Abstract
In the present study, we investigated the mechanism by which sphingosine and its analogues, dihydrosphingosine and phytosphingosine, inhibit polymorphonuclear leukocyte (PMN) phagocytosis of IgG-opsonized erythrocytes (EIgG) and inhibit ERK1 and ERK2 phosphorylation. We used antibodies that recognized the phosphorylated forms of ERK1 (p44) and ERK2 (p42) (extracellular signal-regulated protein kinases 1 and 2). Sphingoid bases inhibited ERK1 and ERK2 activation and phagocytosis of EIgG in a concentration-dependent manner. Incubation with glycine, N,N′-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-[2-[(acetyloxy)methoxy]-2-oxoethyl]]-bis[(acetyloxy)methyl]ester (BAPTA,AM), an intracellular chelator of calcium, failed to block either phagocytosis or ERK1 and ERK2 phosphorylation, consistent with the absence of a role for a calcium-dependent protein kinase C (PKC) in ERK1 and ERK2 phosphorylations. Western blotting demonstrated that sphingosine inhibited the translocation of Raf-1 and PKCδ from PMN cytosol to the plasma membrane during phagocytosis. These data are consistent with the interpretation that sphingosine regulates ERK1 and ERK2 phosphorylation through inhibition of PKCδ, and this in turn leads to inhibition of Raf-1 translocation to the plasma membrane. Consistent with this interpretation, the sphingosine-mediated inhibition of phagocytosis, ERK2 activation, and PKCδ translocation to the plasma membrane could be abrogated with a cell-permeable diacylglycerol analog. The increase in the diacylglycerol mass correlated with the translocation of PKCδ and Raf-1 to the plasma membrane by 3 minutes after the initiation of phagocytosis. Additionally, the diacylglycerol analog enhanced phagocytosis by initiating activation of PKCδ and its translocation to the plasma membrane. Because PMN generate sufficient levels of sphingosine by 30 minutes during phagocytosis of EIgG to inhibit phagocytosis, it appears that sphingosine can serve as an endogenous regulator of EIgG-mediated phagocytosis by downregulating ERK activation.
A WIDE RANGE OF surface molecules, including those of the Fcγ receptor family, are expressed on hematopoietic cells.1,2 These receptors are important in host defense because they represent a major mechanism for the detection and phagocytosis of IgG-opsonized particles.3 After receptor engagement, the functional responses involve many intermediate steps. These include a complex cascade of biochemical events that link receptor engagement to the microbicidal response. One of the early events in polymorphonuclear leukocyte (PMN) stimulation is the rapid induction of protein phosphorylation of several proteins by activation of multiple kinases, including tyrosine kinases, protein kinase C (PKC), and MAP kinases.4,5 Cross-linking of the Fcγ receptor induces tyrosine phosphorylation of FcγRIIA itself and has been implicated in the regulation of phagocytosis.6-8 The phosphorylated tyrosines then serve as an interaction domain for protein tyrosine kinases of the Src and syk/ZAP 70 families.6,9 Once stimulated, these kinases catalyze the phosphorylation of several cellular substrates, including MAP kinases.10
The MAP kinases, extracellular signal-regulated protein kinases 1 and 2 (ERK1 and ERK2), a pair of closely related enzymes, are common intermediates in intracellular signaling cascades and are involved in diverse cellular functions. We previously correlated FcγRII engagement during N-formyl-methionyl-leucyl-phenyl-alanine (fMLP)-primed phagocytosis of EIgG in PMN with activation of the MAP kinases, ERK1 and ERK2.5 Activation of ERK1 and ERK2 requires the dual phosphorylation of Thr and Tyr residues after activation of MAP kinase kinases (MEK1 and MEK2). In turn, MEK activation is also regulated by phosphorylation. The signaling pathway activating the MAP kinase cascade has been studied intensively and has been shown to be dependent on c-Raf activation via Ras.11,12 It has been proposed that these kinases are arranged in a linear cascade (Ras → Raf → MEK → ERK).13 MEK1 and MEK2 are directly phosphorylated and activated by c-Raf. Recently, a Ras-independent pathway has been described in which PKCδ has been implicated in activation of the Raf → MEK → ERK cascade.14 Because of the diversity of signals, there are still open questions concerning the mechanism of upstream activation of ERK1 and ERK2 in this cascade as it relates to phagocytosis.
The metabolism of sphingolipids gives rise to second messengers that regulate cell activation, including free sphingosine. Sphingosine has been shown to inhibit PMN by inhibiting PKC and cellular functions (eg, the respiratory burst and protein secretion) dependent on this enzyme.15 Besides inhibiting PKC, other cell signaling pathways may be influenced by sphingosine. In particular, sphingosine can inhibit the enzyme phosphatidic acid phosphohydrolase, which is involved in generating diacylglycerol (DAG) from phosphatidic acid generated by phospholipase D (PLD).16 17
The purpose of the present study was to examine the mechanism by which sphingosine inhibits phagocytosis of antibody-coated red blood cells (EIgG). We assessed the effect of sphingoid bases on ERK1 and ERK2 activation and phagocytosis of EIgG, because we have shown the former to be crucial in mediating phagocytosis. In turn, we examined some of the enzymes upstream of ERK1 and ERK2. Thus, we determined whether sphingosine blocked translocation of PKCδ and Raf-1 to the plasma membrane during PMN phagocytosis. Finally, we examined whether we could abrogate the effect of sphingosine by adding a cell permeable analog of DAG.
MATERIALS AND METHODS
Reagents.
