The transcription factor GATA-1 is essential for normal erythropoiesis. By examining in vitro–differentiated embryonic stem cells, we showed previously that in the absence of GATA-1, committed erythroid precursors fail to complete maturation and instead undergo apoptosis. The mechanisms by which GATA-1 controls cell survival are unknown. Here we report that in erythroid cells, GATA-1 strongly induces the expression of the anti-apoptotic protein bcl-xL, but not the related proteins bcl-2 and mcl-1. Consistent with a role for bcl-xL in mediating GATA-1–induced erythroid cell survival, in vitro–differentiated bcl-xL−/− embryonic stem cells fail to generate viable mature definitive erythroid cells, a phenotype resembling that of GATA-1 gene disruption. In addition, we show that erythropoietin, which is also required for erythroid cell survival, cooperates with GATA-1 to stimulate bcl-xL gene expression and to maintain erythroid cell viability during terminal maturation. Together, our data show that bcl-xL is essential for normal erythroid development and suggest a regulatory hierarchy in which bcl-xL is a critical downstream effector of GATA-1 and erythropoietin-mediated signals.
THE GENERATION of mature hematopoietic cells relies upon the establishment of lineage-specific developmental programs and the maintenance of precursor viability during cellular maturation. Both of these requirements are achieved during erythroid development in embryos and adults by the essential X-linked transcription factor GATA-1. The first erythrocytes, termed primitive (or embryonic), arise in the extraembryonic yolk sac. Later, erythropoiesis occurs in the fetal liver where adult (or definitive) erythrocytes are produced. Finally, at birth, erythrocyte production shifts to the bone marrow (and spleen in mice). Although primitive and definitive erythrocytes exhibit unique patterns of gene expression and growth factor requirements,1-7 GATA-1 is required for both lineages.8,9 Gene knockout studies in mice have shown that in the absence of GATA-1, committed erythroid precursors undergo maturation arrest and apoptosis.9-11 Moreover, inhibition of GATA-1 in erythroleukemia cells causes apoptosis.12 In addition to its functions in erythroid development, GATA-1 is required for normal megakaryocytic differentiation.13 14
GATA-1 recognizes conserved GATA-motifs found in the regulatory regions of virtually all erythroid-expressed genes including globins, heme biosynthetic enzymes, membrane proteins, and red blood cell transcription factors.15-17 It is believed that GATA-1 helps to establish and maintain the erythroid phenotype by activating these genes. How GATA-1 sustains erythroid precursor viability is unknown.
Erythropoietin (Epo) promotes erythroid development by maintaining the survival of committed precursors at the late colony-forming unit-erythroid (CFU-E) stage.4,5 Depriving primary definitive erythrocyte precursors and erythroid cell lines of Epo leads to apoptosis.18-20 The gene encoding the Epo receptor (EpoR) contains functional GATA-binding sites in its promoter and enhancer, suggesting that it is a downstream target of GATA-1.21,22 However, GATA-1−proerythroblasts express a normal level of EpoR mRNA.10Transcription of the EpoR gene and several other GATA target genes presumably occurs through the action of GATA-2, a related transcription factor whose expression is elevated approximately 50-fold in the absence of GATA-1. The formation of GATA-1− erythroid colonies is Epo-dependent, demonstrating that the EpoR signaling pathway is functionally intact in the mutant cells. These observations exclude the simple model that apoptosis of GATA-1−proerythroblasts is due to absence of the Epo receptor, and compelled us to search for additional GATA-1–induced survival pathways in erythroid cells.
The bcl-2 gene family consists of various members with either pro- or anti-apoptotic activity.23 One member, bcl-x,24appears to be especially important for erythropoiesis. bcl-x is upregulated during terminal erythroid maturation,25 and bcl-x−/− embryos exhibit apoptosis of fetal liver hematopoietic cells and pallor.26 However, the erythropoietic defect resulting from loss of bcl-x function has not been well characterized.
bcl-x has been implicated in mediating some effects of Epo signaling. For example, forced bcl-x expression inhibits apoptosis of Epo-deprived erythroid cells, and bcl-x expression in erythroid cells is Epo-dependent.20 27 The latter observation positions bcl-x downstream of the EpoR in a regulatory pathway that supports erythroid development by preventing apoptosis. How EpoR signaling regulates bcl-x and how GATA-1 fits into this scheme are not known.
GATA-1–regulated cellular maturation and viability occur through distinct pathways that have been uncoupled in the GATA-1− erythroid cell line G1E.28 G1E cells were derived from in vitro–differentiated GATA-1− embryonic stem (ES) cells and represent primary GATA-1− erythroblasts as determined by the expression of erythroid, but not myeloid, genes.28 A distinguishing property of G1E cells is that instead of undergoing apoptosis, they proliferate continuously in culture as developmentally arrested erythroid precursors. This feature makes G1E cells ideally suited for genetic and cell biological studies. Restoration of GATA-1 function in G1E cells triggers terminal erythroid maturation, providing a physiologically meaningful assay to determine structure-function relationships within GATA-1, to evaluate protein interactions surrounding GATA-1, and to identify novel genes activated or repressed by GATA-1 during terminal maturation.28 29 To facilitate these studies, we created a conditional (estrogen-responsive) form of GATA-1 by fusing its coding region to the ligand-binding domain of the human estrogen receptor (ER). G1E cells stably expressing the GATA-1/ER fusion protein (termed G1E-ER cells) undergo synchronous erythroid maturation after exposure to β-estradiol.
