Abstract
Few data are available on the long-term effect of bone marrow transplantation (BMT) on growth. This study examines those factors that play a role in the final height outcome of patients who underwent BMT during childhood. Data on 181 of 230 patients with aplastic anemia, leukemias, and lymphomas who had BMT before puberty (mean age, 9.8 ± 2.6 years) and who had reached their final height were analyzed. An overall decrease in final height standard deviation score (SDS) value was found compared with the height at BMT (P < 107) and with the genetic height (P < 107). Girls did better than boys, and the younger in age the person was at time of BMT, the greater the loss in height. Previous cranial irradiation + single-dose total body irradiation (TBI) caused the greatest negative effect on final height achievement (P < 104). Fractionation of TBI reduces this effect significantly and conditioning with busulfan and cyclophosphamide seems to eliminate it. The type of transplantation, graft-versus-host disease, growth hormone, or steroid treatment did not influence final height. Irradiation, male gender and young age at BMT were found to be major factors for long-term height loss. Nevertheless, the majority of patients (140/181) have reached adult height within the normal range of the general population.
THE SUCCESS OF bone marrow transplantation (BMT) in treating malignant and nonmalignant hematological disorders and the improvement of the sophisticated techniques involved in this procedure have extended the indications for transplantation and have increased the number of patients who survive BMT.1 2 Nevertheless, one of the many negative effects that these successfully treated children have to face is the endocrine dysfunction associated with the chemotherapy, radiotherapy, and immunosuppressive treatment they receive before and after marrow transfusion, which could eventually induce growth delay.
To our knowledge, only two single-center studies have dealt with the final height achievement of patients who had BMT during childhood.3 4 Because the BMT procedure is relatively recent, making it difficult to include large numbers of patients who have reached their final adult height, the statistical power of these two studies was frail. The European BMT Working Party for Late-Effects conducted this multicenter study with the aim of evaluating the final height achieved by children who underwent BMT for hematological disorders and identifying those factors that influence the long-term growth in these patients.
PATIENTS AND METHODS
Study design.
The study is based on a retrospective survey using a two-step-questionnaire approach, involving Centers that are part of the European-BMT group.
A first questionnaire was sent to 284 BMT Centers asking for the number of patients with severe aplastic anemia (SAA), leukemia, and lymphomas who underwent BMT before onset of puberty (breast stage 1 in girls and testicular volume less than 4 mL in boys) and who had reached their final adult height. Final height was defined either on a documented closure of the hand, wrist, or iliac crest epiphyses or growth velocity less than 1 cm/yr.5
One hundred of the 284 Centers (35%) completed and sent back the first questionnaire form. Sixty-two centers (22%) confirmed that they did not have cases that met the inclusion criteria. A second questionnaire was sent to the remaining 38 centers that claimed to have patients eligible for the study. Twenty-two of the 38 centers answered the second questionnaire, providing data on a total of 230 patients.
The questionnaire included queries regarding the primary hematological disorder, irradiation therapy used during first-line treatment (between diagnosis and pre-BMT conditioning treatment), BMT-related data (age and type of BMT, conditioning regimen, grading of acute and chronic graft-versus-host disease [GVHD], and type and duration of immunosuppression therapy), and endocrine-related data that included parental height, patient’s height and weight at BMT, final adult height achieved, age at latest measurement, growth hormone treatment, and sex hormone replacement therapy performed.
Patients’ characteristics.
Of the 230 forms received, 49 patients were excluded from the study either because the onset of puberty was before BMT or because of insufficient key data necessary for a correct and comprehensive statistical analysis. BMT was performed between October 1973 and October 1993. The characteristics of the 181 patients who met the inclusion criteria are summarized in Table1. The type of irradiation applied to the patients in relation to the primary disorder is shown in Table 2. Fifty patients received cranial radiation therapy (CRT) as prophylaxis or treatment of central nervous system involvement (<18 Gy in 4 patients, 18 Gy in 29 patients, 24 Gy in 16 patients, and 36 Gy in 1 patient). Irradiation during conditioning regimen included single-dose total body irradiation (sTBI) in 52 patients at a median dose of 8 Gy (range, 3 to 10 Gy; 3 SAA patients received 3 to 4 Gy, whereas the remaining patients received 7 to 10 Gy); fractionated TBI (fTBI) in 73 patients (6 to 13.2 Gy) administered in 2 to 8 fractions; thoraco-abdominal irradiation (TAI) in 17 patients (5 to 11 Gy); and total lymphoid irradiation (TLI) in 2 patients (7.5 Gy). Seventeen children with acute lymphoblastic leukemia (ALL) received a booster dose of 4 to 10 Gy to the testicles.
Patients subjected to irradiation as part of the pre-BMT conditioning regimen also received cyclophosphamide (Cy) alone or in combination with other cytotoxic drugs (cytarabine, etoposide, and vincristine). Of the 36 patients who did not receive irradiation, 10 children (7 acute myeloid leukemia [AML], 2 chronic myeloid leukemia [CML], and 1 SAA) were conditioned with busulfan and cyclophosphamide only (BuCy).
Steroid therapy for acute and/or chronic GVHD was administered in 87 patients for a median period of 4 months (range, 0.5 to 168 months); 62 of them stopped treatment within 12 months, whereas 14 patients had treatment for periods longer than 24 months. Cyclosporin-A was administered in 90 patients for a median period of 6.5 months (range, 2 to 84 months); 67 of them stopped treatment within 12 months, whereas 11 patients had treatment for periods longer than 24 months.
Sex hormone replacement therapy was administered to 55 patients (24 male and 31 female), starting at 14.0 ± 3.3 years of age (range, 12 to 18 years of age) in males and at 14.4 ± 1.8 years of age (range, 11 to 18.8 years of age) in females. Growth hormone (GH) treatment was administered in 28 patients for a median period of 3.5 years (range, 0.3 to 7 years), starting at 13.2 ± 2.1 years of age (range, 9.8 to 17.9 years of age), 3.8 ± 2.0 years from BMT (range, 0.9 to 8.3 years).
Statistical analyses.
Height measurements of each patient both at the time of BMT and final height were expressed as the standard deviation score (SDS) from the mean of the normal population.5 The genetic height of each patient was calculated as the mean of the mother’s and the father’s height-SDS (genetic height = [mother’s SDS + father’s SDS]/2).
The difference between the height-SDS value at BMT and that of the final height was calculated for each patient and was regarded as the delta-SDS value, expressing the gain (zero or positive values) or the loss of height (negative values) after transplantation in terms of SDS.
Statistical analyses for the comparisons of delta-SDS values were performed according to the type and age at BMT, gender, pretransplant conditioning regimens, complications, and therapies applied. The relationships between dependent and explanatory covariates were investigated by using the analysis of variance and the χ2statistics for continuous and categorical covariates, respectively.
The association between delta-SDS, as dependent variable, with the type of BMT, age at transplant, gender, radiotherapy, chronic GVHD severity, and GH treatment were also investigated using the multiple logistic regression analysis.6 This multivariable technique permits identification of covariates that are associated with the probability of the studied outcome and expresses each covariate association, adjusted for the effect of the other covariates included in the regression model, in terms of relative risk point estimates (RR) and its confidence intervals.
To this aim, the dependent variable delta-SDS was dichotomized to distinguish between subjects who had normal growth after transplant (ie, delta-SDS value ≥0) and those who had growth failure (delta-SDS <0). Patients were also divided into three groups to identify subjects who received CRT (with or without TBI), subjects who received radiation therapy that did not include CRT, and patients who have never received any irradiation therapy (reference group, RR = 1). The age at transplant (continuous covariate) was categorized into three levels according to the 33rd (1.5 to 8.8 years) and the 66th percentile values of its frequency distribution (8.8 to 11 years). Patients with age greater than 11 years at transplantation were used as a reference (ie, RR = 1).
The statistical analyses were performed using the SPSS statistical software, version 8.0 (SPSS Inc, Chicago, IL).7
RESULTS
Final height achievement was documented by closure of hand, wrist, or iliac crest epiphyses in 9 patients who were 18.5 ± 2.4 years of age at latest evaluation and by growth velocity less than 1 cm/yr in the remaining patients, who were 19.1 ± 2.8 years of age. Final height-SDS values of 140 of 181 were within normality for the general healthy population (between −2.0 and +2.0 SDS). Three patients achieved height values greater than +2.0 SDS, whereas the remaining 38 are to be considered as short stature (below −2.0 SDS).
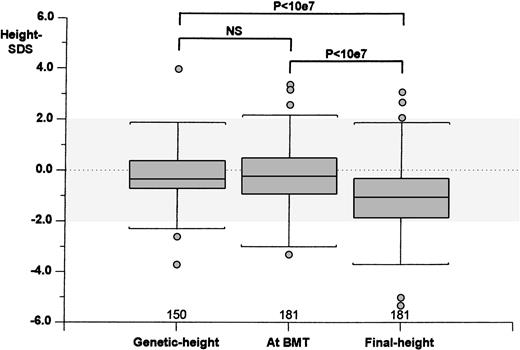
Considering the entire cohort of patients (Fig 1), the height-SDS value at BMT (−0.15 ± 1.16) was significantly higher (paired Student’st-test; P < 107) than the final height-SDS value (−1.09 ± 1.45), resulting in a mean decrease of 0.94 ± 1.30 SDS from transplant to adulthood. Whereas the height-SDS value at BMT was comparable to that of the genetic height (−0.22 ± 1.02 SDS), the final height-SDS value was significantly lower (P < 107).
Correlation between the genetic height, height-SDS at BMT, and final height-SDS. Numerals indicate the number of cases studied in each group. The dotted area indicates the height-SDS distribution for the normal general population. (Box plot) The lower line of the box indicates the 25th percentile, the upper line indicates the 75th percentile, and the horizontal lines above and below the boxes represent the 3rd and the 97th percentile, respectively. Statistical analyses: paired Student’s t-test.
Correlation between the genetic height, height-SDS at BMT, and final height-SDS. Numerals indicate the number of cases studied in each group. The dotted area indicates the height-SDS distribution for the normal general population. (Box plot) The lower line of the box indicates the 25th percentile, the upper line indicates the 75th percentile, and the horizontal lines above and below the boxes represent the 3rd and the 97th percentile, respectively. Statistical analyses: paired Student’s t-test.
The mean delta-SDS value of the whole cohort was −0.94 ± 1.30 (range, −6.9 to +2.6). The 112 boys did worse than the 69 girls, having a mean delta-SDS value of −1.17 ± 1.34 compared with −0.56 ± 1.13, respectively (t-test; P < .002). Girls were younger at BMT compared with boys (8.9 ± 2.7 and 10.3 ± 2.4 years, respectively; P < .0005). Age was found as an additional factor that influences growth, because the younger the age at BMT the higher the delta-SDS value (linear regression analysis; regression coefficient = 0.218; P < 107) and the lower the final height-SDS (regression coefficient = 1.104; P = .01).
The type of BMT was found to have no effect on the delta-SDS value (analysis of variance, one-way ANOVA); in fact, the 28 patients who underwent autologous-syngeneic BMT (for statistical purposes, the 3 children who received a transplantation from a monozygotic twin were considered as part of the autologous group) had a delta-SDS value of −0.95 ± 1.25 compared with that of −0.94 ± 1.31 in the 153 cases who had allogeneic BMT.
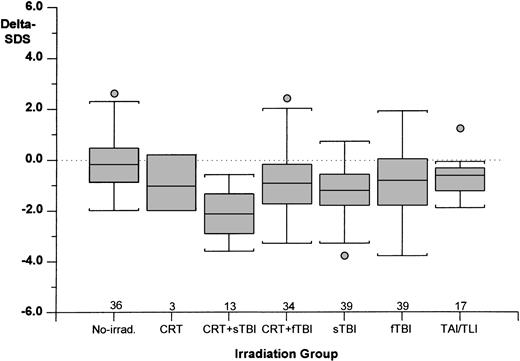
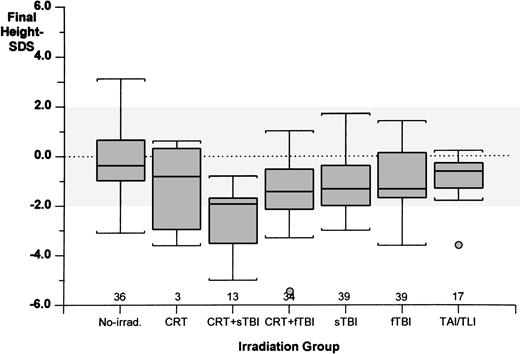
Seven different groups were identified according to the type of irradiation and chemotherapy applied (Table3); the age at BMT was similarly distributed in these groups (analysis of variance). As shown in Fig 2, the most severe growth failure was found in patients who received CRT+sTBI (mean delta-SDS value, −2.07 ± 0.91) followed, respectively, in decreasing degree of severity by sTBI (−1.37 ± 1.06), CRT+fTBI (−1.11 ± 1.61), fTBI (−0.88 ± 1.25), and TAI/TLI (−0.71 ± 0.72). The nonirradiated group had virtually no growth deficit after BMT (−0.07 ± 1.08). A similar pattern was found when final Weight-SDS values were considered (Fig 3).
Delta-SDS (final height-SDS minus SDS at BMT) in the different irradiation groups. Numerals indicate the number of cases studied in each group. (Box plot) The lower line of the box indicates the 25th percentile, the upper line indicates the 75th percentile, and the horizontal lines above and below the boxes represent the 3rd and the 97th percentile, respectively.
Delta-SDS (final height-SDS minus SDS at BMT) in the different irradiation groups. Numerals indicate the number of cases studied in each group. (Box plot) The lower line of the box indicates the 25th percentile, the upper line indicates the 75th percentile, and the horizontal lines above and below the boxes represent the 3rd and the 97th percentile, respectively.
Final height-SDS achievement in the different irradiation groups. Numerals indicate the number of cases studied in each group. The dotted area indicates the height-SDS distribution for the normal general population. (Box plot) The lower line of the box indicates the 25th percentile, the upper line indicates the 75th percentile, and the horizontal lines above and below the boxes represent the 3rd and the 97th percentile, respectively.
Final height-SDS achievement in the different irradiation groups. Numerals indicate the number of cases studied in each group. The dotted area indicates the height-SDS distribution for the normal general population. (Box plot) The lower line of the box indicates the 25th percentile, the upper line indicates the 75th percentile, and the horizontal lines above and below the boxes represent the 3rd and the 97th percentile, respectively.
The delta-SDS value of the 10 nonirradiated patients conditioned with BuCy (+0.05 ± 1.13) was not statistically different from the 26 nonirradiated SAA patients (−0.12 ± 1.08).
Comparing the delta-SDS value among the patients divided into groups according to diagnosis, no significant difference was found between the ALL (73 cases; −1.26 ± 1.35), AML (46 cases; −0.96 ± 1.06), and CML groups (10 cases; −0.74 ± 1.98). The delta-SDS found in the SAA group (48 cases; −0.43 ± 1.09) was significantly better than that of the ALL and AML groups. Dividing the SAA group into nonirradiated (27 cases; −0.12 ± 1.06 delta-SDS) and irradiated (21 cases; −0.82 ± 1.01), a significant statistical difference was found (Wilcoxon rank-sum test;P < .03).
The severity of chronic GVHD (126 cases with no GVHD: −0.84 ± 1.20 delta-SDS; 37 cases with limited GVHD: −1.08 ± 1.30; and 18 cases with extended GVHD: −1.34 ± 1.85) did not significantly influence the delta-SDS mean value (analysis of variance:P = .22), even though an increasing severity of the chronic GVHD showed a tendency toward worsening of delta-SDS.
On a long-term basis, cortisone treatment (87 treated patients [−1.03 ± 1.48 delta-SDS] v 84 not treated [−0.79 ± 1.07]) and cyclosporin-A treatment for GVHD (91 treated [−0.84 ± 1.22] v 80 not treated [−0.99 ± 1.38]) were found to have no effect on delta-SDS values.
The 55 patients who received sex hormone replacement therapy reached a similar final height-SDS (−1.05 ± 1.53) compared with the group of patients who started and completed pubertal development spontaneously (−1.05 ± 1.35).
The mean delta-SDS found in the 28 patients treated with exogenous GH (−1.14 ± 1.24) was not statistically different from that of the remaining 153 patients (−0.90 ± 1.31) not treated with exogenous GH. Because irradiation was the major factor in altering the delta-SDS value in our cohort, this analysis was performed within the same conditioning group. Twelve of 34 patients who received CRT+fTBI and who were treated with GH (delta-SDS −0.75 ± 1.32) were compared with the remaining 22 patients who did not receive GH treatment (delta-SDS −1.31 ± 1.75); 8 of 13 patients who received CRT+sTBI and who were treated with GH (delta-SDS −1.83 ± 0.71) were compared with the remaining 5 patients who did not receive GH treatment (delta-SDS −2.46 ± 1.13); and 7 of 39 patients who received sTBI and who were treated with GH (delta-SDS −1.24 ± 1.38) were compared with the remaining 32 patients who did not receive GH treatment (delta-SDS −1.40 ± 1.0). None of the comparisons was found to be statistically significant, but there seem to be a trend towards better growth in the GH-treated group. We also failed to show differences within the same gender, both between GH-treated (20; −1.21 ± 1.34 delta-SDS) and untreated boys (92; −1.77 ± 1.35) and between GH-treated (8; −0.99 ± 1.01) and untreated girls (61; −0.50 ± 1.14 delta-SDS).
Multiple-logistic regression was used to model the relationship between the dependent variable delta-SDS and the explanatory covariates (gender, age at transplant, type of BMT, irradiation applied, chronic GVHD severity, and GH therapy). Stepwise multiple logistic regression identified irradiation, age at transplant, and gender as statistically relevant explanatory covariates that significantly contributed to the model that was fitted to the data (Table 4). The role of each covariate in determining a relevant growth deficiency (delta-SDS <0) while accounting for the effect of the other covariates included in the logistic regression model and the estimated effect size is reported as relative risk point estimates with their 95% confidence intervals (Table 4).
DISCUSSION
The normal growth process during childhood reflects the child’s general well-being, and it is regulated by and depends on the interaction between genetic, nutritional, metabolic, and hormonal factors. Nevertheless, growth is not always linear, especially in children who have periods of chronic illnesses and/or undergo toxic treatment procedures. The end result of growth is the final adult height, which is used in this study as the long-term marker for treatment-related toxicity in patients who underwent BMT during childhood.
Growth impairment in the short term has been repeatedly reported after BMT,8-12 but data on final height achievement are scarce, and the only two published reports dealt with a limited number of patients.3 4 This is the first multicenter study on final height with a large number of patients that takes into consideration the various potential risk factors that might affect growth after transplantation performed during childhood. Solid tumors and hematological disorders in which short stature is a trait of the disease itself (Fanconi’s anemia, Thalassemia, inborn errors, etc) were excluded.
Our data showed a similarity between the genetic height and the height at BMT on one hand and a decreased value of final height-SDS compared both with the genetic height and the patient’s height-SDS at BMT on the other, suggesting that the growth impairment in transplanted patients occurred mostly during the period after transplantation (Fig1).
The outcome of final height in this study did not change significantly between the different types of hematological malignancies (ALL, AML, and CML), suggesting that, in this cohort of patients, the primary disease itself does not affect growth. This study confirms that irradiation is the major contributor for long-term growth impairment (final height achievement). Patients who were not irradiated had virtually no decrease in final height-SDS compared both with the height at BMT and the genetic height, underlining what was reported in smaller series of patients.3 4 Among the different irradiation settings, leukemia patients who received CRT during first-line treatment and sTBI during pre-BMT conditioning had the most severe long-term impairment of growth (Figs 2 and 3). Moreover, patients with an identical primary disorder (SAA) who were treated with two different conditioning regimens (Cy + irradiation v Cy only) presented two completely different patterns of growth, with the most favorable being the nonirradiated group.
Because the BuCy conditioning regimen has been more recently introduced to reduce the detrimental effects of irradiation,13 the number of the BuCy patients in this study is too small to draw unequivocal conclusions regarding the effect of this regimen on the long-term growth. Nevertheless, and notwithstanding these limitations, final height achieved by these patients was similar to their predicted final height, suggesting that, despite the known radio-mimetic effect of busulfan,14 BuCy pre-BMT conditioning regimen has less interference on the growth process than does irradiation. Published data available on the effect of BuCy regimen on growth are discordant, and report experience on the short-term growth, but not on final height achievement. Whereas Wingard et al15 reported on the similarity between the effect of BuCy and TBI, 2 years after transplant, 8 of 24 patients in that study who were conditioned with BuCy also received CRT during first line treatment. Other studies16-18 found no harmful effect of BuCy on growth. However, these three studies were based on a short-term follow-up (3 to 6 years), whereas the present study reports the final height outcome of BuCy conditioning, albeit on a limited number of patients. Unfortunately, although BuCy conditioning should be encouraged, at least in pediatric patients, the attempt to substitute a TBI-based conditioning regimen with BuCy was not found to be advantageous when applied to patients with ALL.19
The type of BMT (autologous or allogeneic) did not influence final height achievement. In this context, because chronic GVHD is a relatively common complication in patients who receive allogeneic BMT20 and is not encountered after autologous BMT, the severity of chronic GVHD and its treatment (steroids and cyclosporin-A) were also found to have no significant effect on growth, even though a tendency toward worsening delta-SDS with increasing severity of chronic GVHD was documented. Although steroids and severe chronic systemic illness (ie, chronic GVHD) are known to induce growth impairment in children, our study, however, despite being limited by a relatively small sample size, suggests that children surviving after transplantation have an adequate, although partial, capacity to catch-up with growth in the long term.
Being younger at BMT, the female group was theoretically supposed to experience a greater growth impairment than males. Nevertheless, the loss in height-SDS was more profound in boys than in girls, although the two groups were comparable for differences in genetic heights and height at BMT, sex hormone replacement therapy, and age of commencement of sex hormone treatment. This phenomenon therefore remains open for further specific studies.
The loss in growth velocity in patients after BMT seems to be the result of a complex interaction of different factors related to the effect of irradiation and chemotherapy, such as lesions of bone, cartilage, and the epiphyseal growth plate; gonadal damage; delayed or precocious puberty; and hypothyroidism. Growth delay has also been attributed to GH deficiency.8-12,18,21 Data on GH secretion were not included in the questionnaire, and GH therapy was prescribed by some of the BMT centers. Despite the relatively small number of patients who received GH treatment in our cohort, the effect on the final height outcome was less enthusiastic than that reported by others. Thomas et al22 showed that growth impairment after BMT in a homogeneous group of 49 children with leukemia who received CRT and TBI resulted prevalently from severe spinal growth suppression (reduced spinal height) that was unresponsive to GH treatment; also, there was an inappropriate response with absent catch-up growth in their legs. Even in children surviving brain tumors (a group with florid radiation-induced GH deficiency), GH treatment increased the short-term growth velocity but did not significantly improve the final height.23 Furthermore, in our cohort, a reduced final height was also observed in patients with SAA irradiated with TAI/TLI only, ie, with irradiation fields not involving the skull and its neuroendocrine structures. This observation is further emphasized by the finding that patients who received CRT with or without TBI (high cumulative irradiation dose to the hypothalamic-pituitary region) had an equal relative risk for developing growth failure, as those patients who had irradiation that did not include CRT (stepwise multiple logistic regression analysis). GH deficiency, therefore, does not seem to play a major role in growth impairment after BMT. Because patients are already at high risk for secondary tumors after BMT,24,25 and although available data on the safety of GH-treatment in patients with a history of malignancies are reassuring,26 we recommend caution in selecting patients as candidates for GH treatment after BMT, especially because a positive long-term effect of GH treatment on growth is not yet ascertained in such patients. Furthermore, because we found that, in the long term, 140 of 181 patients who attained their final height reached normal heights (within ±2 SDS for the general population), we also suggest that growth should be clinically followed-up once every 6 months and that only a few selected cases of severe and persistent growth deficiency, observed after the interruption of the posttransplant medication, be considered for GH treatment.
This study gathered data on patients who were transplanted during a period when CRT was frequently used as prophylaxis treatment in the majority of ALL patients and single-dose administration of irradiation was widely used. At present, CRT is used in a small and selected group of children, and irradiation schedules encourage fractionated TBI. Because irradiation was found to have a significant role in long-term growth impairment, especially in patients who received cranial irradiation before transplant and received sTBI during pre-BMT conditioning, we expect an improvement in the height prognosis in children transplanted during the 1990s.
APPENDIX
Participating centers.
H. Lackner, Division of Pediatric Hematology-Oncology, University Children’s Hospital, Graz, Austria; J.Y. Cahn, Service D’hematologie Hospital Jean Minjoz, Besancon, France; H. Esperou, G. Socié, Service d’Hematologie-Greffe de Moelle, Hòpital Saint Louis, Paris, France; F. Freycon, Onco-hematologie pediatrique, Hospital Nord Chru, Saint Etienne, France; F. Zintl, Department of Pediatrics University of Jena, Jena, Germany; R. Dopfer, Department of Pediatrics University Hospital, Tubingen, Germany; J. Sanders, Department of Haematology, Canterbury Health Laboratory, Christchurch, New Zealand; J. O’Riordan, Department of Hematology, St. James Hospital, Dublin, Ireland; A. Cohen, A. Gaiero, University Department of Pediatrics, Gaslini Institute Children’s Hospital, Genoa, Italy; M.T. van Lint, A. Bacigalupo, Department of Hematology, Ospedale San Martino, Genoa, Italy; C. Uderzo, A. Rovelli, Clinica Pediatrica, Ospedale San Gerardo, Monza, Italy; S. Varotto, Centro Leucemie Infantili Clinica Pediatrica I, Padova, Italy; W. Arcese, Università La Sapienza, Institute of Hematology, Rome, Italy; J. Prtnar, Department of Hematology, University Medical Center Ljubljana, Ljubljana, Slovenia; A.M. Martinez-Rubio, Hospital Infantil La Paz, Madrid, Spain; A. Verdeguer, Hospital infantil, Valencia, Spain; A. Fast, Department of Pediatrics, East Hospital University of Goeteborg, Goeteborg, Sweden; N. Bekassy, Department of Pediatrics, University Hospital, Lund, Sweden; W. Oostdijk, M. van Weel, J.M. Vossen, Department of Pediatrics, Leiden University Medical Center, Leiden, The Netherlands; I. Roberts, Department of Hematology, Royal Postgrade Medical School, Hammersmith Hospital, London, UK; H.G. Prentice, Department of Hematology, Royal Free Hospital, London, UK; and A.D. Leiper, Great Ormond Street Hospital for Children, London, UK.
ACKNOWLEDGMENT
The authors thank the nonprofit “Associazione CRESCI, a cura e sostegno della bassa statura” for their help in the accomplishment of this study and J. Upton for her technical assistance.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Amnon Cohen, MD, University Department of Pediatrics, Gaslini Institute, Children’s Hospital, Largo Gaslini 5, 16147-Genoa, Italy; e-mail: cohen.amnon@pn.itnet.it.