Abstract
Systemic (nodal) anaplastic large cell lymphoma (ALCL) is a subgroup of T-cell non-Hodgkin’s lymphomas with a relatively favorable clinical outcome. Part of systemic ALCLs harbor a genetic aberration (usually the t(2;5)(p23;q35) translocation) containing the anaplastic lymphoma kinase (ALK) gene at 2p23, which results in aberrant expression of the ALK protein. Recently, we have shown that the presence of high percentages of activated cytotoxic T lymphocytes (CTLs) in tumor biopsy specimens of Hodgkin’s disease (HD) is associated with a poor prognosis. In the present study, we investigated the prognostic value of percentages of activated CTLs in combination with ALK expression in primary nodal ALCL. Primary nodal biopsies of 42 patients with ALCL were investigated for the percentage of activated CTLs (quantified using Q-PRODIT) and the expression of ALK by immunohistochemistry using monoclonal antibodies (MoAbs) directed against T-cell antigen granzyme B (GrB) and ALK, respectively. These parameters were evaluated for their predictive value regarding progression-free and overall survival time. The presence of a high percentage of activated CTLs (ie, ≥15%) was found to be an unfavorable prognostic marker. In combination with a lack of ALK expression, it was possible to identify a group of patients with a very poor prognosis. In this group, 13 of 16 patients died within 2 years as a result of the disease. Of the remaining 26 patients, only three (all ALK negative) died (P < .0001). Furthermore, the percentage of activated CTLs combined with ALK status appeared to be of stronger prognostic value than the International Prognostic Index (IPI). We conclude that a high percentage of activated CTLs present in biopsy material of patients with primary nodal ALCL is a strong indicator for an unfavorable clinical outcome. The combination of ALK expression and percentage of activated CTLs appears to be more sensitive than the IPI in identifying a group of patients with a highly unfavorable clinical outcome who may be eligible for alternative (high dose) therapy schemes.
ANAPLASTIC LARGE CELL lymphoma (ALCL) is a group of non-Hodgkin’s lymphomas characterized by large CD30+ cells with multiple or single prominent nucleoli and T-cell or null cell characteristics.1 Several histologic subtypes have been described, eg, common type, lymphohistiocytic, giant cell rich and small-cell variant.2 Clinically, two types can be recognized: a systemic variant originating mainly from lymph nodes with an intermediate prognosis; and a primary cutaneous variant with a very good prognosis.1,3,4 5
In part of the nodal ALCLs the t(2;5)(p23;q35) is detected, resulting in the fusion of the nucleophosmin (NPM) gene at 5q35 and the anaplastic lymphoma kinase (ALK) gene at 2p23.6 This results in expression of the 80-kD chimeric protein NPM-ALK. Recently, detection of the ALK portion in formalin fixed, paraffin embedded tumor specimens by immunohistochemistry was made possible by the development of the polyclonal antibody anti-p80NPM/ALK and the monoclonal antibody (MoAb) ALK1, respectively.7,8 It was shown that expression of ALK as detected by this ALK1 MoAb correlates strongly with the presence of genetic aberrations in which the ALK locus is involved, including the t(2;5)(p23;q35).8,9Although its function is still unknown, expression of ALK and/or presence of the t(2;5) was found by several investigators to be related to a more favorable clinical outcome10-13; ALK positive cases showing an 80% 5-year surivival rate, as compared with a rate of 30% for ALK negative cases.13
One of the issues to be resolved in the pathogenesis of lymphomas is the fact that tumor cells apparently are not effectively killed by the host’s immune system. The presence of many reactive lymphocytes surrounding the tumor cells in several types of lymphomas (especially in Hodgkin’s disease [HD] and ALCL) suggests that the neoplastic cells are able to elicit an immune response, but that evasion of this immune response is an important pathogenic factor.
Although ALCL and HD are considered to be separate clinicopathologic entities, they have, besides CD30 expression, many morphologic features in common and have a similar bimodal age distribution.1Recently, we and others have shown that in HD different immune escape mechanisms may be involved: downregulation of major histocompatability complex (MHC)-I expression by the tumor cells,14-16 expression of immunomodulatory cytokines such as interleukin-10 (IL-10) by the tumor cells inducing a local T-cell anergy,17-19 and intrinsic resistance of the tumor cells to apoptosis. The latter notion arose from our observation that HD patients with tumor biopsies harboring high percentages of activated cytotoxic T lymphocytes (CTLs), as demonstrated by the presence of granzyme B+ cells, have a far worse prognosis as compared with patients with low percentages of activated CTLs.20 We postulated that one of the possibilities was that in cases with many activated CTLs, this continuous immunogenic pressure selects for tumor cells that are best equipped to resist or inhibit CTL-mediated killing. This acquired resistance to CTL-mediated cytotoxicity might also result in resistance to therapy-induced apoptosis, thus explaining the poor treatment outcome. Indeed, we could show that, to a certain extent, in HD cases with high percentages of activated CTLs, the neoplastic cells expressed the apoptosis inhibiting gene bcl-2, in contrast to HD cases with low percentages of activated CTLs.21
In the present study, we investigated whether the presence of high percentages of activated CTLs in the diagnostic biopsies of patients with primary nodal ALCL is related to poor clinical outcome. Because ALK expression is a strong prognostic marker in ALCL, this was done in relation to ALK expression. Furthermore, we compared its prognostic value with that of the International Prognostic Index (IPI).
MATERIALS AND METHODS
Patients.
Patients with primary nodal ALCL (n = 42) were selected from the files of the Comprehensive Cancer Center Amsterdam (diagnosed between 1985 and 1997) and the Department of Pathology of the St. Elisabeth Hospital, Tilburg, The Netherlands (diagnosed between 1991 and 1994). If during this period a patient presented with recurrent ALCL, the lymph node biopsy was retrieved on which the initial diagnosis of ALCL was made. In our group, one primary tumor was retrieved in this way. Cases were classified according to the Revised European-American Classification of Lymphoid Neoplasms1 and subtyped as described by Benharroch et al.2 Staging at first presentation was determined by physical examination, full blood count, serum lactate dehydrogenase (LDH) concentration, bone marrow aspirate and biopsy, and radiologic imaging of chest and abdomen. Patient and tumor characteristics are summarized in Tables 1 and 2.
Detection of activated cytotoxic T cells.
At present, accurate detection of cytotoxic cells is possible using MoAbs, which react specifically with granzyme B (GrB)22,23and T-cell intracytoplasmic antigen (TIA-1).24 GrB is exclusively expressed in the activated form of CTLs, whereas TIA-1 expression is found in both resting and activated CTLs.25Upon activation, CTLs acquire cytotoxic granules in their cytoplasm. These granules contain, among other, the pore forming protein “perforin” and a family of highly homologous serine proteases, one of them being granzyme B,26-29which is involved in target cell DNA fragmentation and apoptosis. MoAb GrB7 was raised against recombinant human granzyme B protein.22 This antibody detects activated CTLs and natural killer (NK) cells in routinely formalin fixed, paraffin embedded tissue sections by immunohistochemistry.
Detection of both GrB and TIA-1 was performed as described previously, using a three-step staining technique.20,23,24 Moreover, to identify the nature of the GrB+ cells, double-stainings were performed for GrB and CD8 or CD3, as described previously.14
Quantification of the percentage of GrB+ and TIA-1+ CTLs in the reactive infiltrate was performed using a commercially available interactive video overlay-based measuring system (Q-PRODIT; Leica, Cambridge, UK), as described previously.14 20 Per tumor slide, 100 to 150 fields of vision were randomly selected using an automatic scanning stage. Numbers of GrB+ and TIA-1+ lymphocytes were expressed as percentages of all lymphocytes present in a tissue section as judged by morphology. In cases where the activated CTLs were difficult to distinguish from neoplastic cells with a cytotoxic phenotype (n = 4), scoring of activated CTLs was performed with the aid of sections double-stained for GrB and CD8, which helped to differentiate between reactive lymphocytes and neoplastic cells, as the latter were CD8−.
Detection of ALK positive cases.
Expression of ALK was detected in biopsy specimens by immunohistochemistry using the MoAb ALK1, as described previously,8 with minor modifications. Slides were incubated overnight using a 1:75 dilution, and staining was enhanced by the catalyzed reporter deposition (CARD) method, which amplifies biotinylated sites.30 Cases were considered positive if tumor cells showed positive labelling, irrespective of their number.
Analysis of clinical data.
For each patient, the following characteristics were noted from the medical records: age at diagnosis, sex, Ann Arbor stage at presentation, the presence or absence of B symptoms, erythrocyte sedimentation rate (ESR), serum LDH concentration, therapy, response, the occurrence of relapses, and cause of death. Performance status was assessed according to the Eastern Cooperative Oncology Group (ECOG) scale (0 to 4). For each patient, the IPI was determined as described previously.31 The median follow-up time was 25 months (range, 0 to 207 months). Survival time was measured from time of initial diagnosis until death due to ALCL or until end of follow-up. Patients who died of causes unrelated to the disease were censored at the time of death. Progression-free survival time was measured from time of initial diagnosis until time of disease relapse. Patients who did not enter complete remission were assigned a progression-free survival time of zero in the analysis.
Statistical methods.
Survival curves were constructed with the Kaplan-Meier method. Differences between the curves were analyzed using the Log-rank test. Multivariate analysis was performed using the Cox-proportional hazards model32 (enter and remove limits 0.1). Qualitative variables were analyzed by Pearson χ2 test or by the Kruskal-Wallis test, when appropriate. All P values are based on two-tailed statistical analysis. P values below .05 were considered significant. All analyses were performed using the SPSS statistical software (SPSS Inc, Chicago, IL).
RESULTS
Patient characteristics.
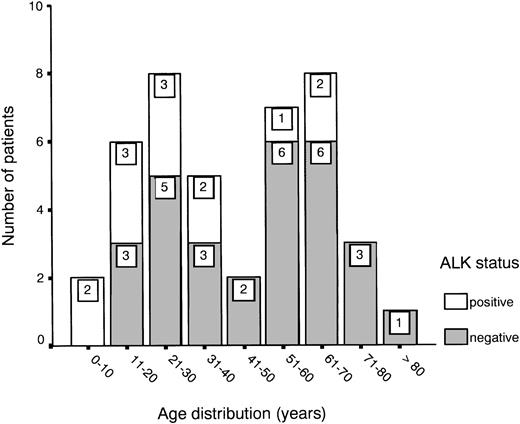
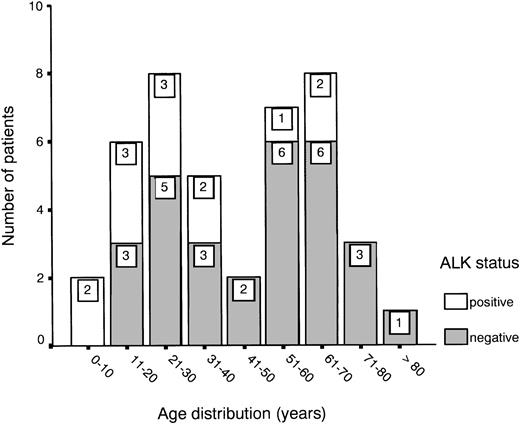
Age distribution showed a bimodal pattern, as has been described for ALCL,1 with one peak between 20 and 30 years and another between 60 and 70 years (Fig 1). ALK expression was found in 31% of all cases (13 of 42) and showed a predilection for the younger age groups (Fig 1 and Table 1). About half of the patients presented with stage III or IV disease, showing either multiple organ involvement or bone marrow dissemination.
With the exception of two cases, all patients received comparable polychemotherapy, consisting of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimens or variants, some receiving involved field radiation. One patient (no. 1) died before therapy could be administered; the other (no. 3) declined therapy on personal grounds. In 33 of 42 cases (78.5%), complete remission was achieved; of these, however, 12 patients (36%) experienced a relapse, with a median progression-free survival time of 9.5 months (range, 2 to 105 months).
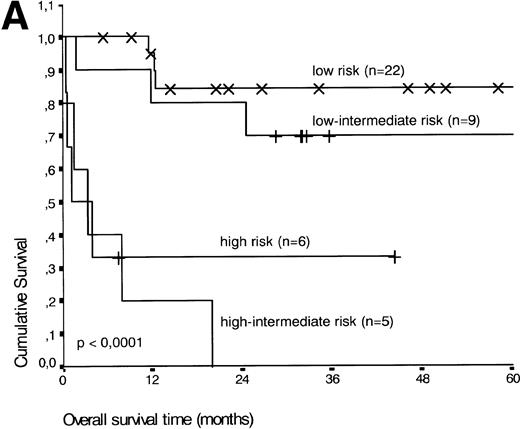
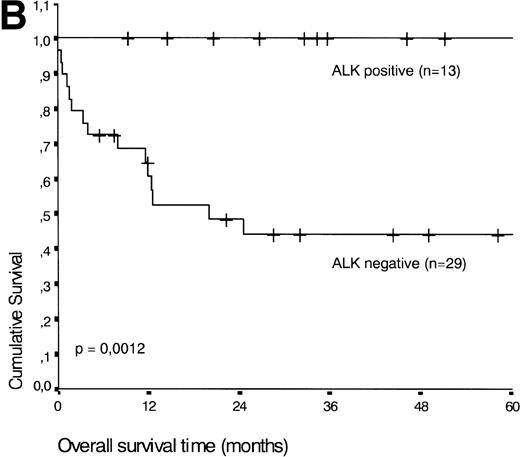
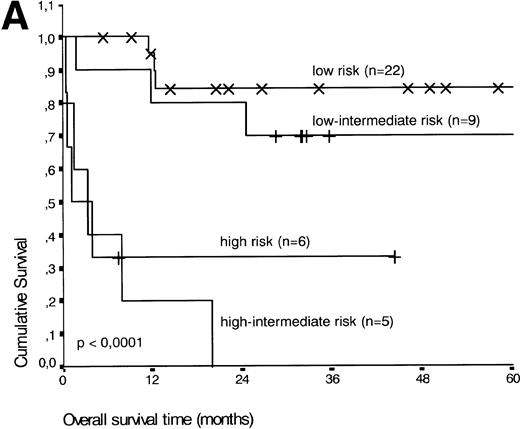
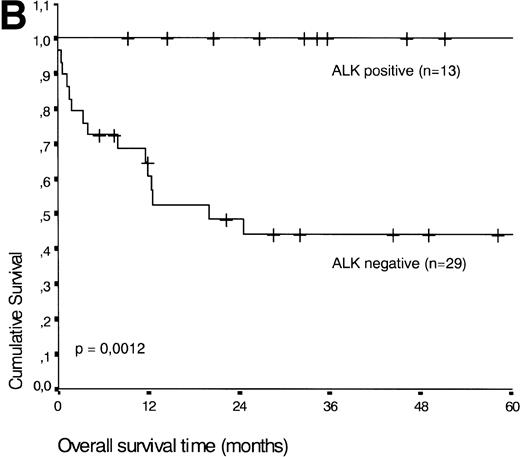
Analysis of overall survival time showed that the IPI has a strong prognostic value (P < .001) (Fig2A). This is in line with previous studies.5,33 34 As expected, ALK expression defined a group with favorable clinical outcome: none of 13 patients with ALK positive ALCL died, whereas 16 of 29 ALK negative patients died as a result of the disease (P < .01) (Fig 2B).
(A) Comparison of overall survival time according to IPI. (B) Comparison of overall survival time, according to ALK status.
(A) Comparison of overall survival time according to IPI. (B) Comparison of overall survival time, according to ALK status.
Tumor characteristics and GrB expression.
Neoplastic cells showed CD30 expression and either a T-cell or null-cell phenotype, as defined by the REAL classification.1 Most cases (30 of 42, 71%) belonged to the common type of ALCL, whereas eight cases (19%) were considered lymphohistiocytic type, one case (2%) as mixed (lymphohistiocytic and giant cell rich), and the remaining three (7%) as giant cell rich variant.2
GrB expression by tumor cells was found in 23 of 42 cases (55%), with the percentage of GrB+ tumor cells ranging from a few to more than 90% of tumor cells (Table 1). In two additional cases, there was TIA-1, but no GrB expression (data not shown).
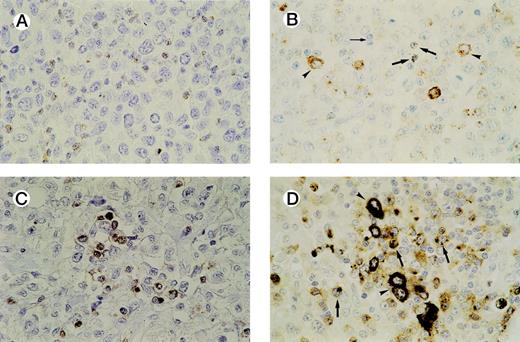
In all cases tested, GrB+ reactive lymphocytes were found interspersed between the tumor cells and displaying a granular cytoplasmic staining pattern, which reflects the granular localization of GrB (see Fig 3A and B). Numbers of GrB+ CTLs ranged between 1% and 62% of reactive lymphocytes, with approximately half of the patients having ≥15% GrB+ CTLs (n = 21).
Detection of activated CTLs. (A) Biopsy specimen of an ALCL patient with ≥ 15% activated CTLs, presenting with stage 1 disease who died 13 months later as a result of the disease. Brown cytoplasmic staining indicates GrB expression. Tumor cells are negative for GrB. (B) Biopsy specimen of an ALCL patient with a cytotoxic phenotype of the tumor cells and ≥ 15% activated CTLs. Both GrB+ tumor cells (arrowheads) and activated CTLs (thick arrows) show brown cytoplasmic staining; thin arrow indicates GrB− reactive lymphocytes. (C) Double-staining for CD8 and GrB in a biopsy specimen of an ALCL patient. The majority of activated CTLs express both CD8 (brown membranous staining) and GrB (black cytoplasmic staining). Tumor cells are CD8− and GrB−. (D) Double-staining for CD8 and GrB in a biopsy specimen of an ALCL patient with cytotoxic phenotype of the tumor cells. Tumor cells (arrowheads) show expression of GrB (black cytoplasmic staining), but not CD8, whereas activated CTLs (thick arrows) show expression of both GrB (black cytoplasmic staining) and CD8 (brown membranous staining).
Detection of activated CTLs. (A) Biopsy specimen of an ALCL patient with ≥ 15% activated CTLs, presenting with stage 1 disease who died 13 months later as a result of the disease. Brown cytoplasmic staining indicates GrB expression. Tumor cells are negative for GrB. (B) Biopsy specimen of an ALCL patient with a cytotoxic phenotype of the tumor cells and ≥ 15% activated CTLs. Both GrB+ tumor cells (arrowheads) and activated CTLs (thick arrows) show brown cytoplasmic staining; thin arrow indicates GrB− reactive lymphocytes. (C) Double-staining for CD8 and GrB in a biopsy specimen of an ALCL patient. The majority of activated CTLs express both CD8 (brown membranous staining) and GrB (black cytoplasmic staining). Tumor cells are CD8− and GrB−. (D) Double-staining for CD8 and GrB in a biopsy specimen of an ALCL patient with cytotoxic phenotype of the tumor cells. Tumor cells (arrowheads) show expression of GrB (black cytoplasmic staining), but not CD8, whereas activated CTLs (thick arrows) show expression of both GrB (black cytoplasmic staining) and CD8 (brown membranous staining).
Double-staining showed that the large majority of GrB+reactive lymphocytes are also positive for CD3 and CD8 and are thus in majority activated CTLs. In all cases, except one, the tumor cells were found to be negative for CD8 (Table 1). This predominantly CD8− phenotype of ALCL has been demonstrated before by us and other groups.35-37 Thus, double-stainings helped to differentiate between (GrB+/CD8+) reactive lymphocytes and (GrB+/CD8−) tumor cells and were used as an aid in quantifying activated CTLs in cases where it was difficult to distinguish tumor cells from reactive lymphocytes (Fig3C and D). In our study, this was the case in four lymphomas, all ALK positive (Table 2, cases 30, 32, 36, and 38).
Prognostic value of percentage activated CTLs.
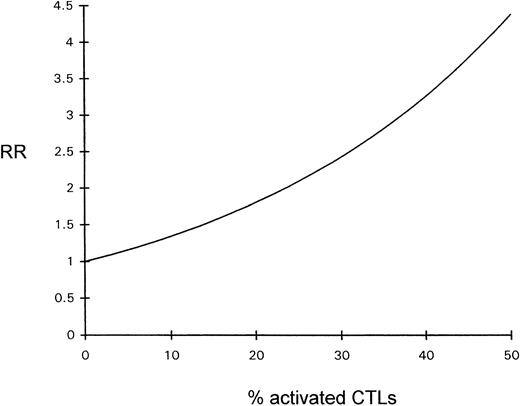
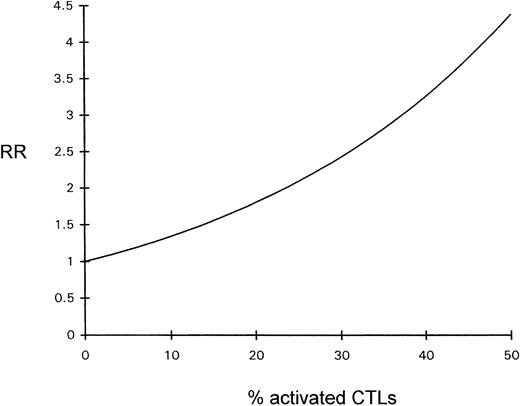
The influence of the percentage of activated CTLs on overall survival time was estimated by Cox regression analysis; the prognosis declined with increasing percentages of activated CTLs (see Fig 4).
Diagram depicting the relative risk (RR) for a fatal outcome of ALCL as a function of the percentage of activated CTLs (Cox regression).
Diagram depicting the relative risk (RR) for a fatal outcome of ALCL as a function of the percentage of activated CTLs (Cox regression).
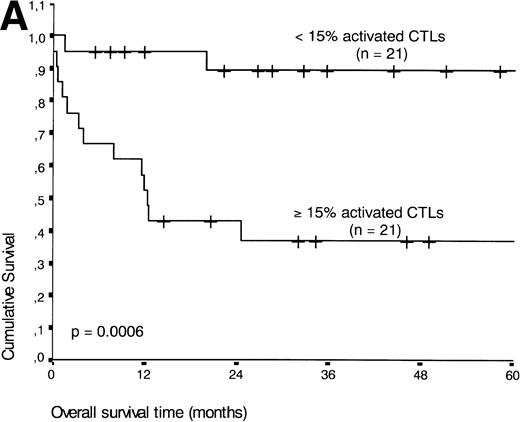
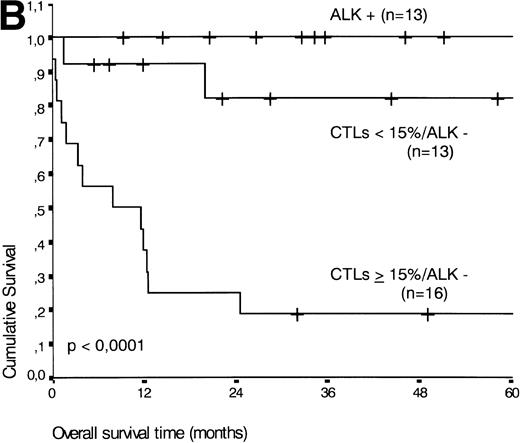
If patients were divided into a group with ≥15% and <15% activated CTLs (the threshold leading to the lowest P value), the presence of ≥15% activated CTLs defined a group of patients with an unfavorable prognosis: 13 of 21 patients with ≥15% activated CTLs died during the follow-up period compared with three of 21 patients with <15% activated CTLs (P < .001) (Fig 5A).
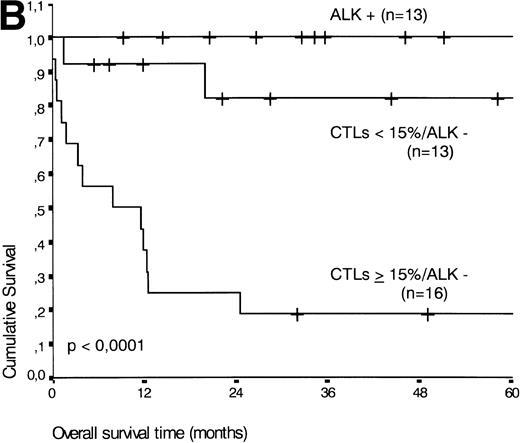
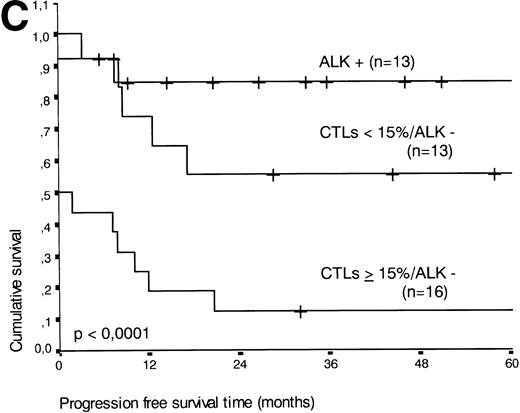
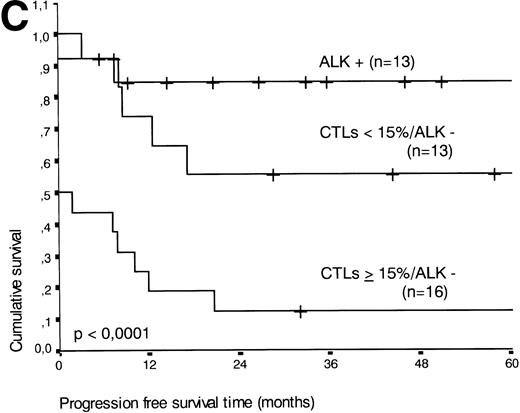
(A) Comparison of overall survival time according to percentage activated CTLs. (B) Comparison of overall survival time according to percentage activated CTLs combined with ALK status. (C) Comparison of progression-free survival time according to percentage activated CTLs combined with ALK status.
(A) Comparison of overall survival time according to percentage activated CTLs. (B) Comparison of overall survival time according to percentage activated CTLs combined with ALK status. (C) Comparison of progression-free survival time according to percentage activated CTLs combined with ALK status.
The percentage of TIA-1+ CTLs (entered as a continuous variable) was not related to progression-free and overall survival time, as estimated by Cox regression analysis.
Multivariate analysis of biological and clinical parameters.
As shown in Table 3, other factors than those mentioned until now were related to survival in univariate analysis: presence of B symptoms, LDH level, and performance status. A multivariate Cox regression analysis was performed using the factors listed in Table 3. Of these, percentage of activated CTLs (P< .001), ALK expression (P < .001) and the IPI (P< .0001) remained independently significant as prognostic markers of overall survival. The other included variables (presence of B symptoms, age, stage, number of extranodal sites, LDH level, and performance status) gave no additional prognostic information.
Prognostic value of percentage of activated CTLs combined with ALK expression.
The two variables, percentage of activated CTLs and ALK expression, were combined (see Table 4). In ALK negative cases, the presence of ≥15% activated CTLs defined a group with a very poor 2-year survival of less than 20%, as compared with ALK negative patients with <15% activated CTLs, of whom more than 80% were still alive after 2 years (P < .0001, Fig 5B). Similar results were obtained for progression-free survival time (P < .0001, see Fig 5C).
Comparison of the prognostic value of percentages activated CTLs/ALK status with the IPI.
As shown above, both indices (percentages of activated CTLs/ALK status and the IPI) were independent, strong prognostic markers in a univariate analysis of overall survival time. In retrospect, both prognostic markers identify largely the same group of patients (see Table 1). However, in a second multivariate analysis of overall survival time using the Cox-proportional hazards model, the percentage of activated CTLs combined with ALK status was a stronger prognostic marker than the IPI. This combination identified six patients who died of the disease within 2 years, although they were at low or low-intermediate risk according to the IPI (nos. 5, 9-13). Using this combined percentage of activated CTLs and ALK status index, only two patients with rapid fatal disease progression were missed (nos. 17 and 18). In contrast to the IPI, our index was also able to identify patients with poor prognosis presenting with low stage disease (nos. 11 through 13).
Thus, in this study, a high percentage of activated CTLs combined with negative ALK status seems to be more sensitive as a marker of poor prognosis in nodal ALCL than the high risk or high-intermediate risk categories of the IPI.
DISCUSSION
In this study, we have shown that the percentage of activated (GrB+) CTLs is a strong prognostic marker in patients with primary nodal ALCL. The same effect has been shown for percentages of activated CTLs in HD.20 Furthermore, we have shown that the expression of ALK is related to a favorable clinical outcome, as was also shown by previous studies.10-13 By combining percentage of activated CTLs with ALK status, it was possible (retrospectively) to accurately identify a group of patients who run a very high risk of dying within 2 years as a result of the disease. In this group, 13 of 16 patients died. In a multivariate analysis of overall survival time, the combination of percentage of activated CTLs and ALK status appeared to be a better prognostic marker than the IPI.
The IPI and other clinical risk factors are well established as prognostic markers in ALCL. In this study, the IPI and our index (percentage activated CTLs/ALK status) identify largely the same group of patients. However, although the IPI is helpful in recognizing most patients who will fail to respond well to therapy, some poorly responding patients are not identified (see Table 1, cases 5 and 9 through 13). Our findings suggest that a biological prognostic marker, as is the percentage of activated CTLs in combination with ALK status, is at least as strong and probably more sensitive in identifying these patients than established clinical markers as combined in the IPI. As such, it may be very helpful in deciding to apply alternative treatment modalities.
However, some caution has to be expressed. Because our study was performed on a relatively small number of patients, separate studies involving larger numbers are indicated to confirm the predictive value of our index.
In those cases where a high percentage of tumor cells show a cytotoxic phenotype, distinction between activated CTLs and tumor cells can be difficult. In our study, this was encountered in four cases, all ALK positive (Table 2, cases 30, 32, 36, and 38). However, double-staining for CD8 and GrB (Fig 3C and D) in these four cases helped to differentiate between activated CTLs and neoplastic cells, as tumor cells did not show CD8 expression (Table 2).
Two previous studies on ALCL with the MoAb ALK1 have shown ALK expression in 39 of 73 (53%) and 13 of 30 (43%) cases, respectively.8,9 The fact that we found less ALK positive cases (31%) is probably due to the relatively high number of elderly patients as compared with the above-mentioned studies. However, our patient group showed a pattern of age distribution consistent with the literature1 and confirmed previous studies with regard to prognostic significance of the IPI and ALK status.5,10-13,33 34 ALK positive ALCL has been uniformly shown to be related to a good prognosis. Thus, the relevance of our study lies in providing a prognostic marker for the ALK negative ALCL, which has not been described in the literature before.
The question remains why primary nodal ALCL patients with a high percentage of activated CTLs and lack of ALK expression show such a highly unfavorable clinical outcome (Fig 5B and C). The following explanations can be given.
(1) Assuming that CTLs are directed against the tumor cells, it is conceivable that in cases with many activated CTLs, only those tumor cells will survive that are best equipped to resist or inhibit CTL-mediated apoptosis. The poor clinical outcome in patients with a high percentage of activated CTLs may then be explained by assuming that resistance to CTL-induced apoptosis also results in resistance to therapy-induced apoptosis due to blockade of the final common apoptosis pathway. Indeed, several independent routes to apoptosis appear to converge on a final common pathway.38,39 CTL-mediated apoptosis is achieved by at least two pathways: the function of perforins and granzymes26-29 and activation of Fas (CD95/APO-1) on target cells,40-42 which induces apoptosis by activation of downstream “caspases”.43 The Fas system was shown also to be involved in drug-induced apoptosis in leukemia cells.44 The downstream antiapoptosis gene, bcl-2, has been shown to reduce sensitivity to both chemotherapy and CTL-induced apoptosis,39,45-47 in the latter case by blocking the apoptosis pathway before caspase activation.48Indeed, in a recent study on HD by our group, cases with a high percentage of activated CTLs (predisposing for poor prognosis) showed bcl-2 expression by tumor cells.21
(2) A possible immune escape mechanism, other than apoptosis resistance, is downregulation of MHC class I expression on the membrane of the tumor cells, preventing recognition of tumor-associated antigens by CTLs. However, in our study, no downregulation of MHC-I expression was found (data not shown).
In addition, tumor cells might circumvent CTL-mediated killing through induction of a local T-cell anergy by expressing certain cytokines. For instance, IL-10 (which has been shown to have direct suppressive effects on cytotoxic T lymphocytes)17-19,49,50 was recently demonstrated in ALCL.51
Finally, tumor cells might escape CTL-mediated killing by neutralizing the function of granzymes. Recently, a serine protease inhibitor (serpin) designated PI-9 was cloned and shown to be a potent inhibitor of GrB-mediated apoptosis.52 Expression of such inhibitory proteins by the tumor cells would also render them resistant to CTL-mediated lysis. However, these mechanisms of immune evasion (MHC I downregulation, local T-cell anergy, and expression of serpins by tumor cells) cannot account for the poor response to therapy.
We conclude that a high percentage of activated CTLs present in biopsy material of patients with ALK negative ALCL is a strong indicator for an unfavorable clinical outcome. In combination with negative ALK status, the presence of high numbers of activated CTLs is a very strong prognostic marker, even more sensitive than the IPI, in identifying a group of ALCL patients with a highly unfavorable clinical outcome. We advise studies with larger numbers of cases to validate the predictive value of our index as described here. Furthermore, studies are indicated to elucidate the putative role of apoptosis resistance as a pathogenic mechanism in ALCL.
ACKNOWLEDGMENT
The authors thank the following persons for their help in collecting tumor material and clinical data: Dr P. van Heerde, Dr J.J.A.M. ten Velden, Dr W.S. Kwee, Dr A.P. Willig, and Dr H. van den Berg. We thank Elly Fieret for her excellent technical assistance.
R.L.T.B. and D.F.D. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Chris J.L.M. Meijer, MD, PhD, Department of Pathology, University Hospital Vrije Universiteit, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.