Here we show that bcl-xL (a splice variant which counteracts apoptotic signals) mRNA and protein are strongly induced following GATA-1 activation in G1E-ER cells. In vitro differentiation of bcl-x−/− ES cells shows maturation arrest and apoptosis of definitive erythroid precursors, similar to what we have observed upon loss of GATA-1 function. Although GATA-1 and Epo stimulate bcl-x expression independently in G1E-ER cells, their combined action is required for maximal bcl-x induction and complete maturation. Our data extend previous work which implicates a role for bcl-x in erythroid development by identifying the stage of erythroid maturation at which bcl-x becomes essential and by establishing a regulatory hierarchy in which GATA-1 and Epo cooperate to stimulate bcl-x expression and prevent apoptosis of incipient erythrocytes.
MATERIALS AND METHODS
Cytokines.
All cytokines used were purchased from R & D Systems (Minneapolis, MN), except for Epo (Amgen, Thousand Oaks, CA).
Cell lines.
G1E-ER cells are G1E cells that stably express murine GATA-1 fused to the ligand-binding domain of the human estrogen receptor.30,31 For the experiments described here, we used the clone G1E-ER2, which expresses a high level of GATA-1/estradiol receptor fusion protein and undergoes nearly complete estradiol-dependent differentiation (>80% benzidine positive cells at 72 hours). Unless otherwise specified, G1E-ER2 cells were grown in IMDM (GIBCO-BRL, Grand Island, NY) with 15% heat-inactivated fetal calf serum (Hyclone, Logan, UT), Epo 2 U/mL, and kit ligand (KL, stem cell factor), 50 ng/mL. For Epo-starvation experiments, cells were washed three times in phosphate-buffered saline, then resuspended in the same medium without Epo. To induce activation of the conditional GATA-1, cells were cultured in the presence of 10−7mol/L β-estradiol (Sigma, St Louis, MO). Benzidine staining was performed according to standard protocol.32 Cell viability was determined using the Live/Dead Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR). bcl-x+/− and bcl-x−/− gene-targeted ES cells were generated as described.33
Cell-cycle analysis.
Cells were fixed in 50% ethanol and stained for DNA content with 7 × 10−5 mol/L propidium iodide. Flow cytometry was performed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Propidium iodide fluorescence was recorded using linear amplification and LYSYS II software, and analyzed using Cellfit software (Becton Dickinson). Data were acquired from intact single cells by using a doublet discriminator. Ten thousand cells were analyzed from each sample.
Plasmids and probes.
Probes for murine bcl-x and bcl-2 were supplied by Stanley Korsmeyer (Dana Farber Cancer Institute, Boston, MA). Probes for α- and β-globins, GATA-2, c-myb, and β-actin were generated by reverse-transcribed polymerase chain reaction (RT-PCR).10 Plasmids encoding murine bcl-xL and bcl-xβ for transcription-coupled translation were supplied by Harvey Cantor and Xiao-Feng Yang (Dana-Farber Cancer Institute).34
RT-PCR for bcl-x mRNA splice variants.
Total RNA was isolated using Trizol reagent (GIBCO-BRL). Complementary DNA was synthesized using Superscript II reverse transcriptase (GIBCO-BRL) per the supplier’s instructions. The following primers, indicated schematically in Fig 2B, were used for PCR: bcl-xL and bcl-xs, LX8 (5′-CTCTCCTACAAGCTTTCCCAG-3′) and LX10 (5′-CCAGCGGTTGAAGCGCTCC-3′); bcl-xβ β1 (5′-AAATGTCTCAGAGCAACCG-3′) and β3 (5′-CACAGAGAAGAGAGACACAAGC-3′). Plasmids containing the bcl-xL coding region and mouse thymus cDNA were used as controls. PCR was performed using Taq DNA polymerase (Boehringer Mannheim, Indianapolis, IN) for 30 cycles under the following conditions: 94°C, 30 seconds; 50°C, 30 seconds; and 72°C, 90 seconds. PCR products were resolved by electrophoresis on 1.6% agarose/Tris acetate gels.
Northern blotting.
Total RNA was isolated using Trizol reagent and fractionated on 1.2% agarose-formaldehyde gels according to standard conditions. Northern blot analysis was performed using Hybond C+ (Amersham, Arlington Heights, IL), according to the manufacturer’s instructions. Blots were washed at a final stringency of 0.5X SSC at 65°C.
Western blotting.
Western blotting was performed according to standard protocols. G1E-ER2 cells were lysed in 1X SDS sample buffer (Sigma) and passed through a 21-gauge needle several times to shear DNA. Fifty micrograms of protein was fractionated on 14% polyacrylamide/sodium dodecyl sulfate (SDS) gel and electrophoretically transferred to nitrocellulose membrane. Equal loading of proteins between samples was verified by Coomassie Blue staining of SDS-polyacrylamide gels in parallel experiments. Two polyclonal rabbit anti-human bcl-x antibodies were used in independent experiments: no. B22630 (Transduction Laboratories, Lexington, KY), 1:1,000 dilution; and bcl-xS/L S-18 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:100 dilution. The secondary antibody was horseradish peroxidase–conjugated donkey anti-rabbit (Amersham) at 1:5,000 dilution. Bound antibody was detected by chemiluminescence using the ECL Western blot kit (Amersham). In vitro transcription and translation of bcl-xL and bcl-xβ was performed using the TNT coupled reticulocyte lysate system (Promega, Madison, WI).
In vitro differentiation of ES cells.
Generation of hematopoietic colonies from ES cells was performed as described.10 35 The specific conditions used to assess various hematopoietic lineages are described in the text and figure legends. Cytokine concentrations used for ES cell replating are as follows: Epo, 2 U/mL; KL, 50 ng/mL; granulocyte colony stimulating factor (G-CSF), 1,000 U/mL; granulocyte macrophage colony-stimulating factor (GM-CSF), 15 U/mL; M-CSF, 100 U/mL.