Sphingosine, DL-erythro-dihydrosphingosine, phytosphingosine hydrochloride, fatty acid free bovine albumin (BSA), fMLP, diethylenetriaminepenta-acetic acid (DETAPAC), ceramide type III, n-octyl β-D-glucopyranoside, 1,2-dioleoyl-sn-glycerol, and diisopropylfluorophosphate (DFP) were purchased from Sigma Chemical Co (St Louis, MO). N-acetyldihydrosphingosine was synthesized from DL-erythro-dihydrosphingosine as previously described.16sn-1,2-Diacylglycerol kinase (Escherichia coli) and dithiothreitol were purchased from Calbiochem (San Diego, CA). The MEK inhibitor, PD098059, was a generous gift of Alan R. Saltiel (Parke-Davis Pharmaceutical Research Division, Ann Arbor, MI).18 Polyclonal antibodies (Abs) against ERK1 and ERK2 (p44/42) recognizing the phosphorylated form of both p42 and p44 were obtained from New England BioLabs (Beverly, MA). Polyclonal Ab against ERK2 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and monoclonal Ab against PKCδ, PKCβ, and Raf-1 from Transduction Laboratories Inc (Lexington, KY). Monoclonal antiphosphotyrosine Ab 4G10 was purchased from Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase (HRP)-conjugated sheep antimouse Abs were from Amersham (Arlington Heights, IL), and HRP-conjugated antirabbit Ab was obtained from Santa Cruz Biotechnology. [3H]-Acetic anhydride was purchased from American Radiolabeled Chemicals Inc (St Louis, MO) and sn-1,2-didecanoylglycerol (DiC10) from Avanti Polar Lipids (Alabaster, AL). Glycine,N,N′-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-[2-[(acetyloxy)methoxy]-2-oxoethyl]]-,bis [(acetyloxy)methyl]ester (BAPTA,AM) was obtained from Molecular Probes (Eugene, OR) and [γ-32P]-adenosine-5′-triphosphate was from ICN Pharmaceuticals, Inc (Irvine, CA).
Cells.
Human PMN were isolated from human peripheral blood as described previously.19 Briefly, fresh whole blood was obtained by venipuncture from healthy volunteers and immediately added to acid citrate dextrose. The PMN were purified by dextran sedimentation followed by hypotonic lysis to remove the majority of erythrocytes and then centrifuged through Ficoll-Paque (Pharmacia LKB Biotechnology Inc, Piscataway, NJ) to remove contaminating mononuclear cells. Before activation of cells and subcellular fractionation, the cells were incubated for 5 minutes on ice with 5 mmol/L diisopropylfluorophosphate (DFP), washed, and resuspended in the desired buffer.
BAPTA,AM loading of PMN.
PMN (2 × 106/mL) were incubated with the intracellular Ca2+ chelator BAPTA,AM at 20 μmol/L in Ca2+-free phosphate-buffered saline (PBS) for 30 minutes at 37°C. The PMN were then washed twice with PBS containing 1 mmol/L Ca2+ and 1 mmol/L Mg2+. The phagocytosis assay was started as outlined below.
Phagocytic targets.
Phagocytosis assay.
The phagocytosis assay was conducted essentially as outlined by Pommier et al.21 For studies with sphingoid bases, lipid stocks as well as DiC10 stock (50 mmol/L) were prepared to form a BSA complex as described by Merrill et al.22 PMN, suspended at 2 × 106/mL in PBS containing 1 mmol/L Ca2+ and 1 mmol/L Mg2+, were incubated with different concentrations of lipids, DiC10, or 50 μmol/L PD098059 for 30 minutes at 22°C. In other experiments, PMN were preincubated with DiC10 and then treated with sphingosine, or PMN were preincubated with sphingosine and then treated with DiC10, or cells were treated with both lipids simultaneously. After the incubation, PMN underwent phagocytosis with EIgG at once or were preactivated with fMLP (10−7mol/L) for 10 minutes at 37°C, and then EIgG (1 × 108/mL) were added to the activated PMN and the incubation was continued for an additional 30 minutes at 37°C. The assays were stopped and counted as described previously.5 Inhibition of phagocytosis in the presence of lipids was expressed as the percentage of control, with control being phagocytosis by fMLP-treated and non–fMLP-treated PMN in the absence of lipid treatment.
Immunoblotting.
PMN lysates (1 to 2 × 106 PMN in 30 to 40 μL buffer) were combined with sample buffer, boiled for 5 minutes, and run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) minigels. The proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Schleicher and Schuell, Keene, NH) for 2 hours at 100 V, and the membrane was blocked with 2% BSA in PBS containing 1 mmol/L EDTA, 0.05% Tween-20, and 1 mmol/L Na3VO4. The membrane was probed with antibody against phosphorylated p44/42 in blocking buffer, washed three times with 0.2% Tween-20 in 50 mmol/L Tris (pH 8.0) and 100 mmol/L NaCl, and then incubated with a second antibody (HRP-conjugated goat antirabbit Ab) in wash buffer containing 5% nonfat dry milk. Phosphorylated bands were visualized using the enhanced chemiluminescence (ECL) system (Amersham). Membranes were stripped with 100 mmol/L β-mercaptoethanol, 2% SDS, and 62.5 mmol/L Tris (pH 6.7) at 50°C, and reprobed with polyclonal anti-ERK. Immunoblotting was also conducted using anti–Raf-1, anti-PKCδ, and anti-PKCβ Ab. HRP-conjugated sheep antimouse Ab served as a second antibody for anti–Raf-1, anti-PKCδ, and anti-PKCβ. For these experiments, PMN were treated as described in the section cell fractionation for immunoblotting. Tyrosine phosphorylation of ERK2 was detected by immunoprecipitating ERK2 as previously described by Suchard et al.5 Subsequent immunoblotting with 4G10 antiphosphotyrosine Ab was conducted and then incubated with HRP-conjugated sheep antimouse Ab.
Immunocomplex ERK activity.
This assay was conducted essentially as outlined by Suchard et al.5 PMN (2 × 106) were lysed in 800 μL of buffer containing 50 mmol/L HEPES (pH 7.5), 100 mmol/L NaCl, 2 mmol/L EDTA, 1% Nonidet P-40, 1 μmol/L pepstatin, 1 μg/mL leupeptin, 0.2 mmol/L phenylmethyl sulfonyl fluoride (PMSF), 0.2 mmol/L Na3VO4, 2 μg/mL aprotinin, and 40 mmol/L 4-nitrophenyl phosphate. Cleared lysates were incubated with 1 μg anti-ERK2 (Santa Cruz Biotechnology) overnight with rotation at 4°C. Protein A-Sepharose was added to each sample and incubated for 30 minutes with rotation at 4°C. Beads were washed twice with cold lysis buffer and twice with 10 mmol/L HEPES (pH 7.5), 10 mmol/L magnesium acetate, and 1 mmol/L Na3VO4. Beads were resuspended in kinase buffer containing 10 mmol/L HEPES (pH 7.5), 10 mmol/L magnesium acetate, 50 μmol/L ATP, 5 μCi/sample [γ-32P]ATP, and 20 μg of myelin basic protein (Life Technologies, Gaithersburg, MD). Samples were incubated for 5 minutes at 30°C and the reaction was terminated by adding sample buffer. Proteins were separated on 12% SDS-PAGE minigels. Phosphorylated myelin basic protein was visualized by autoradiography and the bands were excised and counted in a liquid scintillation counter (Wallac, Gaithersburg, MD). Activity was expressed as the percentage of unstimulated controls.
