Abstract
Human PF4 is a heparin-binding chemokine known to be capable of inhibiting endothelial cell proliferation and angiogenesis. To explore the biological mechanisms responsible for this action, we investigated the effect of PF4 on epidermal growth factor (EGF)-stimulated human umbilical vein endothelial cells (HUVEC), a model system in which stimulation is essentially independent of interaction with cell-surface glycosaminoglycans. Based on previous findings that PF4 blocks endothelial cell cycle entry and progression into S phase, we studied the molecular mechanism(s) of PF4 interference with cell cycle machinery. PF4 treatment of EGF-stimulated HUVEC caused a decrease in cyclin E–cyclin-dependent kinase 2 (cdk2) activity with resulting attenuation of retinoblastoma protein phosphorylation. PF4-dependent downregulation of cyclin E-cdk2 activity was associated with increased binding of the cyclin-dependent kinase inhibitor, p21Cip1/WAF1, to the cyclin E-cdk2 complex. Analysis of total cellular p21Cip1/WAF1 showed that in the presence of PF4, p21Cip1/WAF1 levels were sustained at time points when p21Cip1/WAF1 was no longer detectable in cells stimulated by EGF in the absence of PF4. These findings indicate that PF4 inhibition of HUVEC proliferation in response to EGF is associated with impaired downregulation of p21Cip1/WAF1 and provide the first evidence for interference with cell cycle mechanisms by a chemokine.
HUMAN PLATELET factor four (PF4), a heparin-binding protein contained in platelet α-granules, is secreted upon activation of platelets. Structurally, human PF4 is a symmetrical, tetrameric molecule made up of four identical subunits, each containing 70 amino acid residues. PF4 is a member of the chemokine family, a group of homologous proteins1,2 separated into four branches, designated CXXXC, CXC, CC, and C, based on the relative position of the first two conserved cysteines. Although its structure places it among the members of the CXC family of chemokines, PF4 does not share certain proinflammatory properties of other CXC family members because it is missing a critical N-terminal GLU-LEU-ARG sequence, the “ELR motif,” which precedes the first cysteine residue. In addition, PF4 has not been shown to bind to any characterized chemokine receptor in mammals, with the possible exception of the Duffy antigen.3
The high affinity of PF4 for heparin and heparan sulfate4-6and its competition with antithrombin III (ATIII) for binding to heparan sulfate on the endothelial surface7-9 has provided the basis for a postulated role of this chemokine in the regulation of hemostasis as a procoagulant. However, a recent report has suggested an anticoagulant role for PF4 as a cofactor in the generation of activated protein C by the thrombin-thrombomodulin complex.10 A role for PF4 as a regulator of hemostasis deserves serious consideration because its glycosaminoglycan (GAG) binding properties, coupled with its release from activated platelets, leads to concentration of PF4 on the endothelial surface at sites of vessel injury where effective hemostasis is needed.
In the last decade, several reports have identified PF4 as an inhibitor of both angiogenesis11,12 and hematopoietic progenitor cell proliferation.13,14 Because angiogenesis is critical for tumor growth,15 characterization of naturally occurring angiostatic factors, virtually free of drug-resistance phenomena,16 has become an important focus of cancer research. In vivo studies conducted in several animal models have characterized PF4 as an inhibitor of murine melanoma and human colon carcinoma tumor growth and metastasis.17-20 However, in vitro studies have shown that although PF4 was unable to inhibit proliferation of cultured melanoma or colon carcinoma cell lines, this chemokine could inhibit cultured endothelial cell proliferation,18 leading to the conclusion that PF4 antiproliferative action on the endothelium was responsible for the tumor suppression. At present, clinical trials are being conducted with several angiostatic drugs, including PF4.21 The observations cited above, together with in vivo evidence in animal models demonstrating the preferential binding of PF4 to the endothelium of regenerating vessels,22 suggest a physiologic role for PF4 as a regulator of angiogenesis.15 23
The mechanism by which PF4 inhibits endothelial cell proliferation is unknown. Gupta and Singh24 have reported that PF4 blocks endothelial cell cycle entry and progression into S phase, suggesting that its antiproliferative action may involve direct interference with the cell cycle machinery. Cell proliferation is achieved through a complex and ordered sequence of events controlled by cyclin-dependent kinases (cdks), a group of related serine/threonine kinases whose activation depends on their association with protein subunits, the cyclins, and regulatory phosphorylation.25Each phase of the cell cycle is characterized by a unique pattern of cdk activity. Under the influence of mitogens, progression in early G1 depends on the activity of cyclin D-cdk4/6. The main substrate for cyclin D-cdk4/6 is the retinoblastoma protein (pRb), the product of a tumor suppressor gene. In late G1, phosphorylation of pRb is completed by cyclin E-cdk2. Activation of cyclin D– and cyclin E–associated kinases, and consequent hyperphosphorylation of pRb, may constitute the molecular events that take a proliferating cell over the biological threshold between mitogen-dependent and -independent stages of the G1 phase (restriction point or “R point”). Passage through the R point is required for cellular commitment to the remainder of the growth cycle, unless DNA damage or metabolic disturbances occur.26-29 pRb in its hypophosphorylated active form binds to and inhibits the activity of the E2F family of transcription factors. E2F proteins released from hyperphosphorylated retinoblastoma protein (ppRb) promote transcription of a number of genes with key roles for progression into S phase.26-28
Cyclin-cdk activity is tightly regulated because of its central importance in cell cycle progression. Regulatory mechanisms include: variations in the cyclin levels,29-31 phosphorylation status of the kinase subunit,25 and the action of specific inhibitors, the cyclin-dependent kinase inhibitors (CKIs).32 Two classes of CKIs have been defined according to structural homology. The INK4 (p15, p16, p18, p19) family is specific for cdk4/6; the Cip-Kip (p21, p27, p57) family contains universal inhibitors of all cyclin-cdk pairs. A large body of evidence is accumulating which supports the idea that certain regulators of cell proliferation modulate cyclin-cdk activity by affecting CKI expression. In particular, transforming growth factor-β (TGF-β) and interferons-α and -γ, whose activities extend to endothelial cells, have been reported to upregulate p27Kip1 and/or p21Cip1/WAF1 levels33-35 with consequent inhibition of pRb phosphorylation.
p21Cip1/WAF1 was originally cloned and characterized both as a cdk-binding inhibitor of cdk kinase activity36 and a p53-inducible inhibitor of tumor growth.37p21Cip1/WAF1 was reported to inhibit growth by blocking cells in the G1 phase in a p53-dependent response to DNA damaging agents.38-40 However, its ability to inhibit the cell cycle in a p53-independent manner has also been shown in several recent reports.41,42 p21Cip1/WAF1 has also been shown to inhibit DNA replication, but not repair, by binding to proliferating-cell nuclear antigen (PCNA).43
In this report, we describe studies of the mechanism by which PF4 inhibits the proliferation of mitogen-stimulated human umbilical vein endothelial cells (HUVEC). Epidermal growth factor (EGF) instead of basic fibroblast growth factor (bFGF) was chosen to exclude possible GAG-dependent interference of PF4 with mitogen-receptor interactions. Here we report that PF4 inhibits cell cycle progression by preventing the downregulation of p21Cip1/WAF1, with consequent inhibition of cyclinE-cdk2 activity and pRb phosphorylation.
MATERIALS AND METHODS
Chemicals and reagents.
These were obtained from the following sources: recombinant human bFGF and EGF from R&D Systems Inc (Minneapolis, MN); [methyl-3H]-thymidine (37 MBq/mL) and [γ32P]ATP (370 MBq/mL) from NEN Inc (Boston, MA); heparin derived from porcine intestinal mucosa (10,000 IU/mL, specific activity: 176 IU/mg) and protamine sulfate (10 mg/mL) from Elkins-Sinn, ESI Pharmaceuticals (Cherry Hill, NJ); human PF4, either isolated from human platelets or expressed as a recombinant protein, was purified in our laboratory as previously described,44 45 the specific cDNA encoding human PF4 was a gift from Dr Mortimer Poncz (University of Pennsylvania, Philadelphia); rabbit IgG anti-pRb, mouse monoclonal IgG antibodies to cdk2, cyclin E, p53, agarose beads conjugated with rabbit IgG anti-cdk2, and the glutathione S-transferase-fused murine pRb (GST-Rb, amino acid residues 769-921) from Santa Cruz Biotechnology, Inc (Santa Cruz, CA); mouse monoclonal antibodies to p21Cip1/WAF1 and p27Kip1 from Transduction Laboratories (Lexington, KY); RPMI-1640 medium, EDTA, ethylene glycol bis-(β aminoethyl ether) N,N,N′,N′-tetraacetic acid (EGTA), NaF, NaVO4, leupeptin, aprotinin, phenylmethylsulfonyl fluoride (PMSF), 3-cyclohexylamino-1-propanesulfonic acid (CAPS), Tween-20, Triton X-100, and bovine serum albumin (BSA) from Sigma Chemical Co (St Louis, MO); okadaic acid from Calbiochem-Novabiochem (La Jolla, CA), Immobilon-P, polyvinylidene difluoride (PVDF) transfer membrane from Millipore Co (Bedford, MA); fetal bovine serum (FBS) and horse serum from HyClone Laboratories Inc (Logan, UT); sodium dodecyl sulfate (SDS), acrylamide, N,N′-methylene-bisacrylamide, and Tris from Bio-Rad Laboratories (Richmond, CA); Super Signal Ultra chemiluminescent substrate and bicinchoninic acid (BCA) assay from Pierce (Rockford, IL); enhanced chemiluminescence (ECL) detection system from Amersham Co (Arlington Heights, IL); peroxidase-conjugated goat anti-rabbit and anti-mouse IgG from Jackson Immunoresearch (West Grove, PA). All other chemicals were reagent grade.
HUVEC culture and stimulation.
Endothelial cells were isolated from human umbilical veins by collagenase digestion and were cultured46 47 by the Endothelial Cell Biology Core Laboratory of the Blood Research Institute (Milwaukee, WI). The cells were grown to confluence in T75 flasks (Corning, Corning, NY), coated with 2% gelatin, in RPMI-1640 medium supplemented with 15% horse serum, 100 μg/mL endothelial cell growth supplement (ECGS; Collaborative Research Inc, Lexington, MA), 100 μg/mL heparin, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma Chemical Co). Cell cultures were incubated at 37°C in a water-saturated atmosphere of 95% air-5% CO2, and were used at the third passage. To synchronize the cells in a quiescent state (G0), the cells were cultured in “starvation medium” (RPMI-1640 medium supplemented with 1% horse serum, 0.25% BSA) for 20 to 24 hours and were then stimulated with either EGF (20 ng/mL) alone or EGF (20 ng/mL) and PF4 (2 μg/mL) in “stimulation medium” (RPMI-1640 medium supplemented with 2.5% FBS).
DNA synthesis.
DNA synthesis was assessed by the level of thymidine (TdR) incorporation. Cells, 3 × 103, were seeded in 96-well plates (Corning), coated with 2% gelatin, and cultured to confluence in growth medium. The cells were washed in “starvation medium” and were then incubated in the same medium for 24 hours to synchronize the cells in G0 phase. The cells were labeled with 37 kBq/mL [3H]TdR in “stimulation medium” and then stimulated with either EGF (20 ng/mL) alone or EGF (20 ng/mL) and PF4 (2 μg/mL) in the same medium for 20 hours. Alternatively, bFGF at 2 ng/mL was used instead of EGF. For the experiment shown in Fig1A, EGF was used at concentrations ranging from 20 to 200 ng/mL, bFGF from 1 to 10 ng/mL, and PF4 from 0.2 to 10 μg/mL. The medium was discarded, the cells were obtained using a FilterMate 196 harvester (Packard Co, Meriden, CT), and radioactivity was measured using a Matrix 9600 (Packard) gas scintillation β-counter. Assays were performed in triplicate. [3H]TdR incorporation, determined in starving cells, was considered background and was subtracted from the value obtained in growth factor–stimulated cells. Background values were always less than 10% of the values obtained with growth factor stimulation.
(A) PF4 dose-dependently inhibits bFGF- (left) and EGF-induced (right) DNA synthesis in endothelial cells. HUVEC were plated in 96-well dishes as described in Materials and Methods. After reaching confluence they were kept in starvation medium for 24 hours, then stimulated with growth factor in the presence of the indicated concentrations of PF4. [3H]-thymidine incorporation was evaluated after 20 hours and was expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate). Data shown are mean values ± SE of three independent experiments performed in triplicate. (B) Heparin and protamine inhibit bFGF- but not EGF-stimulated proliferation of HUVEC. HUVEC were treated as in (A), except that bFGF was used at 2 ng/mL, EGF at 20 ng/mL, and PF4 at 2 μg/mL, in the presence of the indicated concentrations of heparin (H) or protamine. Data are expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate), and are mean values ± SE of three independent experiments performed in triplicate.
(A) PF4 dose-dependently inhibits bFGF- (left) and EGF-induced (right) DNA synthesis in endothelial cells. HUVEC were plated in 96-well dishes as described in Materials and Methods. After reaching confluence they were kept in starvation medium for 24 hours, then stimulated with growth factor in the presence of the indicated concentrations of PF4. [3H]-thymidine incorporation was evaluated after 20 hours and was expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate). Data shown are mean values ± SE of three independent experiments performed in triplicate. (B) Heparin and protamine inhibit bFGF- but not EGF-stimulated proliferation of HUVEC. HUVEC were treated as in (A), except that bFGF was used at 2 ng/mL, EGF at 20 ng/mL, and PF4 at 2 μg/mL, in the presence of the indicated concentrations of heparin (H) or protamine. Data are expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate), and are mean values ± SE of three independent experiments performed in triplicate.
In preliminary experiments, using these assay conditions, we determined that recombinant human PF4 is indistinguishable from human PF4 isolated from platelets in respect to its effects on mitogen-stimulated HUVEC. All subsequent experiments were performed using human recombinant PF4.
Immunoprecipitation and immunoblotting.
To prepare cdk2 immunoprecipitates, 6 × 106 cells, washed twice with ice-cold phosphate-buffered saline (PBS: 0.02 mol/L phosphate buffer, pH 7.4, 0.145 mol/L NaCl), were lysed in 0.5 mL of “lysis buffer” (0.05 mol/L Tris pH 8.0, 0.150 mol/L NaCl, 1% Triton X-100, 0.1 mol/L NaF, 0.0025 mol/L EGTA, 0.001 mol/L EDTA, 0.0014 mol/L phenylmethylsulfonyl fluoride [PMSF], 0.001 mol/L NaVO4, 1 μg/mL aprotinin, 0.5 μg/mL leupeptin, 0.2 μg/mL okadaic acid). Protein concentration was determined by the bicinchoninic acid (BCA) assay. Four hundred micrograms of total protein lysate diluted in “immunoprecipitation buffer” (lysis buffer without okadaic acid) to a final volume of 1 mL was incubated for 1 hour on ice with 50 μL of a 50% suspension of agarose beads conjugated with nonimmune rabbit IgG (preclearing), followed by centrifugation to remove beads. The supernatant was incubated for 1 more hour on ice with 50 μL of a 50% suspension of agarose beads conjugated with rabbit IgG anti-human cdk2. To reduce nonspecific binding, the beads were preincubated for 1 hour in immunoprecipitation buffer containing 3% BSA. After six washes with immunoprecipitation buffer, adsorbed proteins were dissociated by boiling in 1× SDS-sample loading buffer (0.05 mol/L Tris/HCl pH 6.8, 10% glycerol, 1% SDS, 0.02% bromphenol blue, and 5% β-mercaptoethanol), fractionated by 12.5% SDS-polyacrylamide gel electrophoresis (PAGE), and electroblotted (0.01 mol/L CAPS, 10% methanol, pH 11) onto a PVDF membrane. After blocking of nonspecific binding (blocking buffer: 0.02 mol/L Tris pH 7.8, 0.145 mol/L NaCl, 0.1% Tween-20, 20% FBS), the membrane was immunoblotted (0.02 mol/L Tris pH 7.8, 0.145 mol/L NaCl, 0.05% Tween-20) with the indicated antibodies. Secondary antibodies (horseradish peroxidase–labeled goat anti-rabbit or anti-mouse IgG) were visualized using the Super Signal Ultra (Pierce) or ECL (Amersham) chemiluminescent substrates. Immunoblotting of total protein was performed as described above, but only 50 μg of total protein diluted in 2× SDS-sample loading buffer was loaded per lane. In preliminary experiments, equal protein loading was assessed by Coomassie staining of the blotted membrane. Discontinuous 12.5% SDS-PAGE was used in all cases except for pRb analysis, where a 5% to 8.5% SDS-PAGE gradient gel was chosen. Immunoprecipitation and immunoblot analyses were performed for each of three (or more) independent, but identical experiments and demonstrated reproducibility of results.
Cyclin-dependent kinase assay.
To measure the activity of cdk2, a glutathione S–transferase-fused murine pRb fragment (GST-Rb) was used as substrate. The cdk2 immunoprecipitate was suspended in 50 μL of 0.02 mol/L Tris/HCl (pH 7.4) containing 0.01 mol/L MgCl2, 0.001 mol/L dithiotreitol, 50 μmol/L [γ32P]ATP/ATP, and 20 μg/mL GST-Rb. This mixture was incubated for 30 minutes at 30°C with occasional mixing. The reaction was terminated by adding an equal volume of 2× SDS-sample loading buffer. Samples were then centrifuged to remove the agarose beads and the supernatant was boiled for 5 minutes and fractionated by 10% SDS-PAGE. Phosphorylated proteins were visualized by autoradiography and incorporated radioactivity was quantified directly on the dried gel with an AMBIS (Automated MicroBiological Imaging System) computerized imaging/radio scanning 4000 system (CSP Inc, Billerica, MA).
RESULTS
PF4 inhibits HUVEC proliferation induced by either bFGF or EGF.
Figure 1A illustrates the ability of two mitogens, bFGF and EGF, to stimulate, in a dose-dependent manner, DNA synthesis in cultured HUVEC as estimated by [3H]thymidine incorporation. Although bFGF and vascular endothelial growth factor (VEGF) have been reported to be the most potent endothelial cell mitogens, EGF, albeit at a 10-fold higher concentration, stimulated endothelial cell DNA synthesis as well as bFGF. PF4 effectively inhibited the action of both mitogens in a dose-dependent manner (Fig 1A).
EGF-induced HUVEC proliferation is glycosaminoglycan-independent.
bFGF, like PF4, is a heparin-binding protein and the interaction of bFGF with cell-surface GAGs is required for its mitogenic action.48 In agreement with other investigators,23,49,50 we found that heparin dose-dependently inhibits bFGF-induced DNA synthesis and that further inhibition is achieved by adding PF4 (Fig 1B). This finding is consistent with a possible synergistic action by both heparin and PF4 to inhibit bFGF dimerization and to displace bFGF from its cell-surface receptor.51 Protamine, a positively charged protein known to bind to GAGs,48-50 also inhibited bFGF-induced DNA synthesis (Fig 1B), supporting the view that bFGF can be inhibited by competing for its binding to cell-surface GAGs. In contrast, GAGs do not act as cofactors in EGF-dependent stimulation of cell growth.52 As shown in Fig 1B, neither heparin nor protamine alone affected EGF-induced DNA synthesis; however, PF4 inhibited DNA synthesis to the same extent whether or not heparin was present. The data in Fig 1B and our finding that PF4 fails to block EGF-stimulated MAP kinase signaling as reflected by phosphorylation of p44ERK and p42ERK (data not shown) indicate that PF4 does not interfere with the binding of EGF to its receptor or with the initiation of EGF signaling. Therefore, we chose EGF-stimulated endothelial cells as a model for further investigation of the angiostatic effect of PF4.
PF4 inhibits EGF-induced hyperphosphorylation of pRb.
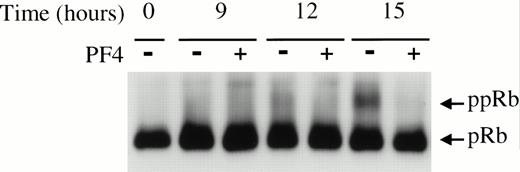
As already noted, a necessary step in G1 progression is the phosphorylation of the retinoblastoma protein by specific cyclin-cdk pairs. Therefore, we investigated the time-dependent accumulation of ppRb in HUVEC stimulated by EGF in the presence and absence of 2 μg/mL PF4. On the basis of its delayed mobility shift in SDS-PAGE, ppRb (114 kD) can be readily separated from pRb (110 kD)53 54 and detected by immunoblotting. As shown in Fig2, there was significant accumulation of ppRb in cells stimulated by EGF for 15 hours. In the presence of PF4, however, this accumulation was impaired.
PF4 attenuates EGF-induced pRb phosphorylation. HUVEC lysates, collected at the specified times after stimulation with 20 ng/mL EGF in the absence (−) or presence (+) of 2 μg/mL PF4, were separated by SDS-PAGE and immunoblotted for pRb as described in Materials and Methods. Results shown are representative of three independent experiments.
PF4 attenuates EGF-induced pRb phosphorylation. HUVEC lysates, collected at the specified times after stimulation with 20 ng/mL EGF in the absence (−) or presence (+) of 2 μg/mL PF4, were separated by SDS-PAGE and immunoblotted for pRb as described in Materials and Methods. Results shown are representative of three independent experiments.
PF4 prevents downregulation of the CKI p21Cip1/WAF1 and enhances its association with cdk2 in EGF-stimulated HUVEC.
In G1, pRb must be phosphorylated sequentially by cyclin-cdk pairs, cyclin D-cdk4/6 (early G1) and cyclin E-cdk2 (late G1),55 to achieve the hyperphosphorylated state necessary for G1/S transition. We immunoprecipitated cdk2 from lysates of HUVEC stimulated for 15 hours with EGF in the presence or absence of 2 μg/mL PF4 and assayed the precipitates for cdk2-associated kinase activity. The 15-hour time point was chosen because in preliminary experiments we observed an increase of cdk2-associated kinase activity beginning after 9 hours and peaking at 15 hours from the time HUVECs were induced with EGF (data not shown). Figure 3A shows that cdk2 immunoprecipitated from extracts of PF4-treated cells was much less effective in phosphorylating GST-Rb than cdk2 immunoprecipitated from PF4-untreated cells. Quantification of cdk2 kinase activity showed that the level in PF4-treated cells was about half that of cells treated with EGF alone. Inhibition of cdk2 kinase activity was also achieved with 10 ng/mL rapamycin (data not shown), a known inhibitor of cyclin-cdk activity.56 57 To determine whether cdk2 activity levels correlated with the amount of cdk2 protein, the cdk2 immunoprecipitates used for kinase activity assays were assayed for cdk2 protein levels by immunoblotting. Figure 3B indicates that cdk2 protein levels were essentially the same in the absence as in the presence of PF4. Because cyclin E is a necessary cofactor for cdk2 activity in G1 phase, we evaluated the amount of cdk2-associated cyclin E in the extracts used for kinase assays. Figure3C shows that PF4 did not affect the amount of cyclin E associated with cdk2. These findings indicate that loss of cdk2 activity associated with PF4 treatment is due to inhibition of function rather than to reduction of cdk2 or cyclin E protein levels.
Enhanced association of cdk2 with p21Cip1/WAF1 occurs in EGF-stimulated HUVEC treated with PF4. (A) Immunoprecipitated cdk2, from HUVEC lysate, collected 15 hours after EGF stimulation in the absence (−) or presence (+) of 2 μg/mL PF4, was assayed with GST-pRB in the presence of [γ32P]ATP/ATP as described in Materials and Methods. Phosphorylated GST-pRb was then analyzed by SDS-PAGE and autoradiography and was quantified with an AMBIS scanner. (B) cdk2 levels in aliquots of the samples used to generate the results shown in panel (A), were determined by SDS-PAGE and immunoblotting. (C) The quantity of cyclin E immunoprecipitated with cdk2 was determined by SDS-PAGE followed by immunoblot analysis. (D) p21Cip1/WAF1associated with cdk2 was similarly assayed. The results shown are representative of three independent experiments.
Enhanced association of cdk2 with p21Cip1/WAF1 occurs in EGF-stimulated HUVEC treated with PF4. (A) Immunoprecipitated cdk2, from HUVEC lysate, collected 15 hours after EGF stimulation in the absence (−) or presence (+) of 2 μg/mL PF4, was assayed with GST-pRB in the presence of [γ32P]ATP/ATP as described in Materials and Methods. Phosphorylated GST-pRb was then analyzed by SDS-PAGE and autoradiography and was quantified with an AMBIS scanner. (B) cdk2 levels in aliquots of the samples used to generate the results shown in panel (A), were determined by SDS-PAGE and immunoblotting. (C) The quantity of cyclin E immunoprecipitated with cdk2 was determined by SDS-PAGE followed by immunoblot analysis. (D) p21Cip1/WAF1associated with cdk2 was similarly assayed. The results shown are representative of three independent experiments.
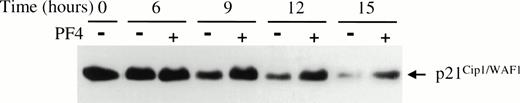
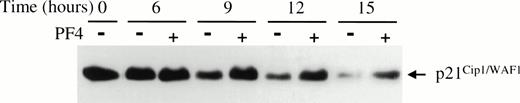
Cyclin-cdk activity can be modulated by specific CKIs, that bind directly to cyclin-cdk pairs to inhibit their activity. p21Cip1/WAF1, a CKI capable of inhibiting all cyclin-cdk pairs, has been shown by in vitro studies to likely have higher affinity for cyclin E-cdk2 than other cyclin-cdk pairs.39Therefore, we determined whether PF4 could affect the amount of p21Cip1/WAF1 associated with cyclin E-cdk2. Immunoblot analysis for p21Cip1/WAF1, from the same immunoprecipitates used to determine cdk2-associated kinase activity, showed that the level of cdk2-associated p21Cip1/WAF1 was significantly higher in EGF-stimulated cells treated with PF4 than in cells incubated without PF4 (Fig 3D). The levels of p21Cip1/WAF1 associated with cdk2 were not appreciably affected by PF4 at early time points, but began to increase relative to control at 9 hours (data not shown), the time at which cyclin E-cdk 2 activity becomes detectable. Serial studies of the time-dependent accumulation of total cellular p21Cip1/WAF1 in EGF-stimulated HUVEC incubated with or without PF4 showed persistence of p21Cip1/WAF1 levels over the course of 15 hours in the presence but not in the absence of PF4 (Fig4). This effect of PF4 was specific for p21Cip1/WAF1 because the cellular content of p27Kip1, another CKI inhibitory toward cyclin E-cdk2, was not altered either in the presence and absence of PF4 (data not shown). Moreover, p21Cip1/WAF1 levels were independent of p53 expression which did not change during the period of observation (data not shown). Together, these findings indicate that PF4 inhibits cell cycle progression into S phase by sustaining high levels of p21Cip1/WAF1 which, in turn, inhibit cyclin E-cdk2 activity and, consequently, pRb phosphorylation.
PF4 prevents p21Cip1/WAF1 downregulation in HUVEC stimulated by EGF. HUVEC lysates collected at the specified times following EGF (20 ng/mL) stimulation with (+) or without (−) 2 μg/mL PF4 were separated by SDS-PAGE and p21Cip1/WAF1 was immunoblotted. Results shown are representative of three independent experiments.
PF4 prevents p21Cip1/WAF1 downregulation in HUVEC stimulated by EGF. HUVEC lysates collected at the specified times following EGF (20 ng/mL) stimulation with (+) or without (−) 2 μg/mL PF4 were separated by SDS-PAGE and p21Cip1/WAF1 was immunoblotted. Results shown are representative of three independent experiments.
DISCUSSION
The goal of these studies was to obtain clues to the mechanism by which PF4 exerts its antiproliferative effect on mitogen-stimulated HUVEC. We chose EGF, a mitogen whose action is independent of GAG, for HUVEC stimulation to eliminate the possibility that inhibition by PF4 might simply be the result of displacement by PF4 of mitogen from GAG, an interaction known to be important for bFGF function.48 We confirmed this phenomenon by showing dose-dependent inhibition of bFGF-induced cell proliferation by PF4, heparin, and the highly cationic protein, protamine (Fig 1). Heparin and protamine exert this effect by binding to bFGF and cell-surface GAGs respectively, thus interfering with bFGF-receptor interaction as shown previously by several groups.24 58-61
Although bFGF is not an ideal mitogen for studying inhibition of cell proliferation by PF4 because of its dependence on GAG, Maione et al18 obtained evidence that PF4 might act to inhibit proliferation by a mechanism independent of GAG by showing that recombinant PF4, modified to delete the major heparin-binding site, retained inhibitory activity. We found that HUVEC proliferation induced by the GAG-independent mitogen EGF was unaffected by heparin and by protamine (Fig 1B), but was inhibited by PF4 in a dose-dependent manner (Fig 1A). Moreover, PF4 failed to block EGF-stimulated MAP kinase signaling, indicating that it does not interfere with the binding of EGF to its cell-surface receptor. These findings are consistent with the possibility that the inhibitory effect of PF4 is exerted through a specific receptor not yet characterized. Alternatively the inhibitory signal could be delivered directly through GAG and their associated protein core.
The transit of proliferating cells through the cell cycle is tightly regulated by various mitogenic and antimitogenic signals.25,32 We examined the possibility that the antimitogenic effect of PF4 is related to regulation of cdk activity in G1. Our findings indicate that the inhibitory effect of PF4 is related to inhibition of cdk2 activity (Fig 3) with resulting failure to phosphorylate the retinoblastoma protein (Fig 2). Reduced cdk2 activity, in turn, appears to result from persistence of the universal CKI, p21Cip1/WAF1 (Figs 3 and 4). These observations provide the first evidence for interference by a chemokine with key regulators of the cell cycle machinery. Future studies are needed to directly link the action of PF4 to the regulation of p21Cip1/WAF1 levels. The inhibitory action of antimitogens, TGF-β,33,62 and interferons-α/γ34 has recently been studied and appears to be related to regulation of cdk activity in G1 by upregulation of specific CKIs. The effect of PF4 on p21Cip1/WAF1 appears to be specific, because there was no change in the cellular content of another CKI, p27Kip1. Sustained levels of p21Cip1/WAF1during growth factor stimulation could be mediated by various mechanisms: decreased protein degradation, increased mRNA production, increased mRNA translation rate, or increased mRNA half-life. p21Cip1/WAF1 mRNA contains three AUUUA sequences36,37 in its 3′ untranslated region. Such sequences contained within A-U rich elements (ARE) are thought to serve as mRNA destabilizing elements that trigger rapid degradation of the messenger, and are typically present in the transcripts of cytokines, oncoproteins, and transcription factors. mRNA stabilization may be correlated with its association to specific RNA-binding proteins.63 Upregulation of p21Cip1/WAF1 protein levels through mRNA stabilization has been proposed as a mechanism of posttranscriptional control.64-66 Very little information is currently available to explain how PF4 might initiate its signal. Although cell-surface receptors have been identified for other CXC chemokines such as interleukin-8 (IL-8), growth-related oncogene α (gro α), stromal cell–derived factor-1 (SDF-1), interferon-inducible protein 10 (IP-10), and others,67 as well as for heparin-binding ligands such as bFGF and VEGF, a cell-surface receptor specific for PF4 has eluded discovery. The high affinity of PF4 for cell-surface heparan sulfate proteoglycans has complicated the isolation of a PF4-specific receptor. The possibility exists that PF4 signals directly through proteoglycan receptors such as syndecan, betaglycan, or perlecan. Even though low-affinity proteoglycan receptors generally serve in an auxiliary manner, presenting certain ligands to high-affinity receptors through which subsequent signaling occurs,68 direct signaling through proteoglycans cannot be ruled out for PF4, especially considering its high affinity for heparan sulfate proteoglycan, on which it recognizes a specific structural motif.69 Added support for this possibility is provided by a recent report in which the ability of GAGs to mediate extracellular signals was shown.70,71 PF4 could be directly internalized via proteoglycan receptors, as has been described for the CXC chemokine, IL-8.72 In this case, initiation of PF4 signaling could be intracellular. Of particular relevance, Luster et al58reported that the CXC chemokine, IP-10, competed with PF4 for binding to cell-surface heparan sulfate proteoglycan and inhibited endothelial cell proliferation, suggesting similar signaling mechanisms for the two chemokines. A specific cell-surface receptor for IP-10 has been identified on T lymphocytes. Although the Duffy antigen erythrocyte chemokine receptor, also expressed on postcapillary venule endothelial cells,73,74 has been possibly implicated as a PF4 binding site,3 it does not appear to be linked to any postreceptor signal transduction mechanisms,75 and is, therefore, not a good candidate for transmission of PF4 antiproliferative signals. Future investigation into how PF4 signaling is initiated is necessary to fully characterize the mechanism of its antiproliferative effects.
PF4 anti-angiogenic action: Implications for cancer therapy.
PF4 is capable of inhibiting tumor growth and metastasis.11,18,19 Using both in vitro and in vivo assay systems, Strieter’s group67 has proposed a model of angiogenesis involving interplay between CXC chemokine angiogenic factors possessing an N-terminal ELR motif (eg, IL-8 and gro α) and CXC chemokine angiostatic factors lacking the ELR motif (eg, PF4 and IP-10). It was postulated that a shift in the balance of angiogenic and angiostatic CXC chemokines contributes to the invasiveness of non–small cell lung cancers and to development of pulmonary fibrosis through dysregulated neovascularization.12,67 The demonstration that PF4 has angiostatic potential justifies its consideration as an agent to treat solid tumors and chronic inflammation-dependent fibroproliferative disorders. Several preclinical studies17-20,76 and a clinical trial21 have been initiated to examine its effectiveness as an antineoplastic agent. A derivative of PF4, truncated at its N-terminus, was reported to be more potent than the wild-type molecule in its antiproliferative action on endothelial cells.77 Maione et al11 found that the antiangiogenic action of PF4 requires a conserved C-terminus. Lecomte-Raclet et al78 found that a peptide corresponding to the central region of PF4, but lacking affinity for heparin, was effective in inhibiting murine hematopoietic progenitors proliferation.
Numerous naturally occurring angiostatic molecules have been characterized, including IP-10, thrombospondin 1, angiostatin, endostatin, and PF4. Although the molecular mechanisms of their action are as yet unknown, inhibition of angiogenesis by angiostatin and PF4 is associated with inhibition of tumor growth and metastasis.18-20,76 79 The present study is the first to define a role for a specific cell cycle regulatory molecule, p21Cip1/WAF1, in mediating the antiangiogenic properties of a CXC chemokine. An understanding of the molecular action of naturally occurring angiostatic agents could lead to the development of targeted therapies effective in various diseases associated with dysregulated angiogenesis.
ACKNOWLEDGMENT
We thank Marco Foschi and Andrey Sorokin for their useful comments and suggestions. We are also indebted to Mortimer Poncz for providing the cDNA clone encoding human PF4 and Cassie Nelson for her excellent technical assistance.
Supported in part by Grants No. HL-13629, HL-51413, and HL-44612 from the National Heart, Lung, and Blood Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Gian Paolo Visentin, MD, Blood Research Institute, The Blood Center of Southeastern Wisconsin, 8727 Watertown Plank Rd, Milwaukee, WI 53226-3548; e-mail: gpvisentin@bcsew.edu.

![Fig. 1. (A) PF4 dose-dependently inhibits bFGF- (left) and EGF-induced (right) DNA synthesis in endothelial cells. HUVEC were plated in 96-well dishes as described in Materials and Methods. After reaching confluence they were kept in starvation medium for 24 hours, then stimulated with growth factor in the presence of the indicated concentrations of PF4. [3H]-thymidine incorporation was evaluated after 20 hours and was expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate). Data shown are mean values ± SE of three independent experiments performed in triplicate. (B) Heparin and protamine inhibit bFGF- but not EGF-stimulated proliferation of HUVEC. HUVEC were treated as in (A), except that bFGF was used at 2 ng/mL, EGF at 20 ng/mL, and PF4 at 2 μg/mL, in the presence of the indicated concentrations of heparin (H) or protamine. Data are expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate), and are mean values ± SE of three independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.25/5/m_blod40147001ax.jpeg?Expires=1766077547&Signature=M00V0TXSaj7woVinHClzJkYuaDdxIfpHE7izc9J89FJ3GJGrgduwvVVs9lB5EqgRMDhXefmI~6I9OLa~RHjpLpjRiLeRd19MY5ZcHMfkQH5gFqXhuPFAb0~H5eMeYtF70YY76yzgfCN0nh4M6JhTOXRlg3k5~Mnn3wVNQYg722~WaSbUrA93Nrdm2l3jvRHz514bEWpUlUR2jit8~~CYce3ZoFMYZ9Uf6RoyVSTKCG5HiqRpQWlp455Z38gWorHsq7Mm--QMKo8bA6ejE0TLMVy7D19Zo8~wvlJQJV7wllXsFIVEmwQby9VO3O2t~u3iUhAuF~2l2-SuO96JjZC5hQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) PF4 dose-dependently inhibits bFGF- (left) and EGF-induced (right) DNA synthesis in endothelial cells. HUVEC were plated in 96-well dishes as described in Materials and Methods. After reaching confluence they were kept in starvation medium for 24 hours, then stimulated with growth factor in the presence of the indicated concentrations of PF4. [3H]-thymidine incorporation was evaluated after 20 hours and was expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate). Data shown are mean values ± SE of three independent experiments performed in triplicate. (B) Heparin and protamine inhibit bFGF- but not EGF-stimulated proliferation of HUVEC. HUVEC were treated as in (A), except that bFGF was used at 2 ng/mL, EGF at 20 ng/mL, and PF4 at 2 μg/mL, in the presence of the indicated concentrations of heparin (H) or protamine. Data are expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate), and are mean values ± SE of three independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.25/5/m_blod40147001bx.jpeg?Expires=1766077547&Signature=gTPRVu0h3YVlCMV3eSy3ZFenEKBYXwuJzyRmKg81SfHhRC8okFyay1O75GutzouZaxTlYv~l~AC12YCxfAuT1lIqBGiRCJbuydNyfjAq5K86iUSDlukzyDjJvnhOtFWcyllsm-ASWeoY5UNp2xpUcCGyJRC1HkmoaIL7281ZTlu~WZjGreiH~ujUmrbVUsEfZjrnmMPNPEshUFS-BQDWfwFwCaUJFmJKqJlVmHwcv26a88EBQQnQFPorlUvXfsaLJLDHgQRX9rcryduTTyNmlVGJhaVOBurP4Dyt5OPp4~~kfqjNb74pDovpaqyxEMGpJtaDv6T9OusXBsQcKbf6hQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Enhanced association of cdk2 with p21Cip1/WAF1 occurs in EGF-stimulated HUVEC treated with PF4. (A) Immunoprecipitated cdk2, from HUVEC lysate, collected 15 hours after EGF stimulation in the absence (−) or presence (+) of 2 μg/mL PF4, was assayed with GST-pRB in the presence of [γ32P]ATP/ATP as described in Materials and Methods. Phosphorylated GST-pRb was then analyzed by SDS-PAGE and autoradiography and was quantified with an AMBIS scanner. (B) cdk2 levels in aliquots of the samples used to generate the results shown in panel (A), were determined by SDS-PAGE and immunoblotting. (C) The quantity of cyclin E immunoprecipitated with cdk2 was determined by SDS-PAGE followed by immunoblot analysis. (D) p21Cip1/WAF1associated with cdk2 was similarly assayed. The results shown are representative of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.25/5/m_blod40147003w.jpeg?Expires=1766077547&Signature=YVv5LbceivBJgxq4TPh~nWNPBgzoqC~qwbzVfPiqWdxZp7T0mbvrhwwInil7copqGwT1D65wTHtETOSvFrbzSlS2Tq6x4tXjSAKTm0Lo48h0IOAo6L25tC1C~pVdnyJyUtvRBvhAJrdRGf0bM2FLBh21a39yMeyrw4OTGeNG4z9CT~qpchuglEh-apm9NpTsdd6y0zxUUq5XY5d3MxZpgXBtab7JvBcpqMGRpJNO6pjruTF9Wqb82KYcY-aZSKoPtQHG-NPiiW3RoOoqY8z4LryFDn0kUEaNQDBnvZbG5pJ8-fbpQzBpuT-rFz4IC3620z1LAiNTHzizqC5Yw3r2zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. (A) PF4 dose-dependently inhibits bFGF- (left) and EGF-induced (right) DNA synthesis in endothelial cells. HUVEC were plated in 96-well dishes as described in Materials and Methods. After reaching confluence they were kept in starvation medium for 24 hours, then stimulated with growth factor in the presence of the indicated concentrations of PF4. [3H]-thymidine incorporation was evaluated after 20 hours and was expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate). Data shown are mean values ± SE of three independent experiments performed in triplicate. (B) Heparin and protamine inhibit bFGF- but not EGF-stimulated proliferation of HUVEC. HUVEC were treated as in (A), except that bFGF was used at 2 ng/mL, EGF at 20 ng/mL, and PF4 at 2 μg/mL, in the presence of the indicated concentrations of heparin (H) or protamine. Data are expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate), and are mean values ± SE of three independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.25/5/m_blod40147001ax.jpeg?Expires=1766400973&Signature=wZs8oz5chWL~HA7A7ob4vEkNLoS-3iQb0mD-BzLpplIOqAPZ3UAEuQfYqlotOtUDme7ThNoOio0EdcQ1qxQvi9CURoD9VS6sliHsiiQYpeDsZETnLhappfXNbWo-QnrxuR~-bNoKxEf8W1IKDSSOvC6qqdb~ArjPfjjLDfv67oGX5nCEbezRStiWfurI0QsMSOLyU~QYA8Dh-6eBAMCC7x6nKX8Q9iwokIbEOXVgT1hYrq3RwhOwbHyhFFGybckPXdGtQBrvxXIfcn3oxn20dw8huD2tcMeSZvaluQc1THTiB0nv5OeeNhQ7EhWJDeToTr1gnr1wjB0-M6AAwXsDFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. (A) PF4 dose-dependently inhibits bFGF- (left) and EGF-induced (right) DNA synthesis in endothelial cells. HUVEC were plated in 96-well dishes as described in Materials and Methods. After reaching confluence they were kept in starvation medium for 24 hours, then stimulated with growth factor in the presence of the indicated concentrations of PF4. [3H]-thymidine incorporation was evaluated after 20 hours and was expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate). Data shown are mean values ± SE of three independent experiments performed in triplicate. (B) Heparin and protamine inhibit bFGF- but not EGF-stimulated proliferation of HUVEC. HUVEC were treated as in (A), except that bFGF was used at 2 ng/mL, EGF at 20 ng/mL, and PF4 at 2 μg/mL, in the presence of the indicated concentrations of heparin (H) or protamine. Data are expressed as a percent of the maximum incorporation achieved with the highest dose of growth factor in the absence of PF4 (ordinate), and are mean values ± SE of three independent experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.25/5/m_blod40147001bx.jpeg?Expires=1766400973&Signature=KKen-rijVLheO8hlg5JfLCV~f87lJ1LLlO0KKADAPP3IQlx57vanV9UKerGJBf4FqNLVypDZz5gvYFSy0IZbGF6D1q1aL6byfA97XfOq3ewMtfmoRj~YAKx5nqFt9ajxEN5Eq8tbyVOtuWej0nJqYxolFsjAMeGnE~Q35SFnH2ZxtPhrarsoZad2tHOwOnDdeqVKTfHbL7rMbRbk10c6OZqDSL7uyLzwzr-bkkSpQHNCOnc5FtPt2z6rc9vx-NGzlPSd05Qk9~p1DEZoDXaMMsD88ZpLdj830ag1alxNYK9h7AaTGhVmt6qHDADq9Mjmt~LYriRldDhOcLFPucdpdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. Enhanced association of cdk2 with p21Cip1/WAF1 occurs in EGF-stimulated HUVEC treated with PF4. (A) Immunoprecipitated cdk2, from HUVEC lysate, collected 15 hours after EGF stimulation in the absence (−) or presence (+) of 2 μg/mL PF4, was assayed with GST-pRB in the presence of [γ32P]ATP/ATP as described in Materials and Methods. Phosphorylated GST-pRb was then analyzed by SDS-PAGE and autoradiography and was quantified with an AMBIS scanner. (B) cdk2 levels in aliquots of the samples used to generate the results shown in panel (A), were determined by SDS-PAGE and immunoblotting. (C) The quantity of cyclin E immunoprecipitated with cdk2 was determined by SDS-PAGE followed by immunoblot analysis. (D) p21Cip1/WAF1associated with cdk2 was similarly assayed. The results shown are representative of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/1/10.1182_blood.v93.1.25/5/m_blod40147003w.jpeg?Expires=1766400973&Signature=fpTKp9q9jBP0CTA~hd77A8Z9alzlLlNbMGiUFXAPAn7EFFr9uq4cqmu70e1HDvoXYbBSvCW4WS70juFZH4es6sgSeoaN7SRJ6BPrLipUlbN1j1sISBIhw2ZO3xd~rG3TiWRBREHYiVjEt9FuhHjGlZINK10seR4wyU7yYDZfyTaPnMeeEzFSmSp3vhvNs5yoR3hPONsv5cgQMVRmmTBDr7gS2tSA99W0us6pug0Ozu6Yb6UNE8-k7-4ZlyEdZZgp8mx6WEk2kcV5yB06n7awK6Zw3Wn0HYKtkKfCCRQ7EaB11IhpbntIcrmjnAiYrzZtyMvOnLtYBJTBni~zd9ThLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)