In situ detection of apoptosis.
Cells from erythroid colonies were deposited onto glass slides by cytocentrifugation. The TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) assay for apoptosis was performed using the ApopTag Apoptosis Detection Kit (Oncor, Gaithersburg, MD) according to the manufacturer’s instructions.
RESULTS
G1E-ER2 cells undergo estradiol-dependent terminal erythroid maturation.
To achieve synchronous activation of GATA-1 in G1E cells, we generated a conditional form of murine GATA-1 in which its coding region was fused to that of the ligand-binding domain of the human ER.29-31 This chimeric protein activates GATA-dependent promoter-reporter constructs in an estradiol-dependent fashion (not shown). After exposure to β-estradiol, G1E cells stably expressing the GATA-1/ER fusion product induced hemoglobin, as measured by benzidine staining. The degree of benzidine staining varied among individual clones and correlated with the level of GATA-1/ER protein expressed (not shown). These observations are consistent with concentration-dependent requirements for GATA-1 observed during erythroid differentiation in vivo.36 A clone expressing a relatively high level of the GATA-1/ER fusion protein, referred to as G1E-ER2, exhibited nearly complete estradiol-dependent maturation (benzidine staining observed in more than 80% of cells after 72 hours); this clone was used in the experiments described below.
Addition of estradiol to G1E-ER2 cultures triggered synchronous erythroid maturation (Fig 1, see page 91). Adult-type globin mRNAs were induced by 6 hours, and within 12 to 24 hours, cell division ceased with concomitant G1-phase cell-cycle arrest, followed by the appearance of benzidine-staining cells with a late normoblast morphology at 24 to 72 hours (Fig 1A and B). Concurrently, mRNA levels of c-myb and GATA-2, markers for less differentiated states associated with higher proliferative capacity,31,37 decreased (Fig1C). Estradiol had no apparent effect on the parental G1E line, which does not express the GATA-1/ER fusion protein. Of note, the partial ER antagonist tamoxifen induced maturation of G1E-ER2 cells (not shown). Tamoxifen binds to the ER moiety of chimeric fusion proteins leading to activation, but in contrast to estradiol, fails to induce interaction of the ER ligand-binding domain with its cellular coactivators.38,39 This indicates that the biological effects of GATA-1/ER are attributable to the GATA-1 moiety of the fusion protein, which is consistent with our previous finding that wild-type GATA-1 introduced into G1E cells via retroviral transfer triggers terminal differentiation.28
Activation of conditional GATA-1 in G1E-ER2 cells triggers terminal erythroid maturation. Conditional GATA-1 was created by fusing the full-length murine cDNA to the ligand-binding domain of the human ER. G1E-ER2 cells are a clone derived from stable expression of the GATA-1/ER chimeric protein in the GATA-1−erythroid cell line, G1E. (A) Kinetics of G1 cell cycle arrest (left) and hemoglobin accumulation (benzidine staining, right) after addition of estradiol. Note that estradiol has no cell-cycle–arresting or hemoglobin-inducing effects on the G1E parental line, which does not express estradiol-inducible GATA-1. (B) G1E-ER2 cell morphology (May-Grünwald-Giemsa staining, top panels) and benzidine staining (bottom panels) before and 72 hours after addition of estradiol. Original magnification: 1,000× top panels; 400×, bottom panels. (C) Northern blot analysis of erythroid-expressed genes. Each lane contains 15 μg of total RNA.
Activation of conditional GATA-1 in G1E-ER2 cells triggers terminal erythroid maturation. Conditional GATA-1 was created by fusing the full-length murine cDNA to the ligand-binding domain of the human ER. G1E-ER2 cells are a clone derived from stable expression of the GATA-1/ER chimeric protein in the GATA-1−erythroid cell line, G1E. (A) Kinetics of G1 cell cycle arrest (left) and hemoglobin accumulation (benzidine staining, right) after addition of estradiol. Note that estradiol has no cell-cycle–arresting or hemoglobin-inducing effects on the G1E parental line, which does not express estradiol-inducible GATA-1. (B) G1E-ER2 cell morphology (May-Grünwald-Giemsa staining, top panels) and benzidine staining (bottom panels) before and 72 hours after addition of estradiol. Original magnification: 1,000× top panels; 400×, bottom panels. (C) Northern blot analysis of erythroid-expressed genes. Each lane contains 15 μg of total RNA.
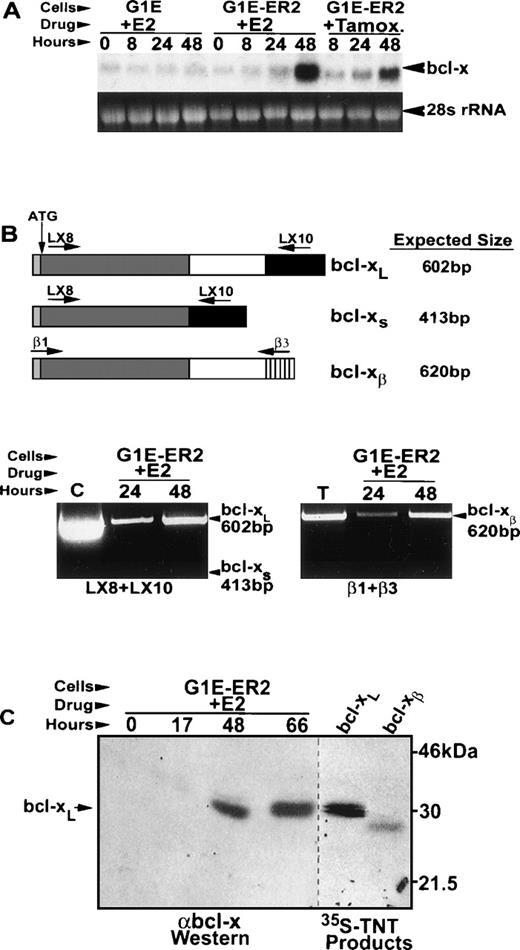
bcl-x mRNA and bcl-xL protein are upregulated during G1E-ER2 cell maturation.