Cell fractionation for immunoblotting with PKCδ and Raf-1.
For fractionation studies, phagocytosis was stopped as described previously 5 minutes after initiating the ingestion of EIgG.5 PMN were resuspended at 2 × 108/mL in extraction buffer (50 mmol/L Tris [pH 7.5], 2 mmol/L EGTA, 1 mmol/L PMSF, leupeptin [1 μg/mL], 10 μmol/L benzamidine, 10 μmol/L pepstatin, and aprotinin [0.2 μg/mL]). The cells were disrupted by sonication on ice, and the resulting homogenate was centrifuged (400g for 10 minutes at 4°C) to remove unbroken cells and nuclei. The supernatant of each sample was applied to a 15% to 40% discontinuous sucrose gradient and centrifuged for 30 minutes at 150,000g at 4°C to obtain cytosolic, membrane, and granule fractions.23 The cytosol was removed from the top of the gradient, and the membrane fraction was collected at the 15% to 40% interface. The granule fraction was seen as the pellet. The cytosol and the membrane fraction were combined with sample buffer and boiled for 5 minutes. Protein was measured in the different samples by the BCA method (Pierce, Rockford, IL) using BSA as a standard.
Cell fractionation for assay of sphingosine formation.
Subcellular fractionation was performed as previously described by Kjeldsen et al.24 For this assay, phagocytosis was stopped 30 minutes after initiating the ingestion of EIgG. PMN were resuspended at 1.5 to 5 × 107/mL and disrupted by nitrogen cavitation, and the postnuclear supernatant was centrifuged over a two-layer Percoll gradient as described.24 This resulted in three visible bands containing azurophilic granules, specific/gelatinase granules, and secretory vesicles and plasma membranes, respectively, with the clear cytosol on top. All fractions were assayed for specific marker proteins as described previously.24 25
Assay for sphingosine, ceramide, and diacylglycerol formation.
Sphingosine was quantitated by acetylation with [3H]-acetic anhydride to form [3H] C2-Ceramide as described previously.26 Ceramide and diacylglycerol were assayed by the method of Preiss et al.27 This assay is based on the formation of [32P]-phosphatidic acid from endogenous diacylglycerol and [32P]-ceramide phosphate from ceramide. The sphingosine, ceramide, and diacylglycerol content in the cell extracts was calculated by extrapolation from standards treated similarly.
Statistical analysis.
Two-tailed Student’s t-tests were used to assess statistical significance.
RESULTS
The effect of DiC10 on phagocytosis.
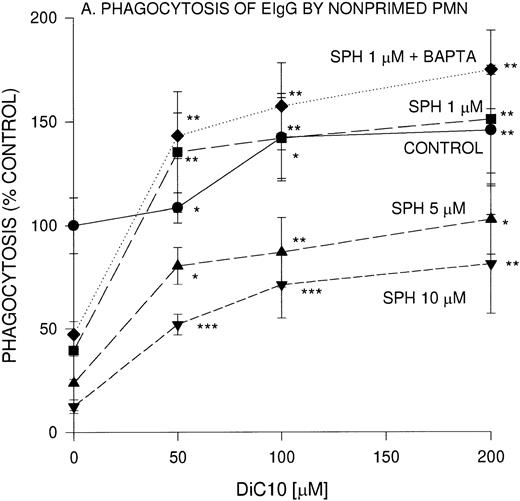
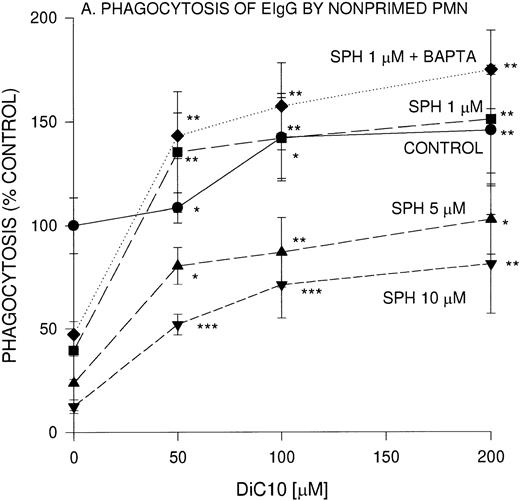
We previously reported that sphingoid bases are potent inhibitors of IgG-dependent phagocytosis in fMLP-stimulated PMN.20Because sphingosine is a competitive inhibitor of DAG, we evaluated the effect of a cell permeable DAG analog, DiC10, on EIgG-mediated phagocytosis. In the presence of 50, 100 and 200 μmol/L DiC10, phagocytosis was significantly increased by 9%, 42%, and 46%, respectively (Fig 1A).
Concentration-dependent restoration of phagocytosis of EIgG by sphingosine-treated nonprimed (A) and fMLP-primed (B) PMN in the presence of DiC10. PMN (2 × 106/mL) were preincubated with different concentrations of sphingosine for 30 minutes at 22°C and washed twice, and then the PMN were incubated with DiC10 at different concentrations at 22°C for 30 minutes. The PMN were then challenged with EIgG (A) or primed with fMLP (10−7 mol/L) and then EIgG (B). The control phagocytic index was 16.4 ± 2.4 (100% control) in nonprimed cells and was 59.2 ± 8 in fMLP-primed PMN. The values represent the mean ± SD for three experiments. Comparisons were made between DiC10 versus cells not treated with DiC10: ***P < .0001, **P < .001, *P < .005.
