2 functions as a growth factor for mature, peripheral T cells such as HT-2 and CTLL, it is tempting to speculate that the lack of permissivity to signaling in the HT-2 and CTLL lines reflects their biologic origins. It would be interesting to determine whether EPOR-mediated signal transduction can occur in normal T cells.
In conclusion, the present report describes a unique cell context in which signaling by the EPOR is impaired, despite the ability of a highly related cytokine (IL-2) to trigger the same signaling pathways. This system will serve as a valuable tool for identifying essential components that link the EPOR to the JAK-STAT, MAPK pathways, and proliferative machinery.
ACKNOWLEDGMENT
The authors acknowledge the valuable assistance of John Carroll, Neile Shea, and Heather Livesay in the preparation of this manuscript, and Dr N. Abraham for critical reading. Annexin-GFP was the kind gift of Dr Joel Ernst, and 371.2 cells were provided by Dr Morris White (Joslin Diabetes Center, Harvard University). IL-2 was a gift from Chiron Corporation, and EPO was a gift from Ortho Biotech.
Supported by the J. David Gladstone Institutes, and National Institutes of Health (NIH) Grants No. GM54351 to M.A.G. and CA75315 to G.D.L. S.L.G. was supported by the Bank of America-Giannini Foundation and the NIH Immunology Training Grant at the University of California, San Francisco. S.Y.L. was in the NIH Medical Scientist Training Program (MSTP) and the Biomedical Sciences Program at the University of California, San Francisco (UCSF), and K.D.L. is in the NIH MSTP and Program in Biological Sciences at UCSF.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mark A. Goldsmith, MD, PhD, Gladstone Institute of Virology and Immunology, PO Box 419100, San Francisco, CA 94141; e-mail: mgoldsmith@gladstone.ucsf.edu.


![Fig. 2. (A) The EPOR fails to support proliferation signaling in HT-2 cells. HT-2 ⊡), HT-2.EPOR.3 (⧫), and HT-2EPOβγ () cells were incubated with no cytokine, various doses of EPO, or 10 nmol/L IL-2 and incorporation of [3H]thymidine was measured at 48 hours. Data are presented as percent of [3H]thymidine incorporation relative to IL-2. (B) The EPOR supports proliferation signaling in 32D cells. 32D (⊡) and 32D.EPOR (⧫) cells were incubated with no cytokine, various doses of EPO, or 10%WEHI-CM (IL-3). Data are presented as percent of [3H]thymidine incorporation relative to IL-3. (C) The EPOR can pair with EPOβ to support EPO-dependent proliferation signaling in HT-2 cells. HT-2.EPOR.3 cells were transfected with 20 μg of pCMV4 vector with no insert (control, ⧫) or pCMV4.EPOβ (EPOβ, ⊡) and [3H]thymidine incorporation measured on days 6-16, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336002ax.jpeg?Expires=1766174793&Signature=1anwtNloHB-Y6mGtR6cb9PCeB6xblCTzcWLO5zgFQQZHXr~ii1yxTXnyOl-DJ2nRZh-IaK3~1-r-gz23RhENqArsux6F8BF0lTO5WU8bytQPAsuZ4zKNDpbOv2Hizx6RWt0udgBM8NcDKQjNsb0XZ-e99aWeLwJV8JO2CwBHuluZpAxtDo9bT2PDqNs8Ddylk5qXfWIiOuMM9TVoXSNfTI76MlYpRZ3F5EEWIv98whzQsQzdfPwa-ldWJd-jvm2e6TWul6B2Te7dGxdUraqhfmTUldMBIKPBCq92qwjyo3rCU4zU6KNzl8Lj8Ddzg6WzvGIGXgyU8nT0ZdoptSmuSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. (A) The EPOR fails to support proliferation signaling in HT-2 cells. HT-2 ⊡), HT-2.EPOR.3 (⧫), and HT-2EPOβγ () cells were incubated with no cytokine, various doses of EPO, or 10 nmol/L IL-2 and incorporation of [3H]thymidine was measured at 48 hours. Data are presented as percent of [3H]thymidine incorporation relative to IL-2. (B) The EPOR supports proliferation signaling in 32D cells. 32D (⊡) and 32D.EPOR (⧫) cells were incubated with no cytokine, various doses of EPO, or 10%WEHI-CM (IL-3). Data are presented as percent of [3H]thymidine incorporation relative to IL-3. (C) The EPOR can pair with EPOβ to support EPO-dependent proliferation signaling in HT-2 cells. HT-2.EPOR.3 cells were transfected with 20 μg of pCMV4 vector with no insert (control, ⧫) or pCMV4.EPOβ (EPOβ, ⊡) and [3H]thymidine incorporation measured on days 6-16, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336002cx.jpeg?Expires=1766174793&Signature=Pd91d1EdIQXsItGGatQ5kAHRAXvOJm4EABLAamY6oym5T2mp6qIPHsgmdvcaDxQZB0Z9eg54yTQlu7EdtV~x9kF7Moff4c~OloYrKSpsGJgflvciwVpR9HJNRnAeJbEUbj~TZX3qwHc3SWeUGHKQyz3-WSSUdSuNyNYaWPV0U6nCtx85W9kUc~wrBElIIWVqMtFD-f5JrAtIoy2nn27gTJmw2H~Wi9KwR995IgGg64yB0wMza~kx07RimVaMzYimQQh3IECoji~88ITo2HL1iXbvuUez98LID7SJEse~kpIn9cdQNR2FjEpoiMKIaQ~3sB2I1GrdPuaw6Ud7ff4r7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006ax.jpeg?Expires=1766174793&Signature=VPCFjKE8miFGJ2UlvdLHbomkuF7NhzXZJDaKZ~4UuwcnUGf25rihCauxeBMCuXukpGGf2iwygpjlrz~QDc-UKCgy-64HRd~ysdezCc~ZHwukRk2m8tgWpmnKHAfki5z6qYHIzG-4x7BClLAlIAPDGkhRPxkkIRPPzIEwrb7sVvC2sUk6HdlqCBrPXbY2frSSi40VMhnwTmgy3JD1Nll94QL3re8nxx0U4yiW7QDfyQWOgYZ-leqBbFfIHpCqAxmHrBuDcpP03AbUMCT1glO1ZF1uDecupEgs1x91~IE6qE7WlB-npP20kuhyQ0KL~~ubGuDQ8e04yZLgqsteWRgnbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006cw.jpeg?Expires=1766174793&Signature=ja3DRNni3xYXKHcYX2PEANBoMtYJB3WzNX0Ehm3qMDjxE2Qn0vrkTAiwWR4TPMd02eWRnA~d-kSdlhS4UJL-YHTZnTX-dKjnyXhZuPtZx~ejTcTRBOpJlXqfdwjkHTEbRunYKNMtCNDbvAQWXtf82ncw2bNTihg2pJz144V~cMsa~1Z6Dj79ovJdJL4yuKF7ejsyykwoUMpo6E8ujxiBePryMp8YAG9XcEsb6~4kaexb2c5eurHjR9l5KYTJAT6bAz4mmPCQPo9zJMaIXZ7Pw0o0cnwp18PsgwsxmDxE0-FaNq~xrs21C1LC3EVv0Gpf9cgX5on9oQcOxQyZxiWvRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006dw.jpeg?Expires=1766174793&Signature=PLcOIrH5Z9nDmiUlUR9OGqIf4ImMChV0AQdVkzNbsr4FxkWtj3yugWRAsiEUSWAX4v9DC6WbKbV-9hmQBgjGc5JXHrJ-nrlHaBsEIp1QNdeXhYPgCoymbVTmO3oC09FHIiCBHMK5E2CRWUcoxUvzcUjqWIk1yWzVvVGeQnH1Iv2x5zzeyJIXNITK8Cj3wQ6ctimKLv8x8ywI3-TCI2CzU9l1LT-A8iZX28PjjmKRWg~gPprjoHsh0mQOFdt~rySIq8f6pf86nfk~g7eD1B5lKpJ9JKF5XZRuQzRpiHQOdg94FH3Q-hj5aFacCcxBs46i5Iczt6ic0iB276duyeuQow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006ew.jpeg?Expires=1766174793&Signature=F-CPnmpPfYMxwdSHFn9c9TTXt6j9fZNTUjS1poJsbVUMNhPS01LTNC8Fll8v1dK3DFZqKWmtwsvrIkbhwRlvErCQVpyxO~EL2PiKvOvfSpriNaWN9pLcEALKvFGf1svy1PwyF1IjC4OnK6olcy-I9U0BPEN2IfhB86qICIJiDu9TzXPs4WSolvYNQBQRcE2DSzxACLidIUULLtvxFARiklJZ7CcQymRIuqfnbeF~NK92VSb2TKHzA2akoWU3Mf-OIvsEDUdipWS8ONadDLJNavyhgsry88ZEptQrKz8GNKyvyVpob04JNSJ38CAYAtVLKcGMT~sP8TmoFRwavmWo~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 2. (A) The EPOR fails to support proliferation signaling in HT-2 cells. HT-2 ⊡), HT-2.EPOR.3 (⧫), and HT-2EPOβγ () cells were incubated with no cytokine, various doses of EPO, or 10 nmol/L IL-2 and incorporation of [3H]thymidine was measured at 48 hours. Data are presented as percent of [3H]thymidine incorporation relative to IL-2. (B) The EPOR supports proliferation signaling in 32D cells. 32D (⊡) and 32D.EPOR (⧫) cells were incubated with no cytokine, various doses of EPO, or 10%WEHI-CM (IL-3). Data are presented as percent of [3H]thymidine incorporation relative to IL-3. (C) The EPOR can pair with EPOβ to support EPO-dependent proliferation signaling in HT-2 cells. HT-2.EPOR.3 cells were transfected with 20 μg of pCMV4 vector with no insert (control, ⧫) or pCMV4.EPOβ (EPOβ, ⊡) and [3H]thymidine incorporation measured on days 6-16, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336002ax.jpeg?Expires=1766338550&Signature=YAEjYt~kGbgJLnVs8WblXfXUUev26gQQdRrS8toczAFCUu0ZW5St9TNrDrOyhcrIJSF0EZtvzDGhUOkvSxH6no4Td6XDo6V7z4h-jNmLo7HNNa8VsowVhXRXStYso0yncpYk2HTCLjnsjeHmhNeRqgPK19l~48La0cq1xLnY67~-DXjnxaJnqR7fU-A41XpVtI1ujmPCzfVUAp4BgNyAP5NtHG9VfCr-ll7-42VNLZNCu6n0-agi-LoyDg0ivUL~RPYQbyx5ACZoPlG3dAeJ7wVSeuTixU~fGfZYAHKxifMkUYrtK9sqblktmPoe7XZgzkXYcSElSWbHG9epY9eS~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. (A) The EPOR fails to support proliferation signaling in HT-2 cells. HT-2 ⊡), HT-2.EPOR.3 (⧫), and HT-2EPOβγ () cells were incubated with no cytokine, various doses of EPO, or 10 nmol/L IL-2 and incorporation of [3H]thymidine was measured at 48 hours. Data are presented as percent of [3H]thymidine incorporation relative to IL-2. (B) The EPOR supports proliferation signaling in 32D cells. 32D (⊡) and 32D.EPOR (⧫) cells were incubated with no cytokine, various doses of EPO, or 10%WEHI-CM (IL-3). Data are presented as percent of [3H]thymidine incorporation relative to IL-3. (C) The EPOR can pair with EPOβ to support EPO-dependent proliferation signaling in HT-2 cells. HT-2.EPOR.3 cells were transfected with 20 μg of pCMV4 vector with no insert (control, ⧫) or pCMV4.EPOβ (EPOβ, ⊡) and [3H]thymidine incorporation measured on days 6-16, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336002cx.jpeg?Expires=1766338550&Signature=OxFhTyzFg269W3eYNK~Xau9u61RKdEQKhcSlzIUpfjmNUaufgSPQ2LeRSWuw3d6sWZoaxDpeAIglw9k89Y5zcDHSaI4Ojzr1xqyoB~AttWCr4X6s1ongPAHdj1zo~tv9JP1hY0Sok9eLBsZJT0f-cnrsaEZKc3nAF2OJATn6x-SKLlf9pFKGEkvMs9F5RjwLG6aalms6FdrKQQaYehUCfs290DyaiNua-vxh5HqiPxW0jnLKd8Ok5H62F-qpTxjfG8h7OA0bFoZrL0OhnC-Jd1UyIHSFxTugVN8MQE6-CAxDJFnv6D6MS5617L9IEyiOjni1JjiYBVfpECfhvgwbyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006ax.jpeg?Expires=1766338550&Signature=H-BU4StTyIBioU0Fbc9TrFJceh5vRKkkmYN9wo7a0EZ5EhP7kv6cMpBijbpC8V8jBaHquXnQ25zwEFiFkTuE8tvF7EHfVXUjB7pK8vLFPQlSapSq4ZGbe-u3nxPYphZod93KeAzgwtD9IKjdNe1SWNKmsZJqdU26k~X7Pn1~YHtr8Chy60BvVEEoKQKYiboNGLxVmcFSM1tx34vYFQoK0hObGoYckwjWTfD~3DCF4WhsLWFwQ-ecwkuKFL0LVoFG69tPu~R6HyfRG-vQaQBgFFFJhL79mewvXKie2COE0gd4H9DFowFKh~WFcDshwvWNTZ3hVOaIQUFnJHc9QlZY7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006cw.jpeg?Expires=1766338550&Signature=IbotcjN9Eoj-H5KoiQ2ynBbc1Q~JdFSaS8iGJFITG9-YABY~XMF2RN6j~0teGS8v10yITV-mVkisaak9JrYO3obsP-j6xgjKHWxt~DiN~rfFBQHzqpR6EpRCyKEus17dPUd5Vj89rRS2xbZi6CSM-Ck-kn1Xz74-FvSUXDt-7HjU2RJXFx7ga0T8OAWZa7HjrgHLLVBjVN2py0a7lPvRHnOxSSDuMZaS-D032CbKZpLKdTBUboGa43FZa26~xHs6K~xo2lpg0mnaNna1ncZiroo9JYub-84ah1nKsfzEMNNBh4P34yxitD79La7-x94LHoTngEhDS8bZhOs182x1Pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006dw.jpeg?Expires=1766338550&Signature=VahM5ZJIv3sKNKH8camROH4EiyuXTrb~tK~Kr6X0r9CWNY-biwYh0Rg89CwpUDPDqxhocTjCmlNwCtODzN7MZ2UICc4met6NiMD3C0EhsqNMCvutVfS-2PF7nVQdkP7sykp~UmpZRNmSoxhE7rqmlMsC1HyvnA7XMnAQd5nal0EYrhRZ~ZU9~FAVre6~bTpEYCOHYdZ8V0yZebwV5SxcHd9jqnJC-E4wgBzYx8Id1xjN43m~DpxjnDiGdrKKde2xS53Pj1sAyX0ZKQ722HnKWUqHU7PDKZv1-Fi5luaXLtpjbjsMfwp~aZ-R3apiZZPmh0uEp1nKiqvmDrSupRF49Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Somatic fusions between HT-2.EPOR.3 and Ba/F3 cells restore EPO-dependent signaling. (A) Indicated cell lines were incubated without cytokine (U), 50 U/mL EPO or 10% WEHI-CM (as a source of IL-3), and [3H]thymidine incorporation measured after 48 hours. (▪), HB40; (▨), HB55; (▤), Ba/F3; (□), HT-2.EPOR.3. (B) Indicated cell lines were incubated without cytokines or with various doses of EPO, and [3H]thymidine incorporation was measured after 48 hours. (▪), HB40; (•), HB55. (C) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated for 15 minutes with no cytokine (U), 50 U/mL EPO (E), or 10 nmol/L IL-2 (2). Nuclear extracts were subjected to EMSA with the FcγRI probe. Note that all lanes were derived from the same gel, but in the photograph, irrelevant intervening lanes were omitted between lanes 3 and 4. (D) HT-2.EPOR.3, HB40, and HB55 cells were rested and stimulated with no cytokine (U), EPO (E), or IL-2 (2) and lysates prepared and immunoprecipitated with anti-STAT-5A and -5B antibodies. Lysates were separated on 8.75% SDS-PAGE, transferred to nitrocellulose membranes, and probed with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti–STAT-5A and -5B antibodies to confirm equivalent loading. (E) HT-2.EPOR.3, HB40, and HB55 cells were starved and stimulated with no cytokine (lanes 1, 5, and 9) or 50 U/mL EPO (lanes 2 through 4, 6 through 8, and 10 through 12) as described in the legend to Fig 5. RNA was separated on a denaturing agarose/formeldehyde gel, transferred to Zeta Probe membranes, and probed with 32P-labeled c-fos orGAPD cDNA probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/1/10.1182_blood.v94.1.74.413k36_74_86/6/m_blod41336006ew.jpeg?Expires=1766338550&Signature=HhHfLdG9VDftMtngV7ciVz1NdEXWXdikQWsMgvr-EQ09ErITqnE7oVvxmpiWFhRk6NJ9jcGXP6eaNYx1FAuUFwWPsBkeTAS-fz7h5UqESKmDyAfDrSwKw0UNEIFCZLMhGi~utfOZwMYkbXFfmau4GDBNEzjWyxXikoQJXR1KcfqQ7esSe8on781UtOBbVYikdk1v0JwyOJ35Si3FU1gO1Uxsn68fnYIQizgchLrpQLDr5hnR1L4tQJDV6LrxnSSGIErSvapP~grv3R0XxdhHZRPMJ52Z7pWZQr52pPqsLtqmRANbmfPreKl6fXpa3lKgp9zThR5VcGN1CWqWBO4uwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)