We examined expression patterns of various anti-apoptotic bcl-2 gene family members during estradiol-induced G1E-ER2 cell maturation. Among several candidates tested—including bcl-2, mcl-1, and bcl-x—only bcl-x was strongly upregulated upon GATA-1/ER activation (Fig 2 and not shown). After addition of estradiol, bcl-x mRNA induction was detected at 24 hours and became more substantial by 48 hours (Fig 2A). In addition, bcl-x mRNA was upregulated by tamoxifen in G1E-ER2 cells, but not by estradiol in parental G1E cells, excluding the possibility that bcl-x is induced by estradiol in a GATA-1–independent manner (see above).
Induction of bcl-xL mRNA and protein by GATA-1 in G1E-ER2 cells. G1E-ER2 and parental G1E cells were treated with estradiol (E2) or tamoxifen (Tamox) and sampled for bcl-x mRNA and protein at the indicated times. (A) Northern blot analysis using a bcl-xL cDNA probe. Each lane contains 20 μg of total RNA. (B) Expression of splice variants bcl-xL, bcl-xs, and bcl-xβ in maturing G1E-ER2 cells. The top panel shows a schematic of the various bcl-x mRNAs examined and the primers used for detection by RT-PCR. The bottom panels show the RT-PCR reactions for bcl-xL and bcl-xs (left) and bcl-xβ (right). The positive controls are bcl-xL cDNA plasmid (C) and mouse thymus cDNA (T). (C) bcl-x protein expression in G1E-ER2 cells. For Western analysis (left panel), 50 μg of protein from whole-cell lysates was fractionated on a 14% SDS polyacrylamide gel. 35S methionine-labeled in vitro transcribed/translated (TNT) bcl-xL and bcl-xβ isoforms were fractionated in adjacent lanes (right panel).
Induction of bcl-xL mRNA and protein by GATA-1 in G1E-ER2 cells. G1E-ER2 and parental G1E cells were treated with estradiol (E2) or tamoxifen (Tamox) and sampled for bcl-x mRNA and protein at the indicated times. (A) Northern blot analysis using a bcl-xL cDNA probe. Each lane contains 20 μg of total RNA. (B) Expression of splice variants bcl-xL, bcl-xs, and bcl-xβ in maturing G1E-ER2 cells. The top panel shows a schematic of the various bcl-x mRNAs examined and the primers used for detection by RT-PCR. The bottom panels show the RT-PCR reactions for bcl-xL and bcl-xs (left) and bcl-xβ (right). The positive controls are bcl-xL cDNA plasmid (C) and mouse thymus cDNA (T). (C) bcl-x protein expression in G1E-ER2 cells. For Western analysis (left panel), 50 μg of protein from whole-cell lysates was fractionated on a 14% SDS polyacrylamide gel. 35S methionine-labeled in vitro transcribed/translated (TNT) bcl-xL and bcl-xβ isoforms were fractionated in adjacent lanes (right panel).
Differential splicing of primary bcl-x transcripts leads to the generation of proteins with varying effects on apoptosis.24,40,41 RT-PCR analysis showed that bcl-xL, the anti-apoptotic form, and bcl-xβ, which can be either pro- or anti-apoptotic depending on cellular context, were induced during G1E-ER2 cell maturation (Fig 2B). mRNA encoding the short isoform, bcl-xs, which antagonizes anti-apoptotic effects of bcl-2 or bcl-xL, was undetectable. A similar pattern of bcl-x mRNA expression is observed in day 15.5 fetal liver, which consists primarily of erythroid cells.41
Western blots using two different polyclonal antibodies against bcl-x detected the induction of a single protein of approximately 29 kD, paralleling the increase of bcl-x mRNA (Fig 2C and not shown). This bcl-x protein migrated at a position identical to in vitro transcribed/translated bcl-xL, and more slowly than bcl-xβ. Therefore, bcl-xL appears to be the predominant form expressed in G1ER-E2 cells. These data show that bcl-xL is a direct or indirect downstream target of GATA-1, suggesting a mechanism by which GATA-1 maintains the viability of developing erythrocytes.
bcl-x is essential at a late stage of definitive erythroid maturation.
