Abstract
A distinct pathologic entity (ALK+ lymphoma) that is characterized by expression of the anaplastic lymphoma kinase (ALK) protein has recently emerged within the heterogeneous group of CD30+ anaplastic large-cell lymphomas. Information on clinical findings and treatment outcome of ALK+ lymphoma is still limited, and no data are available concerning the value of the International Prognostic Index when applied to this homogeneous disease entity. To clarify these issues, a recently developed monoclonal antibody ALKc (directed against the cytoplasmic portion of ALK) was used to detect expression of the ALK protein in paraffin-embedded biopsies from 96 primary, systemic T/null anaplastic large-cell lymphomas, and the ALK staining pattern was correlated with morphological features, clinical findings, risk factors (as defined by the International Prognostic Index), and outcome in 78 patients (53 ALK+ and 25 ALK−). Strong cytoplasmic and/or nuclear ALK positivity was detected in 58 of 96 ALCL cases (60.4%), and it was associated with a morphological spectrum (common type, 82.7%; giant cell, 3.5%; lymphohistiocytic, 8.6%; and small cell, 5.2%) that reflected the ratio of large anaplastic elements (usually showing cytoplasmic and nuclear ALK positivity) to small neoplastic cells (usually characterized by nucleus-restricted ALK expression). Clinically, ALK+ lymphoma mostly occurred in children and young adults (mean age, 22.01 ± 10.87 years) with a male predominance (male/female [M/F] ratio, 3.0) that was particularly striking in the second-third decades of life (M/F ratio, 6.5) and usually presented as an aggressive, stage III-IV disease, frequently associated with systemic symptoms (75%) and extranodal involvement (60%), especially skin (21%), bone (17%), and soft tissues (17%). As compared with ALK+ lymphoma, ALK− cases occurred in older individuals (mean age, 43.33 ± 16.15 years) and showed a lower M/F ratio (0.9) as well as lower incidence of stage III-IV disease and extranodal involvement at presentation. Overall survival of ALK+ lymphoma was far better than that of ALK− anaplastic large-cell lymphoma (71% ± 6%v 15% ± 11%, respectively). However, within the good prognostic category of ALK+ lymphoma, survival was 94% ± 5% for the low/low intermediate risk group (age-adjusted International Prognostic Index, 0 to 1) and 41% ± 12% for the high/high intermediate risk group (age-adjusted International Prognostic Index, ≥2). Multivariate analysis identified ALK expression and the International Prognostic Index as independent variables that were able to predict survival among T/null primary, systemic anaplastic large-cell lymphoma. Thus, we suggest that such parameters should be taken into consideration for the design of future clinical trials.
CD30+ ANAPLASTIC large-cell lymphoma is a widely recognized clinico-pathological entity that is characterized by frequent occurrence in children (∼40% of all large-cell lymphomas),1,2 preferential paracortical and intrasinusoidal lymph node involvement by large anaplastic tumor cells expressing the CD30 molecule (previously named Ki-1),3,4and highly aggressive clinical course usually associated with systemic symptoms and extranodal involvement, especially skin and bone.1,2,4-13 Anaplastic large-cell lymphomas of B- and T-cell type were initially recognized in the updated Kiel Classification.14 The recently proposed Revised European-American Lymphoma (REAL) classification15 included the B-cell type anaplastic large-cell lymphoma among the morphological variants of diffuse large B-cell lymphoma, limiting the term of anaplastic large-cell lymphoma only to cases with T and null phenotype.
Anaplastic large-cell lymphoma is associated with a t(2;5)(p23;q35) chromosome translocation16 that causes the anaplastic lymphoma kinase (ALK) gene on chromosome 2 to fuse with theNPM (nucleophosmin) gene on chromosome 5.17 The NPM-ALK fusion gene encodes for a 80-kD NPM-ALK chimeric protein17-19 that consists of the N-terminal portion of the NPM molecule (aminoacids 1-117)17,18 linked to the entire cytoplasmic domain of the neural-specific receptor tyrosine kinase ALK.20,21 The NPM-ALK hybrid protein is thought to play a key role in lymphomagenesis by aberrant phosphorylation of intracellular substrates.17,18 22-25
Polyclonal26,27 and monoclonal antibodies (MoAbs)28,29 directed against the cytoplasmic portion of the ALK protein have been recently used to detect in tumor biopsies the NPM-ALK fusion protein [generated by the t(2;5) translocation]28,29 or full-length ALK.30 In two large studies,29,31 expression of the ALK protein was demonstrated in approximately 60% of anaplastic large-cell lymphomas. These cases occurred most frequently in the first three decades of life and consistently showed a T/null phenotype associated with a morphological spectrum, ranging from the common type to the lymphohistiocytic or small-cell variant.29,31 For these tumors, we and others have recently proposed the term of ALK+ lymphoma29,31 and Nakamura et al32 proposed that of primary classical anaplastic large-cell lymphoma. This lymphoma is more likely to represent a single homogeneous disease (based on the presence of the genetic abnormality) than are neoplasms selected on the basis of morphologic and phenotypic features, eg, CD30 expression.
In recent years there has been growing interest to identify specific molecular features that, in addition to histologic type and clinical status, may help to define prognosis in patients with aggressive lymphomas. One example includes the rearrangement of the BCL-6 gene that has been associated with a favorable outcome in diffuse large B-cell lymphomas.33 More recently, Shiota et al34 35 reported for the first time that CD30+anaplastic large-cell lymphoma expressing the p80 (NPM-ALK) protein (as defined by an anti-p80 polyclonal antibody) has a better prognosis than p80− anaplastic large-cell lymphoma.
The role of the International Prognostic Index36,37 for predicting outcome of CD30+ anaplastic large-cell lymphoma is matter of debate.12,13 Reasons for these conflicting results may lie in the lack of reliable morphologic and immunophenotypic criteria for defining anaplastic large-cell lymphoma, as reflected by the description in literature of at least eight putative subtypes.38-41 This points to the importance of assessing prognostic factors in the context of a more homogeneous disease entity, eg, ALK+ lymphoma.
In this study, routinely processed biopsies from 96 cases of primary, systemic CD30+ anaplastic large-cell lymphomas with proven T/null phenotype were investigated for expression of the ALK protein using a highly specific MoAb ALKc that we recently generated against a fixative-resistant epitope on the cytoplasmic portion of ALK.29 The ALK immunostaining pattern was then correlated with the histological features of the tumor as well as with the clinical findings, risk factors, and outcome to assess whether ALK expression and the International Prognostic Index may identify different prognostic groups among CD30+ anaplastic large-cell lymphomas.
MATERIALS AND METHODS
Selection of cases.
Pathological samples from 96 patients with CD30+ anaplastic large-cell lymphoma were retrieved from the files of the Hemopathology Section, Institute of Hematology, University of Perugia (Perugia, Italy); the Hemopathology Section, Institute of Hematology “Lorenzo & Ariosto Seragnoli”, University of Bologna (Bologna, Italy); and the Institutes of Pathology at the following Institutions: Centro di Riferimento Oncologico (Aviano, Italy), University of Pavia (Pavia, Italy), University of Verona (Verona, Italy), the Istituto Nazionale Tumori (Milan, Italy), the University of Leuven (Leuven, Belgium), and the Free University of Berlin (Berlin, Germany). The material included 60 cases of T/null anaplastic large-cell lymphoma that had been reported in a previous study.29 Most tissue samples were fixed in 10% buffered formalin, while a percentage of them were fixed in B5 or Bouin. Slides from routinely paraffin-embedded tissues were stained with hematoxylin-eosin, Giemsa, and Gordon-Sweet. Paraffin sections from all cases had been stained by immunoperoxidase or immuno-alkaline phosphatase (APAAP)42techniques for the following antigens: CD45, CD45RO, CD20/L26, CD79a, and CD3 (purchased from Dako, Glostrup, Denmark); CD8 (a gift from Prof David Mason, Oxford, UK); CD30/Ber-H2 (kindly provided by Prof Harald Stein, Berlin, Germany); and CD68.43 Additional phenotyping could be obtained on frozen sections in 25 cases.
Only cases with unequivocal diagnosis of CD30+ anaplastic large-cell lymphoma were included in the study. Diagnostic immuno-morphological criteria were those defined in the REAL classification,15 eg, the presence of large anaplastic tumor cells with horseshoe-shaped or multiple nuclei containing multiple or single prominent nucleoli and abundant, frequently vacuolated cytoplasm that tended to grow in a cohesive pattern and preferentially involved the lymph node sinuses. By definition, all large tumor cells showed dot-like positivity in the Golgi area and/or surface expression of the CD30 molecule.3,4 These criteria, corresponding to the common or classic form of anaplastic large-cell lymphoma, were integrated to include other morphological variants of anaplastic large-cell lymphoma, eg, lymphohistiocytic, small-cell, and giant cell.38-41 Based on these criteria, the cases could be subdivided as follows: common type (n = 79), lymphohistiocytic variant (n = 5), small-cell variant (n = 3), giant-cell rich (n = 8), and signet-ring (n = 1).
The following cases were excluded from the study: (1) primary cutaneous T/null anaplastic large-cell lymphoma; (2) anaplastic large-cell lymphoma with Hodgkin’s like appearance15; (2) anaplastic large-cell lymphoma with B-cell phenotype; and (4) anaplastic large-cell lymphoma occurring in patients with previous diagnosis of lymphoma or documentation of human immunodeficiency virus (HIV) infection. At the end, the analysis was restricted to T/null primary, systemic CD30+ anaplastic large-cell lymphoma with morphology other than Hodgkin’s like that occurred in the immunocompetent host.
Immunohistological detection of the ALK protein.
Expression of the ALK protein was detected with the MoAb ALKc29 that recognizes a fixative-resistant epitope on the cytoplasmic portion of the ALK protein and is appliable to routinely processed, paraffin-embedded samples. All cases were also stained in parallel with the ALK1 MoAb (kindly provided by Prof David Y. Mason, Oxford, UK) raised against a fragment (AA 419-520) of the cytoplasmic portion of ALK.28
Both the antibodies were applied for 30 minutes to dewaxed paraffin sections (3- to 5-μm thick) after microwave antigen retrieval (3 times for 5 minutes at 700 W) in 1 mmol/L EDTA buffer, pH 8.0, as previously described.44 Sections were then washed with Tris-buffered saline, pH 7.6, and immunostained by the immuno-alkaline phosphatase (APAAP) technique.42 The endogenous alkaline phosphatase was blocked by adding levamisole to the substrate solution at the final concentration of 1 mmol/L.45 Slides were then counterstained for 5 minutes in Gill’s hematoxylin and mounted in Kaiser’s gelatin.
Clinical data.
Detailed information on the clinical characteristics, treatment, and outcome was available in 78 of 96 cases (53 ALK+ and 25 ALK−). The clinical features evaluated for potential prognostic importance were sex, age, Ann Arbor tumor stage, B symptoms, performance status, type and number of extranodal sites (as assessed by physical examination, computed tomography (CT) scan, and bone marrow biopsy), maximum diameter of the largest tumor mass (bulky >10 cm), and serum level of lactate dehydrogenase (LDH) level. B symptoms were defined as recurrent fever (temperature >38.3°C), night sweats, or the loss of greater than 10% of body weight within 6 months. The performance status was defined according to the World Health Organization (WHO). The serum LDH level was expressed as the ratio of the measured value to the upper limit of the normal range reported in the laboratory of each participating institution.
The initial therapy and therapeutic response, details of remission, progression or relapse, and subsequent therapies and follow-up were recorded in each patient. The patients received different types of therapy, depending on the Institution. Two cases in stage IA were treated with radiotherapy only. Sixty-eight patients received various combination-chemotherapy regimens containing doxorubicin. Eight pediatric ALK+ lymphomas were treated with different intensive chemotherapy regimens used for acute lymphoblastic leukemia. Complete response (CR) was defined as the total disappearance of signs and symptoms due to the disease as well as the normalization of all previous abnormal findings. Partial response (PR) was defined as the reduction of at least 50% of known disease with disappearance of the systemic manifestations. No response (NR) was anything less than a PR.
Patients were stratified according to the International Prognostic Index.36 37 Because all patients were less than 60 years of age, a simplified age-adjusted International Prognostic Index was applied that only considered three risk factors: tumor stage (I-IIv III-IV), performance status (0-1 v >1), and LDH level (low v high). Based on these criteria, patients were subdivided in two risk groups: (1) low/low-intermediate (simplified International Prognostic Index, 0 to 1), as defined by the absence of risk factors or the presence of one of them; and (2) high/high-intermediate (simplified International Prognostic Index, ≥2), as defined by the presence of two or more risk factors.
Overall survival was measured from diagnosis to death from any cause, with surviving patient follow-up censored at the last contact date. Disease-free survival was defined as the time from therapy to the first occurrence of relapse. Follow-up of patients not experiencing one of these events was censored at their date of last contact. Estimates of overall and disease-free survival distribution was calculated using the method of Kaplan and Meier.46 Survival curves were compared using the log-rank test.47 Multivariate survival analysis was performed applying the Cox regression model.48
RESULTS
Pathological and immunohistological findings.
The results of ALK immunostaining in 96 anaplastic large-cell lymphomas with T/null phenotype are summarized in Table 1. Strong expression of the ALK protein was observed in 58 of 96 (30 with T and 28 with null) cases (60.4%). The ALKc and ALK1 MoAbs gave identical results, but ALKc tended to react more strongly than ALK1 with the nuclei of tumor cells, especially those of small size. In approximately 81% of ALK+ cases the subcellular distribution pattern of the ALK protein (nucleus and cytoplasm of large anaplastic elements and nucleus of small atypical cells) strongly suggested that tumor cells contained the NPM-ALK chimeric product.25,29 The remaining 19% ALK+ lymphomas displayed a cytoplasm-restricted expression of the ALK protein, suggesting that in these cases the ALK gene may have fused with a genes(s) other than NPM.29,31 49Expression of CD30 closely paralleled that of ALK in the large anaplastic cells, whereas small tumor cells with nucleus-restricted ALK-positivity were usually CD30−.
Morphological and Immunohistological Features of 96 T/null CD30+ Anaplastic Large-Cell Lymphoma
| Type . | No. . | Morphological Variants . | Pattern of ALK Positivity in Tumor Cells . |
|---|---|---|---|
| ALK+ | 58 | Common type (48/58) | 29/58 all (c + n)* |
| 10/58 many (c + n)†, few (n)‡ | |||
| 9/58 all (c)* | |||
| Lymphohistiocytic (5/58) | 5/5 few (c + n)†, many (n)‡ | ||
| Small cell (3/58) | 3/3 few (c + n)†, many (n)‡ | ||
| Giant cell (2/58) | 2/2 all (c)* | ||
| ALK− | 38 | Common type (31/38)1-153 | — |
| Giant cell (6/38) | — | ||
| Signet ring (1/38) | — | ||
| Total | 96 |
| Type . | No. . | Morphological Variants . | Pattern of ALK Positivity in Tumor Cells . |
|---|---|---|---|
| ALK+ | 58 | Common type (48/58) | 29/58 all (c + n)* |
| 10/58 many (c + n)†, few (n)‡ | |||
| 9/58 all (c)* | |||
| Lymphohistiocytic (5/58) | 5/5 few (c + n)†, many (n)‡ | ||
| Small cell (3/58) | 3/3 few (c + n)†, many (n)‡ | ||
| Giant cell (2/58) | 2/2 all (c)* | ||
| ALK− | 38 | Common type (31/38)1-153 | — |
| Giant cell (6/38) | — | ||
| Signet ring (1/38) | — | ||
| Total | 96 |
Abbreviations: c, cytoplasmic; n, nuclear.
Only large anaplastic cells (no small tumor cells were observed in these cases).
Mostly large anaplastic cells.
Mostly small cells.
Tumor cells were frequently more pleomorphic than in common type ALK+ lymphoma.
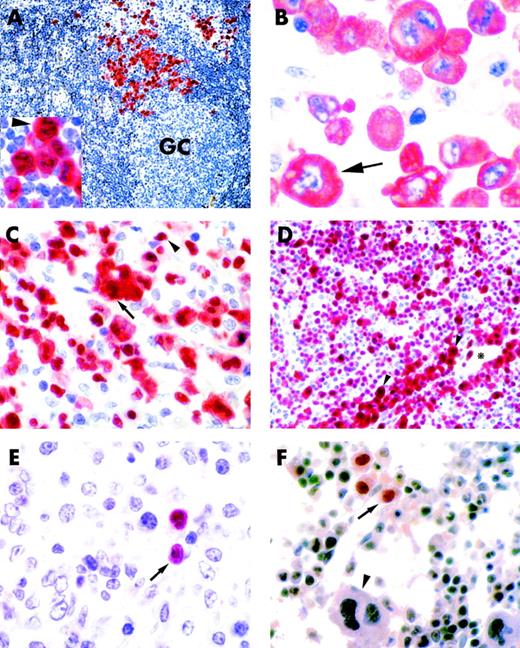
The 58 ALK+ lymphomas showed a morphological spectrum that included variants of common type (82.7%), giant cell (3.5%), lymphohistiocytic (8.6%), and small cell (5.2%) (Fig 1A through D). In 5 of 58 ALK+ cases, more than one histological pattern was found within a single biopsy at the time of initial diagnosis (3 with common plus small cell, 1 with common plus lymphohistiocytic, and 1 with lymphohistiocytic plus small cell). One ALK+ case showed a common type morphology at diagnosis but was lymphohistiocytic at relapse.
ALK+ lymphoma, common type (lymph node, paraffin section). (A) Scattered ALK+ anaplastic tumor cells (labeled in red) are present in the paracortical area. Residual lymphoid tissue is ALK−. GC indicates a germinal center (×150). (Inset) Higher magnification (×800) of the same case showing cytoplasmic and nucleolar positivity of tumor cells for the ALK protein (arrowhead). (B) ALK+ lymphoma, common type (lymph node, paraffin section). Large anaplastic tumor cells show cytoplasm-restricted positivity for the ALK protein (arrow; ×800). (C) ALK+ lymphoma, common type (lymph node, paraffin section). Tumor cells consists of a mixture of large anaplastic elements expressing the ALK protein both in the nucleus and cytoplasm (arrow) and small tumor cells showing nucleus-restricted ALK positivity (arrowhead; ×800). (D) ALK+ lymphoma, small cell variant (lymph node, paraffin section). The tumor cell population mostly consists of small neoplastic elements showing nucleus-restricted ALK-positivity. A small percentage of large neoplastic cells that express the ALK protein both in the nucleus and cytoplasm (arrowheads) is present around blood vessels (*; ×500). (E and F) ALK+ lymphomas (paraffin sections). Rare small-size tumor cells showing nuclear-restricted ALK-positivity are detectable in the cortical area of the lymph node (E, arrow) and in the bone marrow (F, arrow). The arrowhead in (F) indicates an ALK−megakaryocyte (both ×800). (A through F) Immunostaining with the ALKc MoAb; APAAP technique.
ALK+ lymphoma, common type (lymph node, paraffin section). (A) Scattered ALK+ anaplastic tumor cells (labeled in red) are present in the paracortical area. Residual lymphoid tissue is ALK−. GC indicates a germinal center (×150). (Inset) Higher magnification (×800) of the same case showing cytoplasmic and nucleolar positivity of tumor cells for the ALK protein (arrowhead). (B) ALK+ lymphoma, common type (lymph node, paraffin section). Large anaplastic tumor cells show cytoplasm-restricted positivity for the ALK protein (arrow; ×800). (C) ALK+ lymphoma, common type (lymph node, paraffin section). Tumor cells consists of a mixture of large anaplastic elements expressing the ALK protein both in the nucleus and cytoplasm (arrow) and small tumor cells showing nucleus-restricted ALK positivity (arrowhead; ×800). (D) ALK+ lymphoma, small cell variant (lymph node, paraffin section). The tumor cell population mostly consists of small neoplastic elements showing nucleus-restricted ALK-positivity. A small percentage of large neoplastic cells that express the ALK protein both in the nucleus and cytoplasm (arrowheads) is present around blood vessels (*; ×500). (E and F) ALK+ lymphomas (paraffin sections). Rare small-size tumor cells showing nuclear-restricted ALK-positivity are detectable in the cortical area of the lymph node (E, arrow) and in the bone marrow (F, arrow). The arrowhead in (F) indicates an ALK−megakaryocyte (both ×800). (A through F) Immunostaining with the ALKc MoAb; APAAP technique.
No expression of the ALK protein was detected in 38 of 96 anaplastic large-cell lymphomas. Most of these cases showed the histological features of common type, but there was some tendency of ALK− anaplastic large-cell lymphomas to be more pleomorphic than ALK+ cases, sometimes with giant cell appearance. No lymphohistiocytic and small cell variants were observed in the series of ALK− anaplastic large-cell lymphomas.
Diagnostic impact.
Five of 58 ALK+ lymphomas were initially misdiagnosed as metastatic carcinoma (n = 2, due to anaplasia and cohesive pattern of growth of tumor cells), malignant histiocytosis and reactive lymphadenopathy (n = 2, because of the exuberant hyperplasia of reactive histiocytes), and peripheral T-cell lymphoma other than anaplastic large-cell lymphoma (n = 1, due to mixed proliferation of small and large tumor cells). Immunohistochemistry for CD30 and ALK antigens helped to reach a correct diagnosis in these cases.
Immunohistological labeling for the ALK protein was also particularly valuable for detecting a low number of tumor cells, especially those of small size (usually CD30−), in the paracortex of lymph nodes (Fig 1E), bone marrow (Fig 1F), spinal fluid and skin (not shown). In 1 patient with the lymphohistiocytic variant of ALK+ lymphoma, the administration of nonsteroid anti-inflammatory drugs (to control high fever) caused a marked regression of the lymphadenopathy at the time the lymph node biopsy was scheduled. Because of the small amount of tissue available, the diagnosis was only possible with the use of the anti-ALK antibody that showed the presence of rare ALK+ tumor elements in the context of an inflammatory background of neuthrophils, macrophages, and plasma cells.
Clinical findings.
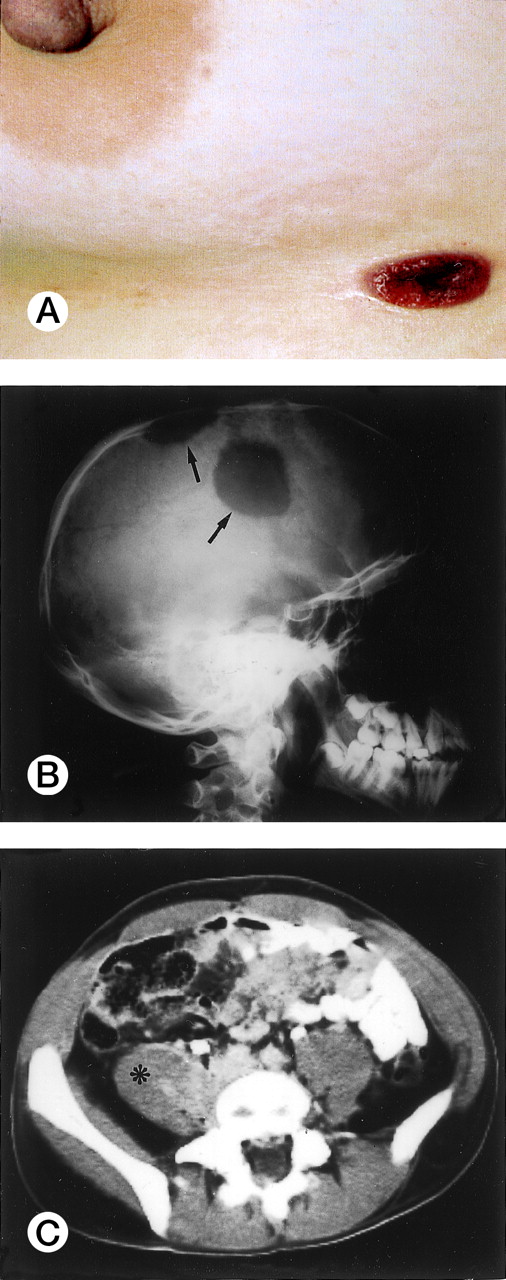
Clinical and laboratory findings were available in 78 of 96 cases (53 ALK+ and 25 ALK−) and are summarized in Table 2. ALK+ lymphoma frequently occurred in the first three decades of life (Fig 2; mean age, 22.01 ± 10.87 years; age range, 3 to 52 years). The present series included 12 pediatric patients and 41 adult patients (>16 years of age). The male/female (M/F) ratio was 3.0, with male predominance being particularly striking in the second to third decades of life (M/F ratio, 6.5; Fig 2). According to the Ann Arbor staging system, 28% of patients had stage I-II and 72% had stage III-IV disease. Most cases (75%) presented with systemic symptoms (high fever and/or weight loss in the absence of pruritus). Lymphadenopathy was present in 92% of patients; 40% had exclusively nodal disease. Extranodal involvement was frequent (60%), with 41% of patients showing two or more extranodal sites. Skin (usually nodules or ulcerated lesions; Fig3A), bone (Fig 3B), and soft tissues (Fig 3C) were the most frequently involved sites (skin, 21%; bone, 17%; soft tissues, 17%), followed by bone marrow (11%), lung (11%), and liver (8%). Tumor cells infiltrating the marrow ranged in size from small atypical cells to large anaplastic elements, and in one case involvement was only evident by ALK immmunostaining of the bone marrow biopsy (Fig 1F). Three of the 6 patients with bone marrow involvement had concomitant tumor cells circulating in the peripheral blood. None of the 6 cases with affected bone marrow showed concomitant skin lesions. The following extranodal sites were rarely involved: pleura (n = 3), central nervous system (CNS; n = 2), gut (n = 1), testis (n = 1), and parotid (n = 1).
Clinical Findings of T/null Primary, Systemic ALK+ and ALK− Anaplastic Large-Cell Lymphoma
| Characteristic . | ALK+ (N = 53) . | ALK− (N = 25) . | P . |
|---|---|---|---|
| Sex | |||
| M | 40 | 12 | .016 |
| F | 13 | 13 | |
| Age (yr) | |||
| ≤35 | 47 | 10 | .001 |
| >35 | 6 | 15 | |
| Performance status | |||
| 0-1 | 25 | 16 | NS |
| >1 | 21 | 7 | |
| B symptoms | |||
| No | 13 | 10 | NS |
| Yes | 40 | 15 | |
| Bulky disease | |||
| No | 40 | 15 | NS |
| Yes | 13 | 10 | |
| Extranodal sites | |||
| No | 21 | 17 | .019 |
| Yes | 32 | 8 | |
| Extranodal sites | |||
| Skin | 11 | 1 | |
| Bone | 9 | 1 | |
| Bone marrow | 6 | 0 | |
| PB | 3 | 0 | |
| Lung | 6 | 0 | |
| Liver | 4 | 1 | |
| Pleura | 3 | 0 | |
| Soft tissues | 9 | 0 | |
| Muscle | 3 | 0 | |
| CNS | 2 | 0 | |
| Gut | 1 | 1 | |
| Testicle | 1 | 0 | |
| Parotid | 1 | 0 | |
| Ann Arbor stage | |||
| I-II | 15 | 14 | .018 |
| III-IV | 38 | 11 | |
| LDH level | |||
| <1× normal | 27 | 13 | NS |
| >1× normal | 20 | 10 | |
| International Prognostic Index | |||
| 0-1 (low/low intermediate) | 25 | 17 | NS |
| ≥2 (high/high intermediate) | 25 | 7 |
| Characteristic . | ALK+ (N = 53) . | ALK− (N = 25) . | P . |
|---|---|---|---|
| Sex | |||
| M | 40 | 12 | .016 |
| F | 13 | 13 | |
| Age (yr) | |||
| ≤35 | 47 | 10 | .001 |
| >35 | 6 | 15 | |
| Performance status | |||
| 0-1 | 25 | 16 | NS |
| >1 | 21 | 7 | |
| B symptoms | |||
| No | 13 | 10 | NS |
| Yes | 40 | 15 | |
| Bulky disease | |||
| No | 40 | 15 | NS |
| Yes | 13 | 10 | |
| Extranodal sites | |||
| No | 21 | 17 | .019 |
| Yes | 32 | 8 | |
| Extranodal sites | |||
| Skin | 11 | 1 | |
| Bone | 9 | 1 | |
| Bone marrow | 6 | 0 | |
| PB | 3 | 0 | |
| Lung | 6 | 0 | |
| Liver | 4 | 1 | |
| Pleura | 3 | 0 | |
| Soft tissues | 9 | 0 | |
| Muscle | 3 | 0 | |
| CNS | 2 | 0 | |
| Gut | 1 | 1 | |
| Testicle | 1 | 0 | |
| Parotid | 1 | 0 | |
| Ann Arbor stage | |||
| I-II | 15 | 14 | .018 |
| III-IV | 38 | 11 | |
| LDH level | |||
| <1× normal | 27 | 13 | NS |
| >1× normal | 20 | 10 | |
| International Prognostic Index | |||
| 0-1 (low/low intermediate) | 25 | 17 | NS |
| ≥2 (high/high intermediate) | 25 | 7 |
Abbreviation: NS, not significant.
Extranodal involvement in ALK+ lymphoma. (A) Umbelicated skin lesion in a 38-year-old women. (B) Large ostelytic lesions of the skull (arrows) in a 14-year-old boy. (C) Involvement of the right psoas muscle (asterix) in a 25-year-old man.
Extranodal involvement in ALK+ lymphoma. (A) Umbelicated skin lesion in a 38-year-old women. (B) Large ostelytic lesions of the skull (arrows) in a 14-year-old boy. (C) Involvement of the right psoas muscle (asterix) in a 25-year-old man.
As compared with ALK+ lymphoma, ALK−cases in this series were characterized by occurrence at older age (mean age, 43.33 ± 16.15 years), lower M/F ratio (0.9), and lower incidence of stage III-IV disease and extranodal involvement at presentation (Table 2).
Response to treatment and survival.
Overall, 77.3% of ALK+ lymphoma obtained a CR and 15.0% a PR, with a major response (CR + PR) rate of 92.3%. Four patients (7.7%) were resistant to chemotherapy. Of 25 ALK−anaplastic large-cell lymphomas, 56% obtained a CR and 28% a PR, with a major response (CR + PR) rate of 84%. Four patients (16%) were resistant to therapy. No patient died of therapy-related effects.
The median follow-up for all patients was 2.10 years (range, 0.07 to 13.17 years). Overall survival of ALK+ lymphoma was significantly better than that of ALK− lymphoma (71% ± 6% v 15% ± 11%, respectively; P < .0007; Fig 4). Disease-free survival of ALK+ lymphoma was significantly better than that of ALK− lymphoma, being at 10 years of follow-up 82% ± 6% and 28% ± 14%, respectively (P < .0001; not shown).
The age-adjusted International Prognostic Index predicted survival within the good prognosis group of ALK+ lymphomas (Table 3). Overall 5-year survival was 94% ± 5% for the low/low intermediate risk group versus 41% ± 12% for the high/high intermediate group (P < .0001; Fig 5).
Clinical Findings and Outcome of ALK+Lymphoma
| Characteristic . | ALK+ . | ||
|---|---|---|---|
| Alive (N = 40) . | Died (N = 13) . | P . | |
| Sex | |||
| M | 30 | 10 | NS |
| F | 10 | 3 | |
| Age (yr) | |||
| ≤35 | 36 | 11 | NS |
| >35 | 4 | 2 | |
| Performance status | |||
| 0-1 | 22 | 3 | .008 |
| >1 | 11 | 10 | |
| B symptoms | |||
| No | 11 | 2 | NS |
| Yes | 29 | 11 | |
| Bulky disease | |||
| No | 30 | 10 | NS |
| Yes | 10 | 3 | |
| Extranodal sites | |||
| No | 21 | 0 | .001 |
| Yes | 19 | 13 | |
| Extranodal sites | |||
| Skin | 6 | 5 | |
| Bone | 6 | 3 | |
| Bone marrow | 3 | 3 | |
| Lung | 3 | 3 | |
| Ann Arbor stage | |||
| I-II | 14 | 1 | NS |
| III-IV | 26 | 12 | |
| LDH level | |||
| <1× normal | 21 | 6 | NS |
| >1× normal | 15 | 5 | |
| International Prognostic Index | |||
| 0-1 (low/low intermediate) | 24 | 1 | .001 |
| ≥2 (high/high intermediate) | 13 | 12 | |
| Characteristic . | ALK+ . | ||
|---|---|---|---|
| Alive (N = 40) . | Died (N = 13) . | P . | |
| Sex | |||
| M | 30 | 10 | NS |
| F | 10 | 3 | |
| Age (yr) | |||
| ≤35 | 36 | 11 | NS |
| >35 | 4 | 2 | |
| Performance status | |||
| 0-1 | 22 | 3 | .008 |
| >1 | 11 | 10 | |
| B symptoms | |||
| No | 11 | 2 | NS |
| Yes | 29 | 11 | |
| Bulky disease | |||
| No | 30 | 10 | NS |
| Yes | 10 | 3 | |
| Extranodal sites | |||
| No | 21 | 0 | .001 |
| Yes | 19 | 13 | |
| Extranodal sites | |||
| Skin | 6 | 5 | |
| Bone | 6 | 3 | |
| Bone marrow | 3 | 3 | |
| Lung | 3 | 3 | |
| Ann Arbor stage | |||
| I-II | 14 | 1 | NS |
| III-IV | 26 | 12 | |
| LDH level | |||
| <1× normal | 21 | 6 | NS |
| >1× normal | 15 | 5 | |
| International Prognostic Index | |||
| 0-1 (low/low intermediate) | 24 | 1 | .001 |
| ≥2 (high/high intermediate) | 13 | 12 | |
Abbreviation: NS, not significant.
Overall survival of ALK+ lymphoma according to age-adjusted International Prognostic Index (0 to 1, low/low intermediate risk group; ≥2, high/high intermediate risk group).
Overall survival of ALK+ lymphoma according to age-adjusted International Prognostic Index (0 to 1, low/low intermediate risk group; ≥2, high/high intermediate risk group).
Thirteen of 53 ALK+ lymphomas died of disease (Table 3). All 13 patients were initially treated with aggressive polychemotherapy (N = 6, 2° generation regimens; N = 6, 3° generation regimens; N = 1, regimen for acute lymphoblastic leukemia). Four of the 13 patients achieved a CR after combination therapy but relapsed immediately after (median time to relapse, 3 months). Relapse sites included sites of previous disease in 2 cases and new sites in 2 cases. Three of these cases were promptly treated with high-dose therapy followed by peripheral stem cell support that only resulted in a transient response (median survival time from relapse to death, 3 months). Five of the 13 patients responded partially to combination chemotherapy. Two of them received only supportive therapy and died of progressive disease. The remaining patients were treated with radiotherapy only, additional chemotherapy, and high-dose chemotherapy followed by peripheral stem cell rescue, respectively. All of them showed transient response and died of disease. Four of the 13 patients were resistant to chemotherapy and died of rapidly progressive disease without receiving any further treatment.
Cox multivariate analysis related to all patients indicated ALK expression and the International Prognostic Index as the only two independent factors able to predict survival (Table 4).
Cox Multivariate Analysis of Overall Survival in CD30+ Anaplastic Large-Cell Lymphoma
| Factor . | Relative Risk . | P . |
|---|---|---|
| ALK expression (ALK+v ALK−) | .29 | .001 |
| International Prognostic Index (≥2 v 0-1)4-150 | 3.50 | .001 |
| Factor . | Relative Risk . | P . |
|---|---|---|
| ALK expression (ALK+v ALK−) | .29 | .001 |
| International Prognostic Index (≥2 v 0-1)4-150 | 3.50 | .001 |
Age-adjusted.
DISCUSSION
A distinct pathologic entity (ALK+ lymphoma) that is characterized by expression of the anaplastic lymphoma kinase (ALK) protein has recently emerged within the heterogeneous group of anaplastic large-cell lymphomas.29,31,32 The clinical significance of ALK protein expression among anaplastic large-cell lymphomas is still limited,32,34 and no data are available concerning the value of the International Prognostic Index36 37 when applied to the homogeneous category of ALK+ lymphoma.
In this retrospective study, we report on clinical findings, risk factors, and treatment outcome of a large series (53 cases) of ALK+ lymphomas, as defined by a highly specific MoAb (ALKc) that is appliable to routine biopsies. The availability of MoAbs (ALK1 and ALKc)28,29 directed against the cytoplasmic portion of the ALK protein represents an obvious advantage over the anti-p80 (NPM/ALK) polyclonal antibodies32 34 that are prone to problems of nonspecific reactivity and variations between different samples and are available only in limited amounts. Our results clearly show that analysis of ALK expression by immunohistochemistry has important diagnostic and prognostic implications, and it should be extensively applied to the study of CD30+ anaplastic large-cell lymphoma.
This study confirms previous observations from our group29and other groups31 that ALK+ lymphoma is a single disease with a morphological spectrum, with the different variants being defined by the ratio of small to large anaplastic tumor cells and the presence of accompanying inflammatory cells (eg, histiocytes in the lymphohistiocytic variant).29 Some problem cases were encountered in this study and solved by immunocytochemical detection of the ALK protein. In particular, anti-ALK antibodies helped to recognize anaplastic large-cell lymphoma of the lymphohistiocytic type (frequently misdiagnosed as reactive condition or malignant histiocytosis), to distinguish between the small cell variant of anaplastic large-cell lymphoma and peripheral T-cell lymphoma of small size, and to define cutaneous infiltrates of uncertain nature. Moreover, taking advantage of the fact that the ALK protein is normally not expressed in human tissues (with the exception of a few cells in the CNS),28 29 it was possible to identify rare ALK+ tumor cells in needle aspirates of lymph nodes (allowing early diagnosis of disease relapse in 1 patient) or in bone marrow and spinal fluid at the time of initial diagnosis. These results also point to the potential of anti-ALK antibodies for monitoring minimal residual disease post-therapy.
Clinically, ALK+ lymphoma occurred in the first three decades (in keeping with previous data)29,31,32 34 and frequently presented with stage III-IV disease, usually associated with fever that possibly reflects the release of cytokines by tumor cells. Administration of steroids to decrease fever (one of the most troublesome symptoms in these patients) should be avoided until a pathological diagnosis is established, because ALK+lymphoma is usually highly responsive to these agents. This policy should probably apply also to nonsteroid anti-inflammatory drus that, in 1 patient with lymphohistiocytic variant of ALK+lymphoma, caused a marked regression of the lymphadenopathy, probably acting on the inflammatory component.
There was a higher frequency of extranodal involvement in ALK+ than in ALK− lymphoma. The most commonly involved sites in ALK+ lymphoma were skin (21%), bone (17%), and soft tissues (17%). High frequency of skin and bone involvement was originally recognized as a characteristic of systemic anaplastic large-cell lymphoma occurring in childhood,5 and the presence of cutaneous lesions has been associated with an increased risk of treatment failure at this age.10 Skin involvement was not found to be a risk factor in our series of ALK+lymphomas, but this issue needs to be further addressed in future prospective studies. The psoas muscle was a frequent site of involvement among soft tissues, and this was often responsible for the clinical picture at presentation (eg, low back pain), sometimes misinterpreted as osteo-articular disease. We observed bone marrow involvement by conventional histology in 11% of ALK+lymphomas. This figure is higher than that reported in two previous large series of anaplastic large-cell lymphomas in childhood (no cases with marrow infiltration)10 and in adults (1/31 with involved marrow).6 In at least one study,50immunostaining for CD30 was found to be more sensitive than conventional morphology for detecting marrow infiltration by a low percentage of anaplastic lymphoma cells. In this respect, immunostaining for the ALK protein could result even more informative, because small tumor cells are usually CD30−.
Anaplastic large-cell lymphoma often has an aggressive clinical course for which combination therapy is warranted. An excellent outcome has been reported for anaplastic large-cell lymphoma occurring in pediatric and adult patients treated with polychemotherapy.2,10,12,13Data on ALK expression were not available in those studies. More recently, Shiota et al34 reported on the prognostic importance to distinguish between anaplastic large-cell lymphomas expressing and not expressing the p80 (NPM-ALK) protein, with p80+ cases usually showing a better survival. The results presented in this report further confirm and extend these observations on a larger series of patients. Moreover, we provide evidence that the age-adjusted International Prognostic Index is able to predict outcome within the homogeneous, prognostically favorable, group of ALK+ lymphomas. This is in contrast with a recent report by the non-Hodgkin’s Lymphoma Classification Project12 in which the International Prognostic Index was regarded as not important for predicting survival of patients with T/null anaplastic large-cell lymphoma that, even with a high prognostic index, showed a surprisingly good outcome. Lack of strict criteria (eg, ALK immunostaining) for defining anaplastic large-cell lymphoma may have been responsible for these differences.
Because of its similarities with Burkitt’s lymphoma in terms of clinical aggressiveness and comparable high growth fraction,10 there has been the tendency to treat anaplastic large-cell lymphoma occurring in childhood with the highly aggressive polychemotherapy regimens used for lymphoblastic leukemia/lymphoma that also involve prophylaxis for CNS involvement.7,10 In contrast, less intensive regimens have been usually employed for the treatment of anaplastic large-cell lymphoma occurring in young adults.12,13 Clearly, the optimal strategy for the treatment of anaplastic large-cell lymphoma is yet to be established. The possibility to recognize by analysis of ALK expression anaplastic large-cell lymphoma with good-prognosis (ALK+ lymphoma) and to further dissect this homogeneous disease into low and high risk cases according to the International Prognostic Index might be of great relevance for the design of future prospective clinical trials. The excellent outcome of low-risk ALK+ lymphoma (age-adjusted International Prognostic Index, 0 to 1) warrants randomized comparison of less versus more intensive conventional chemotherapy and certainly does not support the use of high-dose therapy followed by autologous bone marrow transplantation as a front-line treatment.51 In contrast, patients with high-risk ALK+ lymphoma (age-adjusted International Prognostic Index, ≥ 2) or ALK− anaplastic large-cell lymphoma should be probably enrolled in clinical studies aimed to compare the efficacy of conventional polychemotherapy versus high-dose chemotherapy followed by stem cell support.51,52 Other potentially interesting and innovative therapeutic strategies in this category of patients include (1) anti-CD30 immunotoxins53,54; (2) anti-CD30–targeted tyrosine kinase inhibitors (to block anaplastic lymphoma kinase)55,56; (3) induction of T-cell immune response against the tumor-specific ALK protein; and (4) use of cis-retinoic acid.57
Based on the above-noted findings, we suggest that, within the category of CD30+ anaplastic large-cell lymphoma (T/null), ALK+ lymphoma should be separated from the ALK− cases, because they represent a distinct disease entity with favorable outcome. However, even for ALK+lymphoma, prognostic factors, as defined by the age-adjusted International Prognostic Index, must be taken into consideration for predicting patient survival.
ACKNOWLEDGMENT
The authors thank Claudia Tibidó for the excellent secretarial assistance.
Supported by AIRC (Associazione Italiana per la Ricerca sul Cancro) and MURST. M.F. is supported by the Fondazione Antonio Castelnuovo.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Brunangelo Falini, MD, Istituto di Ematologia, Policlinico, Monteluce, 06122 Perugia, Italy.