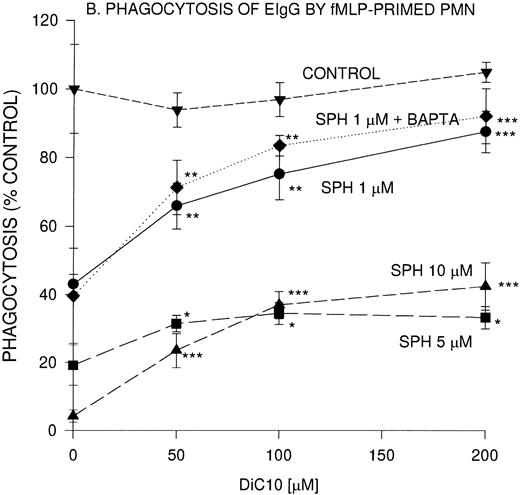
Concentration-dependent restoration of phagocytosis of EIgG by sphingosine-treated nonprimed (A) and fMLP-primed (B) PMN in the presence of DiC10. PMN (2 × 106/mL) were preincubated with different concentrations of sphingosine for 30 minutes at 22°C and washed twice, and then the PMN were incubated with DiC10 at different concentrations at 22°C for 30 minutes. The PMN were then challenged with EIgG (A) or primed with fMLP (10−7 mol/L) and then EIgG (B). The control phagocytic index was 16.4 ± 2.4 (100% control) in nonprimed cells and was 59.2 ± 8 in fMLP-primed PMN. The values represent the mean ± SD for three experiments. Comparisons were made between DiC10 versus cells not treated with DiC10: ***P < .0001, **P < .001, *P < .005.
We next examined whether the addition of DiC10 could abrogate the inhibition by sphingosine of phagocytosis of EIgG by PMN. PMN were preincubated with sphingosine at different concentrations, and then varying concentrations of DiC10 were added. A concentration of 1 μmol/L sphingosine inhibited EIgG-mediated phagocytosis by 50% (Fig1A). After the addition of 50 μmol/L DiC10, phagocytosis was normalized. At higher concentrations of DiC10 (100 and 200 μmol/L), phagocytosis was further enhanced above controls. When 5 μmol/L sphingosine was used, 200 μmol/L DiC10 was required to restore phagocytosis to control value (P was not significant). When PMN were incubated with 10 μmol/L sphingosine, the addition of 200 μmol/L DiC10 was only able to restore phagocytosis to 69% of control value. These results indicate that the DAG analog was able to reverse sphingosine inhibition of phagocytosis in a concentration-dependent manner and that the presence of DAG is a likely requirement for Fc-receptor–mediated phagocytosis to occur.
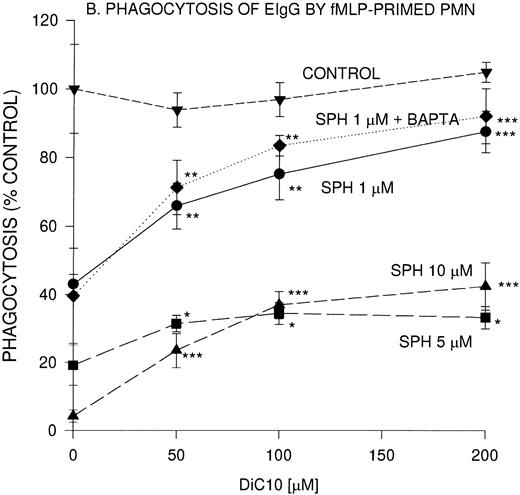
The effect of DiC10 on PMN, incubated with sphingosine was compared between phagocytosis of EIgG by nonprimed PMN and fMLP-primed PMN (Fig1B). The addition of DiC10 significantly increased phagocytosis in a concentration-dependent manner. The addition of all concentrations of DiC10 augmented fMLP-primed phagocytosis of EIgG in the presence of 5 μmol/L sphingosine to the same extent as observed with 10 μmol/L sphingosine (Fig 1B). In fMLP-primed PMN, DiC10 did not restore phagocytosis to control values in the presence of 5 or 10 μmol/L sphingosine. In contrast, the addition of 200 μmol/L DiC10 augmented phagocytosis to control values in PMN treated with 1 μmol/L sphingosine.
To determine whether the lipid-mediated inhibition of phagocytosis was [Ca2+]i-dependent, PMN were incubated with an intracellular Ca2+ chelator, BAPTA,AM. BAPTA,AM-treated PMN were not impaired in their ability to ingest EIgG. The concentration-dependent inhibition of phagocytosis by 1 μmol/L sphingosine was unaffected by Ca2+ chelation (Fig 1A and B). Similar to Ca2+ replete cells, the BAPTA,AM-treated PMN underwent a similar increase in EIgG-mediated phagocytosis in the presence of 1 μmol/L sphingosine with various concentrations of DiC10.
Effect of sphingoid bases on ERK1 and ERK2 activation during Fc-receptor–mediated phagocytosis in fMLP-primed PMN.
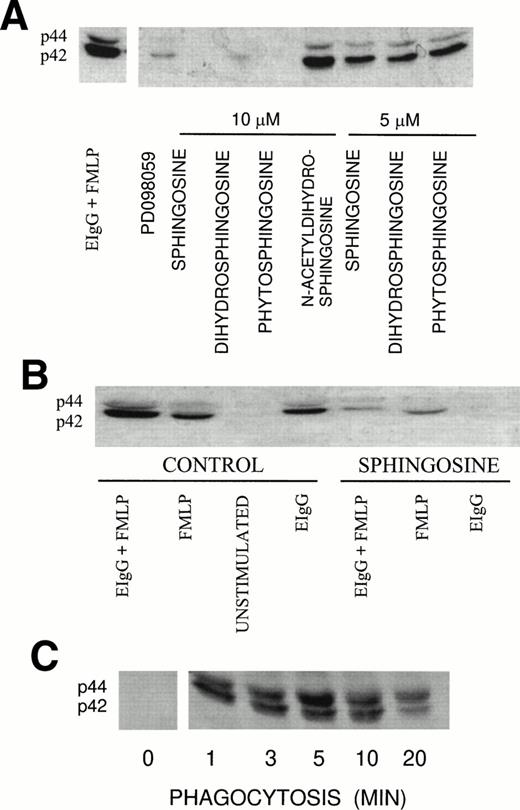
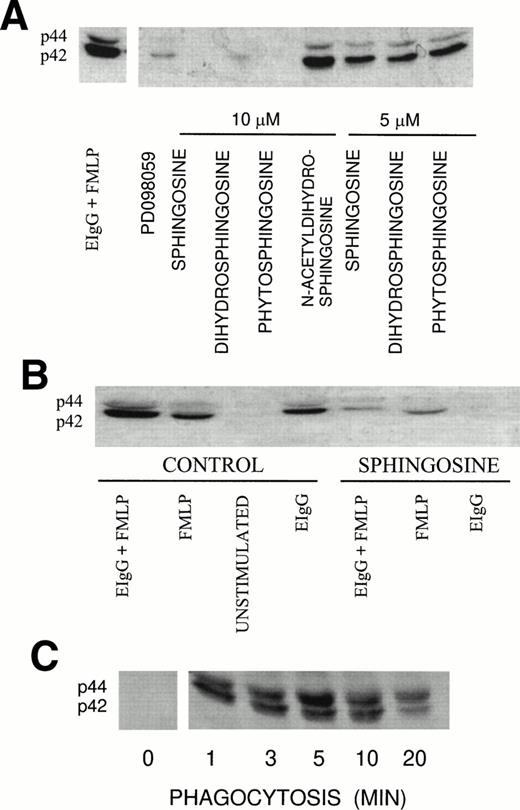
Tyrosine phosphorylation is one of the earliest responses in PMN activation and is required for FcR-mediated phagocytosis by macrophages.28 Recently, we correlated ERK1 and ERK2 phosphorylation with the engagement of FcγRII.5 We investigated the effect of sphingoid bases on ERK1 and ERK2 activation during fMLP-primed phagocytosis using an antibody that recognizes the phosphorylated forms of both ERK1 and ERK2, which have molecular masses of 44 and 42 kD, respectively. ERK1 and ERK2 phosphorylation was suppressed by sphingosine and its analogs dihydrosphingosine and phytosphingosine in a concentration-dependent manner (>95% inhibition at 10 μmol/L and 55% inhibition at 5 μmol/L; Fig 2A). At 1 μmol/L sphingosine and 1 μmol/L dihydrosphingosine, ERK1 and ERK2 activation was reduced by 17% and 11%, respectively; whereas phytosphingosine failed to inhibit ERK1 and ERK2 activation (data not shown). These observations were correlated with the effect of these compounds at 1 μmol/L on their ability to modulate the phagocytic response. We also measured ERK2 activity using myelin basic protein as the substrate (Table 1). ERK2 activity increased during phagocytosis, which was reduced to basal levels in the presence of 10 μmol/L sphingosine. In our previous studies, we found that PD098059, a specific MEK inhibitor, blocked ERK1 and ERK2 activation by greater than 80% which corresponded to a reduction in phagocytosis of 63%.5 In contrast, the tyrosine phosphorylation of ERK1 and ERK2 was not reduced in the presence of N-acetyldihydrosphingosine (Fig 2A). As expected, N-acetyldihydrosphingosine had no influence on FcR-mediated phagocytosis.
(A) Effect of sphingoid bases on ERK1 and ERK2 activation during fMLP-primed phagocytosis of EIgG in PMN. PMN (2 × 106/mL) were incubated with different sphingoid bases (5 and 10 μmol/L), buffer (control), or 50 μmol/L PD098059 for 30 minutes at 22°C. The PMN were primed with fMLP (10−7mol/L) for 10 minutes at 37°C, followed by the addition of EIgG (1 × 108/mL) for 3 minutes at 37°C. The membranes were probed with anti-MAP kinase Ab that recognizes both phosphorylated isoforms, ERK1 (p44) and ERK2 (p42). (B) Effect of sphingosine on ERK1 and ERK2 activation. PMN (2 × 106/mL) were preincubated with 10 μmol/L sphingosine or buffer (control) and subsequently activated with 10−7 mol/L fMLP and EIgG. Phagocytosis was allowed to proceed for 3 minutes. The samples were run on 10% SDS-PAGE and protein transferred to PVDF membranes. The membranes were probed with Ab against phosphorylated ERK1 and ERK2. The figure is representative of three experiments. (C) Kinetics of ERK1 and ERK2 phosphorylation in PMN during phagocytosis of EIgG. PMN were incubated with EIgG. At the indicated times, phagocytosis was terminated and the PMN were treated as noted in (B).
(A) Effect of sphingoid bases on ERK1 and ERK2 activation during fMLP-primed phagocytosis of EIgG in PMN. PMN (2 × 106/mL) were incubated with different sphingoid bases (5 and 10 μmol/L), buffer (control), or 50 μmol/L PD098059 for 30 minutes at 22°C. The PMN were primed with fMLP (10−7mol/L) for 10 minutes at 37°C, followed by the addition of EIgG (1 × 108/mL) for 3 minutes at 37°C. The membranes were probed with anti-MAP kinase Ab that recognizes both phosphorylated isoforms, ERK1 (p44) and ERK2 (p42). (B) Effect of sphingosine on ERK1 and ERK2 activation. PMN (2 × 106/mL) were preincubated with 10 μmol/L sphingosine or buffer (control) and subsequently activated with 10−7 mol/L fMLP and EIgG. Phagocytosis was allowed to proceed for 3 minutes. The samples were run on 10% SDS-PAGE and protein transferred to PVDF membranes. The membranes were probed with Ab against phosphorylated ERK1 and ERK2. The figure is representative of three experiments. (C) Kinetics of ERK1 and ERK2 phosphorylation in PMN during phagocytosis of EIgG. PMN were incubated with EIgG. At the indicated times, phagocytosis was terminated and the PMN were treated as noted in (B).
In unstimulated PMN, there was no evidence of basal ERK1 and ERK2 phosphorylation (Fig 2B). We observed greater phosphorylation of ERK1 and ERK2 in PMN when primed with fMLP and then challenged with EIgG (100%) compared with activation of ERK1 and ERK2 treated with fMLP alone (63%) or by EIgG challenge alone (49%) (Fig 2B and Table 1). Sphingosine (10 μmol/L) suppressed the phosphorylation of ERK1 and ERK2 in cells either stimulated with fMLP alone or cells stimulated with fMLP followed by addition of EIgG. In contrast, the addition of sphingosine completely inhibited ERK1 and ERK2 activation in cells stimulated with EIgG alone (Fig 2B).
The kinetics of ERK1 and ERK2 phosphorylation were monitored in PMN challenged with EIgG alone (Fig 2C). At time 0, using nonstimulated PMN, there was no activation of ERK1 and ERK2. After the addition of EIgG, we observed increased phosphorylation of these proteins beginning at 60 seconds, which reached maximal phosphorylation by 5 minutes and was sustained for 10 minutes. At 20 minutes, dephosphorylation of ERK1 and ERK2 was apparent. Chelation of [Ca2+]iwith BAPTA,AM had no influence on the activation of ERK1 and ERK2, indicating a [Ca2+]i-independent process (data not shown).
After the subcellular fractionation of PMN into primary and secondary granules, cytosol, and plasma membranes, an antibody to ERK2 was used to determine its intracellular location. ERK2 was distributed in both the cytosol and plasma membrane (data not shown). ERK2 failed to translocate to the plasma membrane during phagocytosis.
Effect of DiC10 on sphingosine-induced inhibiton of ERK activation.
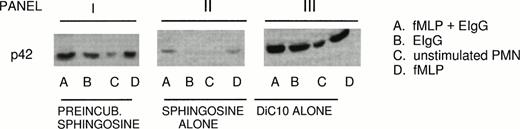
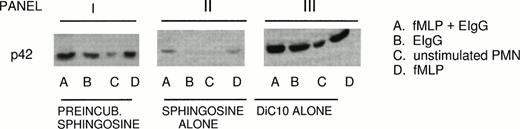
Because ERK2 activation rather than ERK1 activation is primarily involved in mediating phagocytosis, the role of DiC10 in restoring ERK2 activation in the presence of sphingosine was studied.5 PMN were incubated initially with 10 μmol/L sphingosine, followed by 200 μmol/L DiC10. DiC10 alone increased phosphorylation of ERK2 during fMLP-primed phagocytosis of EIgG (condition A) 32% above the control. The control consisted of fMLP-primed PMN challenged with EIgG alone. After fMLP treatment alone (condition D), ERK2 phosphorylation was increased by DiC10 14% above the control. Ingestion of EIgG alone (condition B) by PMN led to an increase of 8% above the control. Even without the addition of fMLP or EIgG, DiC10 led to ERK2 phosphorylation in PMN (condition C; panel III in Fig 3 and Table 2).
Restoration of ERK2 phosphorylation by DiC10 in PMN treated with sphingosine. PMN (2 × 106/mL) were pretreated for 30 minutes with 10 μmol/L sphingosine followed by incubation for 30 minutes with 200 μmol/L DiC10 (panel I) or treated with 10 μmol/L sphingosine alone (panel II) or 200 μmol/L DiC10 alone (panel III). The PMN were then primed with 10−7mol/L fMLP and then challenged with EIgG (column A), challenged with EIgG alone (column B), were not treated (column C), or were stimulated with 10−7 mol/L fMLP (column D). ERK2 phosphorylation was determined by Western blotting. See Fig 2B for an example of control values.
Restoration of ERK2 phosphorylation by DiC10 in PMN treated with sphingosine. PMN (2 × 106/mL) were pretreated for 30 minutes with 10 μmol/L sphingosine followed by incubation for 30 minutes with 200 μmol/L DiC10 (panel I) or treated with 10 μmol/L sphingosine alone (panel II) or 200 μmol/L DiC10 alone (panel III). The PMN were then primed with 10−7mol/L fMLP and then challenged with EIgG (column A), challenged with EIgG alone (column B), were not treated (column C), or were stimulated with 10−7 mol/L fMLP (column D). ERK2 phosphorylation was determined by Western blotting. See Fig 2B for an example of control values.
When PMN were preincubated with 10 μmol/L sphingosine followed by addition of 200 mmol/L DiC10 (panel I in Fig 3 and Table 2), an increase in ERK2 phosphorylation was observed under all conditions (A, B, C, and D; panel I). As demonstrated in panel II of Fig 3 and Table2, the addition of sphingosine alone inhibited ERK2 phosphorylation under conditions in which PMN were stimulated with various agonists (A, B, and D). These results indicate that DiC10 was able to restore ERK2 phosphorylation and to improve phagocytosis when sphingosine was present.
Sphingosine, ceramide, and diacylglycerol generation in PMN during phagocytosis.
Because exogenous sphingosine inhibited phagocytosis of EIgG by PMN, we measured endogenous sphingosine levels during phagocytosis to ascertain whether cessation of phagocytosis correlated with a rise in sphingosine levels. Subcellular fractions of unstimulated (control) and EIgG-phagocytosing PMN were analyzed for sphingosine content. Sphingosine was associated with azurophilic and specific granule subsets and with the plasma membrane fraction (Table 3). After 30 minutes of phagocytosis of EIgG, the amount of sphingosine increased significantly in PMN to 275% above control in isolated azurophilic granules after phagocytosis, to 237% above control in specific granules, and to 222% above control in plasma membranes (Table 3). When the free sphingosine generation was adjusted for the volume and water content in PMN, it was about 5 μmol/L, which correlated with the concentration of sphingosine used in several of our experiments.
Priming of PMN with FMLP did not alter the time at which maximal levels of diacylglycerol, ceramide, and sphingosine were generated in PMN compared with nonprimed cells. The diacylglycerol mass increased from 20.2 pmol/106 PMN to 40.2 ± 13.8 and 34.1 ± 6.1 pmol/106 PMN (n = 4) and decreased to control levels at 10 minutes in nonprimed and primed PMN, respectively. The ceramide level increased 2.6% by 1 minute from a basal level of 0.8 pmol/nmol Pi and achieved a maximal level of 4.8 pmol/nmol Pi by 30 minutes, which was similar in our previous study using primed PMN.20
In nonprimed PMN challenged with EIgG, the ceramide level increased by twofold at 30 minutes. The levels of sphingosine, which is derived from ceramide, increased from a basal value of 14.4 ± 3.4 pmol/106 PMN to 47.3 ± 12.8 pmol/106 PMN (n = 6) at 30 minutes in cells primed with fMLP followed by EIgG addition. In PMN challenged with EIgG alone, the sphingosine amount increased by 1.9-fold. Between 0 and 30 minutes, sphingosine levels gradually increased and did not achieve maximal levels before ceramide reached its maximal level.
Translocation of Raf-1 and PKCδ to PMN plasma membrane during phagocytosis.
Because the generation of DAG is a prerequisite for phagocytosis, the effect of DAG on PKC isoenzyme translocation and function in phagocytosing PMN was evaluated. Of the DAG-stimulated PKCs, only PKCδ is functional in the absence of [Ca2+]i in PMN; therefore, the translocation of PKCδ to the plasma membrane during phagocytosis was studied. Raf-1 was also studied as a possible intermediate between PKCδ and MEK.
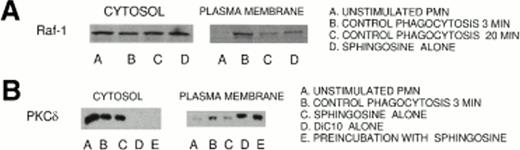
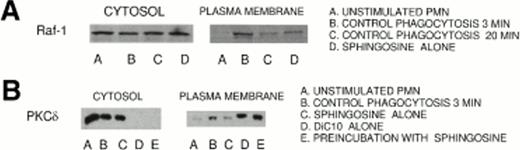
Both Raf-1 and PKCδ were present in the cytosol (Fig 4A and B). Raf-1 and PKCδ were translocated to the plasma membrane as early as 1 minute and reached maximal levels by 3 minutes after the initiation of phagocytosis (data not shown), which correlated with the increase in the diacylglycerol mass. The translocation to the plasma membrane was quantified on the resultant autoradiographs by densitometry on the basis of protein equivalents. The sum of the control peptides (unstimulated PMN) in the cytosol and plasma membrane was assigned 100%. EIgG challenge of PMN resulted in the greatest loss of Raf-1 and PKCδ from the cytosol about 39% ± 18% (mean ± SD, n = 4,P < .02) and 31% ± 12% (mean ± SD, n = 5, P< .005), respectively, at 3 minutes after initiation of phagocytosis. The increase of Raf-1 and PKCδ was about 30% and 27%, respectively, in the plasma membrane at 3 minutes after initiation of ingestion of EIgG. At concentrations of 10 μmol/L sphingosine, Raf-1 and PKCδ translocation to the plasma membrane was inhibited after 3 minutes of phagocytosis by greater than 80%. As shown in Fig 4B, the role of DiC10 restoring PKCδ translocation in the presence of sphingosine was examined. DiC10 alone led to increased translocation of PKCδ greater than 63% to the plasma membrane during phagocytosis of EIgG. When PMN were preincubated with 10 μmol/L sphingosine followed by the addition of 200 μmol/L DiC10, an increase in translocation of PKCδ to the plasma membrane occurred during phagocytosis of EIgG compared with unstimulated PMN or PMN treated with sphingosine alone (Fig4B). These results demonstrate that DiC10 was able to restore PKCδ translocation during phagocytosis of EIgG when sphingosine was present. The ability of DiC10 to restore PKCδ translocation correlated with the restoration of both phagocytosis and ERK2 activation in the presence of sphingosine. Similar to the findings of others, less than 100% of the total PKCδ and Raf-1 content of PMN was recovered from the cytosol and membrane fraction of EIgG-stimulated cells compared with control PMN.23 These findings suggest that these enzymes may be proteolyzed or translocated to other subcellular fractions.
(A) Inhibition by sphingosine of the translocation of Raf-1 to the plasma membrane during phagocytosis of EIgG in PMN. PMN (1 × 108/mL) were incubated with EIgG (5 × 109/mL) at 37°C. The PMN were then separated into cytosolic and membrane fractions. The fractions were analyzed for the presence of Raf-1 by employing SDS-PAGE and immunoblotting using specific Abs. (A) indicates the translocation of Raf-1 to the plasma membrane within 3 minutes in column B, a decrease in translocation at 20 minutes phagocytosis in column C, respectively; columns D indicate PMN pretreated 10 μmol/L sphingosine and activated with EIgG for 3 minutes. (B) Restoration of PKCδ translocation from cytosol to the plasma membrane in PMN treated with sphingosine by DiC10. (B) indicates translocation of PKCδ to the plasma membrane at 3 minutes from the cytosol in the presence or in the absence of 10 μmol/L sphingosine and DiC10.
(A) Inhibition by sphingosine of the translocation of Raf-1 to the plasma membrane during phagocytosis of EIgG in PMN. PMN (1 × 108/mL) were incubated with EIgG (5 × 109/mL) at 37°C. The PMN were then separated into cytosolic and membrane fractions. The fractions were analyzed for the presence of Raf-1 by employing SDS-PAGE and immunoblotting using specific Abs. (A) indicates the translocation of Raf-1 to the plasma membrane within 3 minutes in column B, a decrease in translocation at 20 minutes phagocytosis in column C, respectively; columns D indicate PMN pretreated 10 μmol/L sphingosine and activated with EIgG for 3 minutes. (B) Restoration of PKCδ translocation from cytosol to the plasma membrane in PMN treated with sphingosine by DiC10. (B) indicates translocation of PKCδ to the plasma membrane at 3 minutes from the cytosol in the presence or in the absence of 10 μmol/L sphingosine and DiC10.
These experiments were also performed in [Ca2+]i-depleted PMN using BAPTA,AM and were not different, indicating that Raf-1 and PKCδ translocation to the plasma membrane was a [Ca2+]i-independent process. The experiments using Ca2+ chelation were performed to examine specially the role of Ca2+-independent PKC isozymes in phagocytosis. To assure that sufficient BAPTA,AM was used to inhibit translocation of the PKC isoenzyme, PKCβ, a [Ca2+]i-dependent isoenzyme, the translocation of PKCβ to the plasma membrane during phagocytosis was also studied. When [Ca2+]i was chelated with BAPTA,AM, PKCβ was not translocated to the plasma membrane during phagocytosis of EIgG (data not shown).
DISCUSSION
The stimulation of human PMN with EIgG occurs via binding of FcγRII.5 It was shown that FcγRII-mediated engagement and associated signal transduction steps led to ERK1 and ERK2 phosphorylation and activation. In turn, ERK2 activation was better correlated with phagocytosis of EIgG than ERK1 activation.5The upstream signaling events leading to ERK1 and ERK2 phosphorylation during EIgG-mediated phagocytosis have not been completely elucidated. In contrast, an extensively studied model for ERK1 and ERK2 phosphorylation exists for fMLP-primed activation of PMN. Ligation of the fMLP receptor results in the activation of Ras. In turn, Ras activates the serine/threonine kinase Raf.11 Upon activation, Raf phosphorylates MEK1 and MEK2. MEK, in turn, phosphorylates ERK1 and ERK2. Ueda et al14 showed that a constitutively active mutant form of PKCδ activated MEK and then ERK in a Ras-independent but Raf-dependent manner. This provides a basis linking PKCδ activation to the phagocytic response.
PKCδ is a [Ca2+]i-independent isoenzyme of PKC. PKCδ is found in PMN and is one of four PKC isoenzymes that translocate to the plasma membrane during phagocytosis.23,29 Unlike PKCζ, which is also [Ca2+]i-independent and found in PMN, PKCδ is diacylglycerol dependent. In our study, we found that EIgG is a potent stimulus for the translocation of PKCδ to the plasma membrane fraction of PMN during phagocytosis of EIgG, where we observed ERK in part to be located. The association of ERK1 and ERK2 with membrane and cytoskeletal structures has been suggested. Gonzalez et al30 observed the localization of ERK2 to membrane ruffles in COS cells. Coincident with the PKCδ translocation to the plasma membrane during phagocytosis, Raf-1 was translocated. The appearance of PKCδ and Raf-1 in the plasma membrane fraction by 3 minutes during phagocytosis of EIgG was sustained over 10 minutes of phagocytosis (data not shown). We confirmed that PKCδ was participating in a [Ca2+]i-independent signaling pathway in these cells because chelation of intracellular Ca2+ with BAPTA,AM did not influence the translocation of PKCδ and Raf-1. Because phagocytosis is [Ca2+]i-independent,31 these findings are consistent with the notion that PKCδ activation is linked to Raf-1 activation.14
Because sphingosine inhibits phagocytosis and PKC activation, the role of sphingosine in blocking activation of key components of the signal transduction pathway affected by PKC activation was studied.15,20 In contrast, C2-ceramide does not directly inhibit PKC activity, which implies that ceramide regulates phagocytosis by a mechanism different than sphingosine.32We previously observed that ceramide completely suppressed PLD activation,16,33 but it only suppressed ERK2 activation by 70% to 80%.5 Sphingosine completely blocked ERK2 activation. Because sphingosine was more efficient in inhibiting ERK activity than ceramide, it is likely that it is the preferred metabolic inhibitor of phagocytosis. At 10 minutes, when the rate of phagocytosis of EIgG was decreasing,20 the free sphingosine level reached 3 μmol/L when adjusted for PMN water content and volume. In contrast, ceramide levels were only minimally elevated in nonprimed PMN ingesting EIgG,20 which again supports the critical role of sphingosine in regulating phagocytosis. Sphingosine inhibited translocation of PKCδ and Raf-1 to the plasma membrane during phagocytosis. Because PKCδ and Raf-1 activation are upstream events leading to MEK activation followed by ERK phosphorylation, these data suggest that PKCδ and Raf-1 activation are key components of the phagocytic pathway. The maximal translocation of PKCδ and Raf-1 to the plasma membrane by 3 minutes after the initiation of ingestion of EIgG correlated with the increase in the endogenous diacylglycerol mass. In accordance with our results, Della Bianca et al34showed that phagocytosis of EIgG in PMN was associated with an increase in the formation of the diacylglycerol mass at 4 minutes after challenge with EIgG, which is largely generated through the activity of phospholipase D. Sphingosine can inhibit phosphatidic acid phosphohydrolase activity in PMN, primed with C5a, phorbol myristate acetate, fMLP, or primed PMN undergoing phagocytosis of EIgG, which results in diminished diacylglycerol formation.16,20,35Because diacylglycerol is a necessary requirement for PKC activation, the sphingosine-induced failure to generate diacylglycerol could explain the failure of PKCδ to translocate to the plasma membrane during phagocytosis.23
Others have observed that sphingosine is a competitive inhibitor of diacylglycerol and prevents the interaction of this activator with the ternary complex of PKC.15 The findings that diacylglycerol can increase phagocytosis, ERK activation, and PKCδ translocation to the plasma membrane in PMN treated with sphingosine would support the notion that diacylglycerol was required to permit PKCδ activation. We also observed that PKCδ translocation to the plasma membrane could occur in PMN pretreated with sphingosine after the addition of DiC10, which supports the hypothesis that diacylglycerol is a competive agonist with sphingosine in terms of regulatory PKC activity. Additionally, it was observed that DiC10 augmented phagocytosis by nonprimed PMN. In contrast, DiC10 failed to enhance phagocytosis beyond control values in PMN primed with fMLP. Because fMLP activation leads to diacylglycerol formation, the fMLP-dependent diacylglycerol formation may contribute to the priming effect mediated by fMLP.36-38 Finally, sphingosine formation may contribute to events terminating phagocytosis in activated PMN. Maximal generation of sphingosine required 30 minutes of phagocytic activation. Others have also reported that sphingosine is formed in PMN during activation.39 The amount of sphingosine that was generated was sufficient to inhibit phagocytosis of EIgG. In conclusion, these studies indicate a mechanism by which endogenous sphingosine generation can modulate PMN activation through inhibition of PKCδ activation and subsequent phosphorylation of kinases that are required for phagocytosis to occur.
Supported by Deutsche Forschungsgemeinschaft Grant No. Ra 789/1-1 (to E.M.B.R.); by National Institutes of Health Grants No. AI20065 (to L.A.B.) and DK41487 and DK39255 (to J.A.S.); and by The Danish Medical Research Council (L.K.). J.A.S is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Laurence A. Boxer, MD, Department of Pediatrics, University of Michigan, F6515 Mott Children’s Hospital, Ann Arbor, MI 48109; e-mail: laboxer@umich.edu.