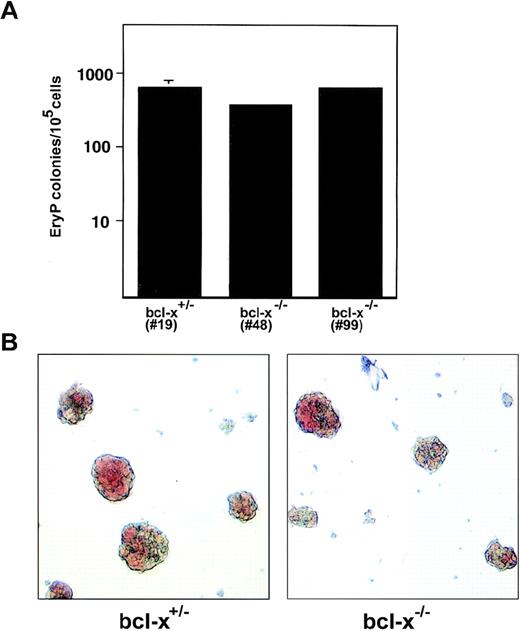
bcl-x knockout mice die at embryonic day 13 with apoptosis of fetal liver hematopoietic cells and pallor.26 To investigate whether failure to produce viable definitive erythroid cells is caused by a cell-autonomous defect of bcl-x-null precursor cells, and to pinpoint the stage at which loss of bcl-x function affects erythropoiesis, we analyzed the developmental potential of heterozygous mutant (bcl-x+/−) and homozygous null (bcl-x−/−) ES cells using a two-step in vitro differentiation protocol.35 Upon differentiation in vitro, ES cells form aggregates, termed embryoid bodies (EBs), which contain numerous differentiated cell types, including hematopoietic precursors of multiple lineages, which appear in a well-defined temporal sequence. Pure hematopoietic colonies are generated by disaggregating EBs and replating the single-cell suspension into cytokine-containing methylcellulose cultures. This technique allowed us to quantitate and evaluate primitive and definitive erythroid precursors from the bcl-x gene-targeted ES cells. To minimize the possibility that any differences between homozygous and heterozygous mutant cells are caused by a mutation unrelated to bcl-x acquired during cell passage, all experiments were performed using two independently derived clones of bcl-x−/− ES cells (nos. 48 and 99). The results obtained from these clones were indistinguishable (not shown).
To generate primitive erythroid (EryP) colonies, 6-day-old EBs were replated into cultures with Epo. Under these conditions, the majority of colonies formed were EryP as determined by histological staining of cytocentrifugation preparations (not shown). bcl-x−/− EryP progenitors formed in normal numbers (Fig 3A), and generated normal-appearing, hemoglobinized colonies when compared with the heterozygous mutant cells (Fig 3B). There were no marked differences in size or extent of hemoglobinization between bclx−/−, bcl-x+/−, and bcl-x+/+ EryP colonies (Fig3 and not shown). These observations are consistent with the phenotype of bcl-x−/− embryos, in which primitive erythropoiesis is not drastically impaired.26
Bcl-x is dispensable for normal development of primitive erythroid colonies. (A) Primitive erythroid (EryP) precursors were enumerated after secondary replating of 6-day-old embryoid bodies derived from in vitro differentiation of bcl-x+/− and bcl-x−/− ES cells. EryP colonies were generated in methylcellulose cultures containing Epo. Similar results were obtained using two independently derived bcl-x−/− ES cell clones (nos. 48 and 99). Bcl-x−/− EryP colonies shown in B are from clone no. 48. Error bars represent standard error of the mean from three separate cultures. (B) bcl-x+/− and bcl-x−/− EryP colonies exhibit similar morphologies. Five-day-old colonies are shown. Original magnification × 400.
Bcl-x is dispensable for normal development of primitive erythroid colonies. (A) Primitive erythroid (EryP) precursors were enumerated after secondary replating of 6-day-old embryoid bodies derived from in vitro differentiation of bcl-x+/− and bcl-x−/− ES cells. EryP colonies were generated in methylcellulose cultures containing Epo. Similar results were obtained using two independently derived bcl-x−/− ES cell clones (nos. 48 and 99). Bcl-x−/− EryP colonies shown in B are from clone no. 48. Error bars represent standard error of the mean from three separate cultures. (B) bcl-x+/− and bcl-x−/− EryP colonies exhibit similar morphologies. Five-day-old colonies are shown. Original magnification × 400.
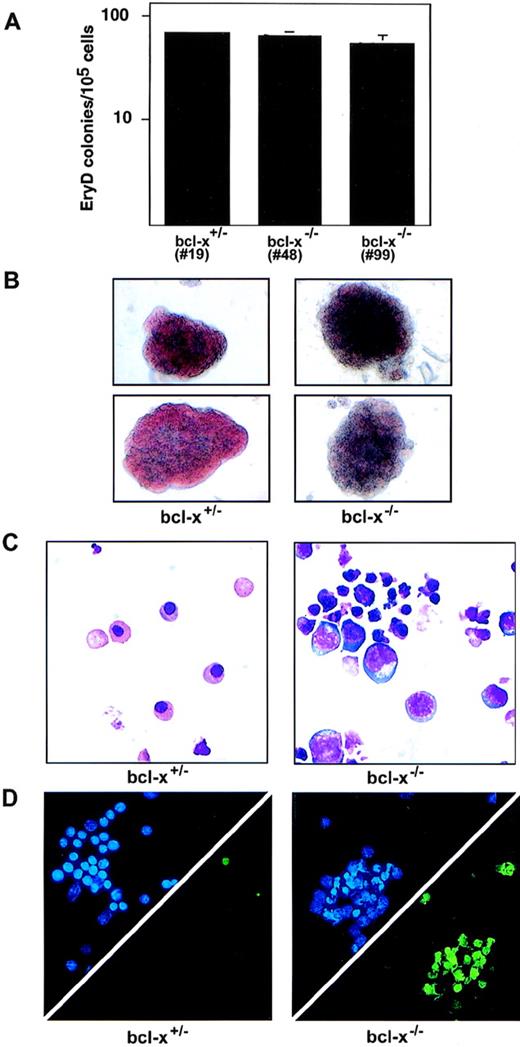
Definitive erythroid (EryD) bcl-x−/− colonies, which were generated from 10-day-old EBs replated into cultures with Epo and KL, were also normal in number. However, in contrast to their EryP counterparts, bcl-x−/− EryD precursors were significantly impaired in their maturation as reflected by their failure to form fully hemoglobinized colonies (Fig 4B), and by their abnormal cellular morphology (Fig 4C). In addition, the delay in maturation was accompanied by excessive apoptosis as shown by TUNEL staining of the bcl-x−/− erythroid cells (Fig 4D). These results are consistent with the observation that loss of bcl-x function impairs viability of fetal liver erythrocytes in mice,26and localize the defect to between the proerythroblast and early normoblast stage of development. Therefore, bcl-x is required for the survival and normal maturation of definitive erythroid cells. The phenotype resulting from bcl-x deficiency in EryD cells is similar to that observed with GATA-1 loss, albeit less severe.
Bcl-x−/− definitive erythroid cells hemoglobinize poorly and undergo excessive apoptosis. (A) bcl-x+/− and bcl-x−/− definitive erythroid (EryD) precursors were enumerated after replating 10-day-old embryoid bodies into cultures containing Epo and Kit ligand (KL, stem cell factor). Similar results were obtained using two independently derived bcl-x−/− ES cell clones (nos. 48 and 99). Analyses in B through D were performed using bcl-x−/−EryD colonies from clone no. 48. Error bars represent standard error of the mean from three separate cultures. (B) bcl-x−/−EryD colonies are normal in size, but exhibit defective hemoglobinization. Colonies shown are 6 days old. Original magnification × 200. (C) May-Grünwald-Giemsa staining of erythroid cells from 6-day-old bcl-x+/− and bcl-x−/− EryD colonies. Note the larger blast-like cells and cells with pyknotic nuclei within bcl-x−/−colonies. Original magnification × 600. (D) Excessive apoptosis within cells derived from bcl-x−/− EryD colonies. Upper triangles show DAPI staining (blue), which displays the nuclei of all cells. The same cells were stained for apoptosis using the TUNEL stain (lower triangles, green).
Bcl-x−/− definitive erythroid cells hemoglobinize poorly and undergo excessive apoptosis. (A) bcl-x+/− and bcl-x−/− definitive erythroid (EryD) precursors were enumerated after replating 10-day-old embryoid bodies into cultures containing Epo and Kit ligand (KL, stem cell factor). Similar results were obtained using two independently derived bcl-x−/− ES cell clones (nos. 48 and 99). Analyses in B through D were performed using bcl-x−/−EryD colonies from clone no. 48. Error bars represent standard error of the mean from three separate cultures. (B) bcl-x−/−EryD colonies are normal in size, but exhibit defective hemoglobinization. Colonies shown are 6 days old. Original magnification × 200. (C) May-Grünwald-Giemsa staining of erythroid cells from 6-day-old bcl-x+/− and bcl-x−/− EryD colonies. Note the larger blast-like cells and cells with pyknotic nuclei within bcl-x−/−colonies. Original magnification × 600. (D) Excessive apoptosis within cells derived from bcl-x−/− EryD colonies. Upper triangles show DAPI staining (blue), which displays the nuclei of all cells. The same cells were stained for apoptosis using the TUNEL stain (lower triangles, green).
Nonerythroid colonies derived from in vitro differentiation of bcl-x−/− ES cells, including macrophage, granulocyte-macrophage, and mast, were indistinguishable from wild-type and heterozygous mutant colonies in number and appearance (not shown).
GATA-1 and Epo are both required for optimal bcl-x expression.
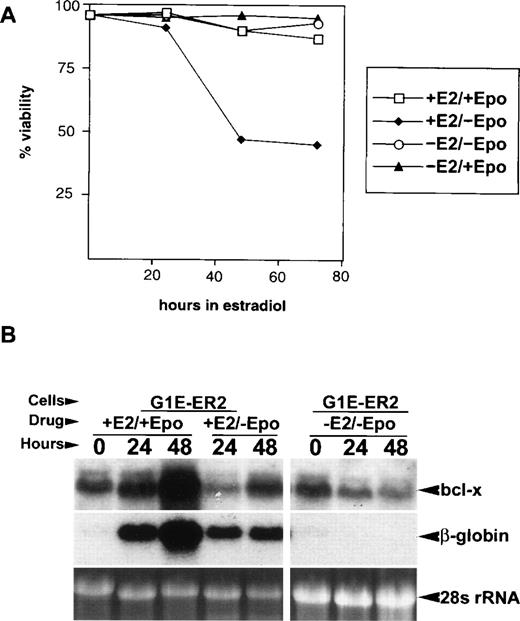
Because both GATA-1 and Epo are required for erythroid cell survival and optimal bcl-x expression4,5,20,25 (and this manuscript), we determined the effects of Epo deprivation on viability and bcl-x expression in G1E-ER2 cells. G1E-ER2 cells are typically grown in Epo and KL. After Epo removal in the absence of estradiol, G1E-ER2 proliferation decreased slightly (not shown), but more than 90% of the cells remained viable (−Epo, −E2; Fig 5A). In contrast, Epo starvation during estradiol-induced maturation reduced viability by approximately 50% at 48 hours (−Epo,+E2; Fig 5A), although induction of globin RNAs (Fig 5B) and benzidine-positive cells still occurred (not shown). Therefore, Epo deficiency impairs survival of G1E-ER2 cells only during GATA-1–induced terminal maturation, but induction of terminal differentiation markers still occurs, consistent with models that assign a supportive, rather than instructive, role for Epo in erythroid differentiation.18,42 43
Epo cooperates with GATA-1 to maximize bcl-x induction and G1E-ER2 cell viability during terminal maturation. (A) Viability of G1E-ER2 cells cultured with various combinations of Epo and E2. Live and dead cells were quantitated using calcein AM and ethidium bromide, respectively. Note that Epo is required for cell survival only during estradiol-induced terminal maturation. (B) Effects of Epo starvation on bcl-x and β-globin mRNA expression in G1E-ER2 cells. Cells were cultured with the indicated combinations of Epo and/or estradiol (E2), sampled at various times and analyzed for gene expression by Northern blotting. Note that Epo and GATA-1 can induce bcl-xL mRNA independently, although both are required for maximal expression. Each lane contains 20 μg of total RNA.
Epo cooperates with GATA-1 to maximize bcl-x induction and G1E-ER2 cell viability during terminal maturation. (A) Viability of G1E-ER2 cells cultured with various combinations of Epo and E2. Live and dead cells were quantitated using calcein AM and ethidium bromide, respectively. Note that Epo is required for cell survival only during estradiol-induced terminal maturation. (B) Effects of Epo starvation on bcl-x and β-globin mRNA expression in G1E-ER2 cells. Cells were cultured with the indicated combinations of Epo and/or estradiol (E2), sampled at various times and analyzed for gene expression by Northern blotting. Note that Epo and GATA-1 can induce bcl-xL mRNA independently, although both are required for maximal expression. Each lane contains 20 μg of total RNA.
Next, we tested whether Epo stimulates bcl-x expression in G1E-ER2 cells (Fig 5B). In the absence of estradiol, removal of Epo reduced bcl-x mRNA levels (−Epo, −E2; Fig 5B) with little or no effect on cell viability (see above) or β-globin expression; readdition of Epo restored bcl-x mRNA level to baseline within 24 hours (not shown). During estradiol-induced maturation, Epo starvation markedly reduced but did not completely block the ability of GATA-1 to induce bcl-x (−Epo,+E2; Fig 5B). Similar results were obtained when G1E-ER2 cells were cultured in serum-free defined medium (not shown), excluding the possibility that trace amounts of Epo present in serum are required for induction of bcl-x expression by GATA-1. Therefore, Epo and GATA-1 exert independent, cooperative effects on bcl-x mRNA induction in erythroid cells. In this cellular model for erythroid development, a strict requirement for Epo becomes manifest mainly during the later stages of maturation.
DISCUSSION
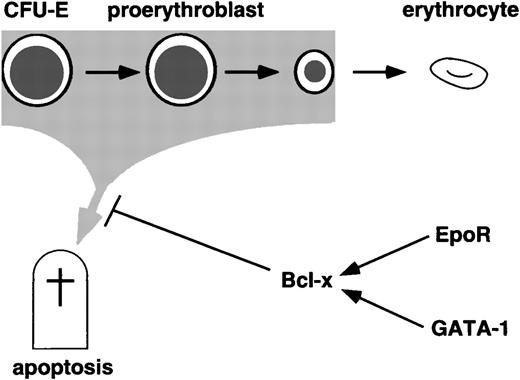
For mature blood cells to form properly, it is essential that committed precursors are protected from cell death. In most cell types, apoptosis is tightly regulated by interacting networks of cytokine signaling pathways, nuclear factors, and other effectors such as members of the caspase and bcl-2 families. Increased anti-apoptotic activity may be required in developing erythrocytes to counter stress caused by accumulation of intracellular iron and consequent production of toxic reactive oxygen species.25,44 Here we establish a regulatory pathway in which EpoR and GATA-1 cooperate to induce bcl-xL expression, which in turn is critical for the survival of late proerythroblasts and early normoblasts (Fig 6). Regulated expression of bcl-2 gene family members is one mechanism by which apoptosis is controlled.45,46 However, it remains possible that bcl-x activity in erythroid cells might also be regulated through posttranslational mechanisms such as phosphorylation and availability of dimerization partners.46
Anti-apoptosis pathways during terminal erythropoiesis. GATA-1 and Epo receptor signaling both induce bcl-x mRNA, and all three genes are required for survival of maturing definitive erythroid cells.
Anti-apoptosis pathways during terminal erythropoiesis. GATA-1 and Epo receptor signaling both induce bcl-x mRNA, and all three genes are required for survival of maturing definitive erythroid cells.
Knockout studies in mice can demonstrate the requirement of certain genes for the formation of a given cell type. One limitation of such studies is that phenotypic analysis of homozygous gene-targeted animals cannot distinguish between cell-autonomous versus indirect effects. Here we have used in vitro differentiation of bcl-x−/− ES cells to make this distinction. Our observation that bcl-x−/− definitive erythroid colonies fail to mature properly indicates that bcl-x is required in a cell-autonomous fashion for normal erythroid development.
The similarity between the phenotypes observed in GATA-1− and bcl-x−/−definitive erythroid cells is consistent with a model which places bcl-xL downstream of GATA-1 in an erythroid cell survival pathway. However, several notable differences exist between the two mutant phenotypes. First, while GATA-1 is required for normal development of both primitive and definitive erythrocytes, bcl-x appears to be essential predominantly for the definitive lineage, as evidenced by our results and the phenotype of homozygous null embryos.26 It is possible that GATA-1 maintains viability of yolk sac erythrocytes by regulating the expression of other bcl-2 family members, although none as yet have been shown to be limiting for primitive erythropoiesis. Second, while GATA-1− EryD colonies exhibit complete maturation arrest with failure to hemoglobinize, some age-matched bcl-x−/−colonies contain a higher proportion of viable cells and hemoglobinize partially. Therefore, it is possible that GATA-1 activates other death-antagonizing genes (or represses death-promoting ones) in definitive erythrocytes, in addition to bcl-x.
An open question remains as to whether bcl-x is a direct transcriptional target of GATA-1. Two GATA consensus motifs reside in the 5′ region of the human and mouse bcl-x genes, upstream of a major transcription start site mapped by primer extension using several hematopoietic cell lines,47 including G1E-ER2 (unpublished data, April 1998). DNA segments from this region activate transcription of reporter genes in several erythroid lines including MEL, G1E-ER2 (unpublished data, April 1998), and K562,47 with the majority of promoter activity residing in a 57-bp fragment that contains the proximal GATA motif.47 Mutations in this motif which prevent GATA-1 binding have little effect on promoter activity in MEL, K562, or G1E cells (unpublished data, August 1998). It is possible that GATA-1 regulates bcl-xL expression through other elements present elsewhere in the gene. Alternatively, bcl-x might be an indirect target of GATA-1. This latter possibility is supported by the delayed kinetics of bcl-xL induction after GATA-1 activation (Fig 2A, above).
Loss of Epo signaling through gene targeting results in apoptosis of committed definitive erythroid precursors at the late CFU-E stage,4,5 and Epo is required for optimal expression of bcl-x in erythroid cells.20,25 Therefore, it is possible that Epo exerts some of its anti-apoptotic effects by inducing bcl-x expression. Epo and several other hematopoietic cytokines are believed to induce bcl-x through a JAK-dependent pathway.48,49 In addition, Epo may upregulate bcl-x by inhibiting the expression of JunB, a member of the AP-1 transcription factor family.50While GATA-1 can induce bcl-xL expression independent of Epo, we do not exclude the possibility that crosstalk also exists. For example, GATA-1 might regulate the expression of genes that modulate EpoR signaling. However, in G1E-ER2 cells, it is unlikely that GATA-1 influences bcl-xL expression by directly stimulating transcription of the EpoR gene, as EpoR mRNA level decreases slightly during the period of maximal bcl-x induction (unpublished data, October 1998), and the half-life of EpoR protein in hematopoietic cells is only about 60 minutes.51 52
Epo requirements vary in G1E cells according to their state of maturation, which in turn is controlled by GATA-1. In the absence of GATA-1 activity, the cells exhibit an immature erythroid phenotype, are relatively Epo insensitive, but require KL for proliferation and survival. Based on these cytokine requirements,4 G1E cells most closely approximate the late BFU-E or early CFU-E stage of erythroid development. Because primary GATA-1−erythroid progenitors die at the proerythroblast stage, it appears that G1E cells were immortalized at a level of differentiation upstream of where the antiapoptotic effect of GATA-1 is exercised.10,11Estradiol-induced GATA-1 activation in G1E-ER2 cells triggers terminal maturation accompanied by transition into a state of Epo-dependency, consistent with the in vivo requirement for Epo during the late CFU-E and proerythroblast stages.4,5,18 GATA-1 activation without Epo causes cellular maturation, but bcl-xL induction is reduced and the cells die. We speculate that Epo is required along with GATA-1 to achieve a critical level of bcl-xL that is required for terminal differentiation. The apparently normal erythroid phenotype in bcl-x heterozygous mutant states implies that either 50% bcl-x protein is sufficient, or that there is a compensatory increase in expression of the intact allele. In contrast to Epo, KL is not required for viability or bcl-xL mRNA induction during GATA-1–stimulated maturation of G1E-ER2 cells (unpublished data, January 1999). The shift from KL to Epo-dependency during terminal differentiation of G1E cells resembles the pattern of cytokine response exhibited during normal definitive erythropoeisis.4
Hematopoietic cytokines, including Epo, are believed to function as survival factors that are supportive for terminal differentiation. Accordingly, forced expression of bcl-2 partially rescues the hematopoietic phenotypes of interleukin-7 (IL-7) receptor- and M-CSF receptor-deficient mice.53-55 The EpoR probably has a similar, supportive role,4,5 yet overexpression of bcl-2 does not rescue Epo-deprived erythroid precursors from death.56 This may reflect a critical function for bcl-x in erythroid cells that cannot be substituted for by related proteins. In contrast to the role for Epo signaling in terminal erythropoiesis, GATA-1 exerts more pleiotropic actions, fostering cell survival and erythroid maturation. Therefore, while forced expression of bcl-x might rescue apoptosis of GATA-1− erythroid precursors in vivo, it would not be expected to overcome the maturation arrest associated with the mutant phenotype.
Induction of bcl-xL by GATA-1 shows a connection between an essential anti-apoptosis pathway and a tissue-specific transcription factor required for erythroid differentiation. Gene targeting and overexpression studies have identified numerous nuclear factors that participate in the determination and differentiation of specific hematopoietic lineages.57 Enhancement of cell survival through regulation of viability genes may be a common mechanism through which transcription factors participate in the developmental control of hematopoiesis.
ACKNOWLEDGMENT
We thank Craig Thompson, Tullia Lindsten, Haidi Yang, and Kay MacLeod for providing bcl-x genomic DNA; Stanley Korsmeyer, Harvey Cantor, and Xiao-Feng Yang for providing plasmid DNAs; and Christopher Guo for performing site-directed mutagenesis on bcl-x promoter sequences. We thank Merav Socolovsky and Gordon Keller for reviewing this manuscript prior to publication.
The first two authors contributed equally to this work.
Supported in part by a grant from the National Institutes of Health (K08 HL03364, to M.J.W.)
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mitchell J. Weiss, MD, PhD, Ontogeny, Inc, 45 Moulton St, Cambridge MA 02138-1118; e-mail:mweiss@ontogeny.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal