Abstract
We examined the potential involvement of two CC chemokine receptors (CCRs), CCR-1 and CCR-3, in the functional activation of granulocyte-macrophage colony-stimulating factor (GM-CSF) plus interleukin-4 (IL-4)–generated human peripheral blood monocyte-derived immature dendritic cells (DCs). Flow cytometric analysis showed that CCR-1, CCR-3, CCR-5, and CXC chemokine receptor (CXCR)-4 were expressed on the cell surface of monocyte-derived DCs. Treatment with a monoclonal antibody (MoAb) to either CCR-1 or CCR-3 but not MoAbs to CCR-5 and CXCR-4 abolished chemotactic migration of monocyte-derived DCs. The DCs treated with either the anti–CCR-1 MoAb or anti–CCR-3 MoAb were less efficient than untreated DCs in proliferation of allogeneic T cells (TCs) and TC-derived secretion of interferon-γ (IFN-γ). The homotypic aggregation of DCs and heterotypic aggregation of DCs with TCs were suppressed by the anti–CCR-1 MoAb or anti–CCR-3 MoAb. These results indicate that CCR-1 and CCR-3 specifically regulate interaction of TCs and DCs in the process of antigen presentation.
DENDRITIC CELLS (DCs) are unique professional major antigen (Ag)-presenting cells (APCs) capable of stimulating resting T cells (TCs) in the primary immune response and are more potent APCs than peripheral blood monocytes or B cells.1 DCs are also critically involved in the autoimmune diseases, graft rejection, and human immunodeficiency virus infection and the generation of T-cell–dependent antibodies (Abs).1-4 They capture and process Ag in nonlymphoid tissues and then migrate to T-cell–dependent areas of secondary lymphoid organs through afferent lymph or the blood stream to prime native TCs and initiate the immune response.5 During this process, DCs lose Ag-capturing/processing ability as they differentiate into mature, fully stimulatory APCs.6
The characterization of DCs is difficult because they represent only a small subpopulation that includes interdigitating reticulum cells in lymphoid organs, blood DCs, Langerhans cells in the epidermis of the skin, and dermal DCs.1 Recently, an in vitro culture system that allows progenitors in peripheral blood, bone marrow, and cord blood to differentiate into DCs has been established, and it has shown the basic mechanisms underlying the properties of DCs.7-13 DCs originate from CD34+pluripotent hematopoietic progenitor cells in the bone marrow and cord blood.7 14-17
The chemokines are crucially involved in inflammatory and immunological responses via their unique capacity to recruit selective leukocyte subsets.18-21 The chemokines have also been implicated not only in regulation of normal leukocyte recirculation and homing but also in certain physiological and pathogenic processes, including hematopoiesis, angiogenesis, allergy, autoimmune diseases, and viral infectious diseases.22 Chemokines are a group of approximately 70 to 90 amino acid structually related polypeptides, most of which contain four conserved cysteine residues in their primary amino acid sequence.23,24 There are two major groups: the CXC chemokines in which the two NH2-terminal cysteines are separated by a single amino acid and the CC chemokines, in which the two NH2-terminal cysteines are adjacent. A third type of chemokine, represented by lymphotactin, contains only two of the four conserved cysteines.23 24
The specific effects of chemokines on the target cell types are mediated by a family of G-protein–coupled seven-transmembrane receptors.22 Ligand specificities of 12 chemokine receptors have thus far been identified; 4 of the receptors are specific for CXC chemokines (CXCR1-4),22 6 of them are specific for CC chemokines (CCR1-6),2 and the Duffy antigen receptor (DARC) binds both CC and CXC chemokines.25 In addition, distinct chemokines appear to act on more than one receptor type in vitro.22 However, there is increasing evidence to suggest that this redundancy does not occur in vivo.26
Recent reports have shown that several CC and CXC chemokines receptors were found to be expressed on some DCs at the transcriptional level,27-29 and these molecules have been implicated to mediate in part the trafficking of DCs from blood to tissues and then to lymph nodes, where they form a close association with TCs in the process of Ag presentation. However, the precise roles of these chemokine receptors in the process of DC-mediated activation of TCs remain still unknown due to the difficulty in analyzing these phenomena using available materials such as specific monoclonal antibody (MoAb) to these molecules.
To better understand how chemokine receptors expressed on the cell surface of DCs regulate their APC functions, we therefore generated MoAbs specific to two human CC chemokine receptors, CCR-1 and CCR-3 (Kawasaki et al, manuscript submitted). In this report, we examined the potential roles of CCR-1 and CCR-3 in the process of monocyte-derived DC-mediated TC activation. We showed here, in this respect, that the two CC chemokine receptors, CCR-1 and CCR-3, were involved in the APC function of immature DCs derived from peripheral blood monocytes by using exogenous granulocyte-macrophage colony-stimulating factor (GM-CSF) plus interleukin-4 (IL-4). Our results suggest that the chemokines and their respective receptors play a crucial role in APC function of DCs.
MATERIALS AND METHODS
Media and reagents.
The medium used throughout was RPMI 1640 supplemented with 2 mmol/L L-glutamine, 50 μg/mL streptomycin, 50 U/mL penicillin, and 10% heat-inactivated fetal calf serum (FCS). GM-CSF was kindly provided by Kirin Brewery (Tokyo, Japan). IL-4 and regulated on activation normal T-cell expressed and secreted (RANTES) were purchased from Pepro Tech (London, UK). Fluorescein isothiocyanate-labeled dextran (FITC-DX) and lucifer yellow (LY) were purchased from Molecular Probes, Inc (Eugene, OR). Phorbol myristate acetate (PMA) and ionomycin (IoM) were purchased from Sigma (St Louis, MO). The specific MoAbs to CCR-5 and CXCR-4 were purchased from Pharmingen (San Diego, CA).
Preparation of MoAbs to CCR-1 and CCR-3.
cDNA for CCR-1 and CCR-3 were polymerase chain reaction (PCR)-amplified from cDNA that had been reverse-transcribed from total RNA of human peripheral blood mononulear cells (PBMCs) stimulated by 5 μg/mL phytohemagglutinin (Sigma) for 3 days. Amplified cDNA fragments were subcloned into EF-1a promoter-driven mammalian expression vector30 and stably expressed in mouse preB-cell lymphoma B300-19.31 The expression of CCR-1 was flow cytometrically verified by staining with rabbit anti–CCR-1 antiserum kindly provided to us by Dr K. Matsushima (University of Tokyo, Tokyo, Japan).32 The expression of CCR-3 was confirmed by ligand-induced calcium influx. Female BALB/c mice were intraperitoneally immunized with 1 × 107transfectants 6 times in total over a 3-month period. Three days after final immunization, mice were killed and spleen cell suspension was fused with P3XAg8.33 Supernatant of growing hybridomas was selected for its ability to positively stain transfectants and negatively stain wild-type B300-19 cell. Anti–CCR-1 clone #141-2 and anti–CCR-3 clone #444-11 were chosen. Both clones were IgG1κ isotype.
In vitro generation and culture of human DCs.
DCs were generated from PBMCs as described previously,8-10with some modification. Briefly, PBMCs were obtained from 30 mL of leukocyte-enriched buffy coat from healthy donors by centrifugation with use of Ficoll-Hypaque (Pharmacia Fine Chemicals, Uppsala, Sweden), and the light-density fraction from the 42.5% to 50% interface was recovered. The cells were resuspended in culture medium and allowed to adhere to 6-well plates. After 2 hours at 37°C, nonadherent cells were removed and adherent cells (∼90% CD14+ cells) were cultured in 3 mL of medium supplemented with GM-CSF (50 ng/mL) and IL-4 (250 ng/mL). After 7 days of culture, DCs were harvested, washed, and used for subsequent experiments. The resulting cell preparation contained more than 90% DCs as assessed by morphology and fluorescence-activated cell sorting (FACS) analysis. The cell differentiation was monitored by light microscopy.
Isolation of TCs from PBMCs.
Peripheral blood TCs were prepared using a T-cell enrichment immunocolumn (Biotex Laboratories, Inc, Edmonton, Alberta, Canada) from leukocyte-enriched buffy coat as described above. T-cell preparations were typically greater than 90% pure by anti-CD3 MoAb.
Flow cytometry.
For surface marker analysis, DCs were cultured with one of the following MoAbs conjugated to FITC or phycoerythrin (PE) for direct fluorescein: CD1a (Coulter Immunology, Hialeah, FL); CD3, CD4, CD11c, CD14, and HLA-DR (all from Becton Dickenson, Mountain View, CA); and CD40, CD80, CD86, CCR-5, and CXCR-4 (all from Pharmingen). Cells were also stained with the corresponding FITC- or PE-conjugated isotype-matched control MoAb (all from Becton Dickinson). In indirect staining, cells were incubated with the anti–CCR-1 MoAb, anti–CCR-3 MoAb, or biotin-conjugated anti-CXCR-4 MoAb for 30 minutes at 4°C, washed twice with cold phosphate-buffered saline (PBS), and subsequently stained with FITC-conjugated antimouse IgG (Becton Dickinson) or FITC-conjugated avidin (Becton Dickinson) for 30 minutes at 4°C. Thereafter, the cells were washed twice and suspended in PBS containing 0.2 μg/mL propidium iodide (Sigma) to allow exclusion of dead cells. Analysis of fluorescence staining was performed with a FACSCalibur flow cytometer (Becton Dickinson) and CELLQuest Software.
Preparation of culture conditioned medium (CM).
Culture CM was prepared as follows. TC-conditioned media were obtained from culture of purified TCs (5 × 107) unstimulated or stimulated with PMA (50 ng/mL) plus IoM (500 ng/mL) in 5 mL of serum-free medium for 24 hours at 37°C. TCs/DCs-conditioned medium was prepared from coculture of TCs (5 × 107) and DCs (5 × 106) in 5 mL of serum-free medium for 5 days at 37°C. DCs-conditioned medium was obtained from culture of DCs (107) in 5 mL of serum-free medium for 3 days at 37°C. Each supernatant was collected, and cell-free supernatants were obtained after centrifugation at 800g for 5 minutes and passage through a 0.22-μm filter (Milipore Corp, Bedford, MA) and then stored at −20°C before use.
Assay for chemotaxis.
The in vitro migration of cells prepared as described above in response to RANTES (1 μg/mL) or CMs prepared as described above was assessed in a Transwell cell culture chamber (Costar 3422; Costar, Cambridge, MA) as described previously,28 with some modification. In brief, polycarbonate filters with 8.0-μm pore size (Nucleopore, Pleasanton, CA) were precoated with 5 μg of gelatin in a volume of 50 μL on the lower surface and dried overnight at room temperature. The coated filters were washed in PBS and then dried immediately before use. DCs were pretreated with various concentrations from 0.1 to 10 μg/mL of the MoAbs to CCR-1, CCR-3, CCR-5, CXCR-4, or control IgG (cont. IgG; Sigma) for 30 minutes at 37°C, and 100 μL of the cell suspension (106) was added to the upper compartment of the chamber. RANTES or CM diluted in serum-free culture medium was loaded in the lower compartment. After 2 hours of incubation, the filters were fixed with methanol and stained with hematoxylin and eosin. The cells on the upper surface of the filters were removed by wiping with cotton swabs. The cells that had migrated to various areas of the lower surface were manually counted under a microscope at a magnification of ×400, and each assay was performed in triplicate. The data were expressed as the number of migrated cells per field.
Endocytosis assay with FITC-DX and LY.
The methods used to determine the endocytotic activity of in vitro-generated DCs have previously been described.10Briefly, FITC-DX or LY was added to a final concentration of 1 mg/mL to the cells, and the cells were cultured for 60 minutes at 37°C. After incubation, cells were washed four times with ice-cold PBS and analyzed by flow cytometry as described above.
Mixed leukocytes reaction (MLR) and detection of interferon-γ (IFN-γ) by enzyme-linked immunosorbent assay (ELISA).
Responding TCs (105) from an unrelated individual (allogeneic MLR) in the presence or absence of various concentrations from 0.1 to 10 μg/mL of anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG were cultured in 96-well flat-bottom microplates (Costar) with different numbers of monocyte-derived DCs. Thymidine incorporation was measured on day 5 by an 18-hour pulse with 0.5 μCi/well of [3H]thymidine (1 μCi/well; specific activity, 5 Ci/mmol; Amersham Life Science, Buckinghamshire, UK). The culture in each well was monitored by light microscopy. In another experiment, the culture supernatant of each well was collected, and assay for IFN-γ production was performed. IFN-γ was detected in the supernatants using a two-site sandwich ELISA (Endogen, Woburn, MA). Samples were analyzed in serial twofold dilutions in duplicate; the sensitivity of the assay was 2 pg/mL.
RESULTS
Cell surface expression of CCR-1 and CCR-3 in monocyte-derived DCs.
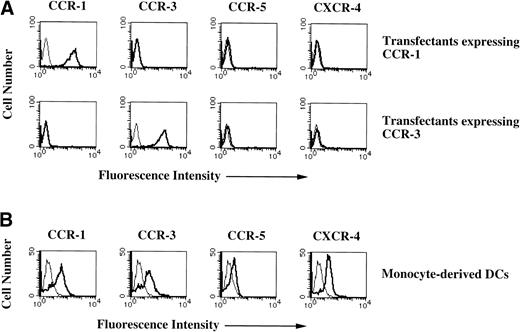
The regulated interactions of TC-derived chemokines with their respective receptors expressed on DCs are thought to mediate the controlled recruitment of DCs from resident sites in nonlymphoid organs into the lymph nodes and spleen to initiate the primary T-cell–dependent immune response of DCs. However, little is known about the involvement of chemokine receptors expressed on DCs in their APC functions. In an attempt to clarify the role of chemokine receptors underlying APC functions of human peripheral blood immature DCs, we generared MoAbs to both human CCR-1 and CCR-3 (Kawasaki et al, manuscript submitted). To investigate specific recognition of MoAbs to CCR-1 and CCR-3 for the respective CCRs, the cell surface expression levels of CCR and CXCR in the transfectants expressing respective CCR-1 or CCR-3 were examined. Figure 1A shows that transfectants expressing CCR-1 or CCR-3 exclusively expressed respective CCR. These results indicate that MoAbs to CCR-1 and CCR-3 specifically recognize the respective CCRs.
Cell surface expressions of chemokine receptors in monocyte-derived DCs. (A) Chemokine receptors expression levels in the transfectants expressing CCR-1 or CCR-3. The transfectants were stained with anti–CCR-1 MoAbs, anti–CCR-3 MoAbs, biotin-conjugated anti–CXCR-4 MoAbs, or FITC-conjugated anti–CCR-5 MoAb (thick lines) or FITC-conjugated mouse Ig (thin lines) for 30 minutes at 4°C. In an indirect staining, the cells were subsequently stained with FITC-conjugated antimouse IgG or FITC-conjugated avidin for 30 minutes at 4°C. (B) Expression levels of chemokine receptors in monocyte-derived DCs. Monocyte-derived DCs were stained with MoAbs to the respective chemokine receptors as described above. The cell surface expression was analyzed by flow cytometry. The results are representative of three experiments performed with similar results.
Cell surface expressions of chemokine receptors in monocyte-derived DCs. (A) Chemokine receptors expression levels in the transfectants expressing CCR-1 or CCR-3. The transfectants were stained with anti–CCR-1 MoAbs, anti–CCR-3 MoAbs, biotin-conjugated anti–CXCR-4 MoAbs, or FITC-conjugated anti–CCR-5 MoAb (thick lines) or FITC-conjugated mouse Ig (thin lines) for 30 minutes at 4°C. In an indirect staining, the cells were subsequently stained with FITC-conjugated antimouse IgG or FITC-conjugated avidin for 30 minutes at 4°C. (B) Expression levels of chemokine receptors in monocyte-derived DCs. Monocyte-derived DCs were stained with MoAbs to the respective chemokine receptors as described above. The cell surface expression was analyzed by flow cytometry. The results are representative of three experiments performed with similar results.
To examine the mechanism by which CCR-1 and CCR-3 exerted in DCs, we established immature DCs from human peripheral blood monocytes with the use of GM-CSF (50 ng/mL) plus IL-4 (250 ng/mL). These cells have a typical dendritic morphology, a phenotype (CD1a+, CD4+, CD11c+, CD14−, CD40+, CD80+, CD86+, and HLA-DR+), a high pinocytic capacity for FITC-DX or LY, and high capacity to cause allogeneic TCs to proliferare (data not shown). Recent studies have shown that DCs express the transcripts for several chemokine receptors27-29; however, their cell surface expression levels remain still unknown. Therefore, we first examined the cell surface expressions of CCR-1 and CCR-3, CCR-5, and CXCR-4 in monocyte-derived DCs with the use of MoAbs to respective chemokine receptors. Flow cytometric analysis showed that monocyte-derived DCs exhibited various expression levels of both CCR-1 and CCR-3 (Fig 1B). On the other hand, we observed that another CC chemokine receptor, CCR-5, was expressed at a low level on monocyte-derived DCs and that these cells also expressed CXCR-4 (Fig 1B).
Previous studies have shown that CCR-1 is predominantly expressed on peripheral blood TCs,32 whereas CCR-3 was found to be expressed only on T-helper 2 (Th2) cells.34 We observed that the MoAb to CCR-1 stained a large population of peripheral blood TCs, whereas the MoAb to CCR-3 weakly reacted with the bulk population and strongly stained only a small proportion (<5%) of peripheral blood TCs (data not shown).
MoAbs to CCR-1 and CCR-3 suppress DC chemotactic migration.
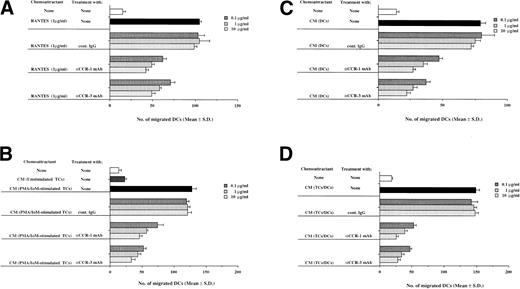
The trafficking of DCs from blood to tissues and then to lymph nodes has been thought to be mediated in part by members of the chemokine superfamily.28 To assess whether CCR-1 and CCR-3 were involved in trafficking properties of monocyte-derived DCs, the effect of MoAb to CCR-1 and CCR-3 on the ability of monocyte-derived DCs to migrate in response to stimuli by various chemoattractants was examined using a Transwell cell culture chamber. Previous reports have demonstrated that RANTES, a member of the CC chemokine family, acts as a potent and selective chemoattractant for monocytes, memory T cells, natural killer cells, eosinophils, and DCs and possesses binding affinity and fidelity to CCR-1 and CCR-3.22 To assess whether monocyte-derived DCs migrated to RANTES via CCR-1 and/or CCR-3, we examined the effects of MoAbs to CCR-1 and CCR-3 on the migratory capacity of monocyte-derived DCs in response to RANTES. Figure 2A shows that both the anti–CCR-1 MoAb and anti–CCR-3 MoAb inhibited chemotactic migration of monocyte-derived DCs to RANTES in a dose-dependent manner. On the other hand, treatment of peripheral blood TCs with either of these MoAbs had a suppressive effect on their migratory capacity to RANTES, although these inhibitory effects were not significant (data not shown). To address the role of other chemokine receptors in chemotactic migratory capacities of monocyte-derived DCs, we examined the effects of MoAbs to CCR-5 and CXCR-4 on chemotactic migration of monocyte-derived DCs to RANTES, because CCR-5 also exhibited binding affinity and fidelity to RANTES, whereas CXCR-4 acts as a receptor for stromal cell-derived factor-1.22 The treatment of monocyte-derived DCs with anti–CCR-5 MoAb slightly inhibited the chemotactic migration to RANTES, whereas anti–CXCR-4 MoAb had little or no suppressive effect on the chemotactic migratory capacities of these cells (Table 1). We further examined the combinations of MoAbs to CCR-1, CCR-3, and CCR-5 on the migratory capacity of monocyte-derived DCs to RANTES. The combinations of these two or three MoAbs to CC chemokine receptors exhibited greater inhibition on the migratory capacity of monocyte-derived DCs to RANTES than those of each MoAb to CCRs (Table 1). These results indicate that CCR-1, CCR-3, and CCR-5 contribute to the migratory capacity of monocyte-derived DCs to RANTES.
CCR-1 and CCR-3 regulate the migratory capacity of monocyte-derived DCs. Monocyte-derived DCs (106) were pretreated with the indicated concentrations of anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG for 30 minutes at 37°C and seeded on the filters precoated on the lower surface with 5 μg of gelatin. (A) RANTES (1 μg/mL), (B) CM derived from unstimulated or PMA (50 ng/mL) plus IoM (500 ng/mL)-stimulated TCs, (C) CM derived from coculture of TCs and DCs, and (D) CM derived from culture of DCs used as a chemoattractant were added to the lower chamber. After 2 hours of incubation, the cells that migrated to the lower surface were visually counted.
CCR-1 and CCR-3 regulate the migratory capacity of monocyte-derived DCs. Monocyte-derived DCs (106) were pretreated with the indicated concentrations of anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG for 30 minutes at 37°C and seeded on the filters precoated on the lower surface with 5 μg of gelatin. (A) RANTES (1 μg/mL), (B) CM derived from unstimulated or PMA (50 ng/mL) plus IoM (500 ng/mL)-stimulated TCs, (C) CM derived from coculture of TCs and DCs, and (D) CM derived from culture of DCs used as a chemoattractant were added to the lower chamber. After 2 hours of incubation, the cells that migrated to the lower surface were visually counted.
Effect of MoAbs to Various Chemokine Receptors on Migratory Capacity of Monocyte-Derived DCs
| Chemoattractant . | Treatment With . | No. of Migrated Cells . |
|---|---|---|
| Mean ± SD . | ||
| None | None | 7 ± 2 |
| RANTES | None | 111 ± 10 |
| Cont. IgG | 114 ± 5 | |
| αCCR-1 MoAb | 42 ± 4 | |
| αCCR-3 MoAb | 45 ± 7 | |
| αCCR-5 MoAb | 81 ± 7 | |
| αCXCR-4 MoAb | 108 ± 5 | |
| αCCR-1 MoAb/αCCR-3 MoAb | 24 ± 4 | |
| αCCR-1 MoAb/αCCR-5 MoAb | 35 ± 4 | |
| αCCR-3 MoAb/αCCR-5 MoAb | 33 ± 3 | |
| αCCR-1 MoAb/αCCR-3 MoAb/αCCR5 MoAb | 19 ± 3 |
| Chemoattractant . | Treatment With . | No. of Migrated Cells . |
|---|---|---|
| Mean ± SD . | ||
| None | None | 7 ± 2 |
| RANTES | None | 111 ± 10 |
| Cont. IgG | 114 ± 5 | |
| αCCR-1 MoAb | 42 ± 4 | |
| αCCR-3 MoAb | 45 ± 7 | |
| αCCR-5 MoAb | 81 ± 7 | |
| αCXCR-4 MoAb | 108 ± 5 | |
| αCCR-1 MoAb/αCCR-3 MoAb | 24 ± 4 | |
| αCCR-1 MoAb/αCCR-5 MoAb | 35 ± 4 | |
| αCCR-3 MoAb/αCCR-5 MoAb | 33 ± 3 | |
| αCCR-1 MoAb/αCCR-3 MoAb/αCCR5 MoAb | 19 ± 3 |
Monocyte-derived DCs (106) were pretreated with stated MoAbs (10 μg/mL) for 30 minutes at 37°C and seeded on the filters precoated on the lower surface with 5 μg gelatin. The RANTES (1 μg/mL) used as chemoattractants were added to the lower chamber. After 2 hours of incubation, the migrated cells on the lower surface were visually counted.
The recruitment of DCs from blood to tissue and then to lymph nodes where DCs present antigenic materials to TCs is believed to be mediated in part by identified and/or unknown TC-derived chemokines. We investigated involvement of CCR-1 and CCR-3 in the chemotactic migration of monocyte-derived DCs to CM derived from peripheral blood TC (Fig 2B). CM of PMA/IoM-stimulated peripheral blood TCs induced a migration of monocyte-derived DCs, although the cells showed little or no migratory capacity to CM of unstimulated TCs. Treatment of monocyte-derived DCs with MoAbs to CCR-1 and CCR-3 suppressed chemotactic migration to CM of PMA/IoM-stimulated TCs. These results indicate that CCR-1 and CCR-3 are involved in trafficking of monocyte-derived DCs to TC-derived chemokines.
CCR-1 and CCR-3 regulate DC-mediated triggering activation of TCs.
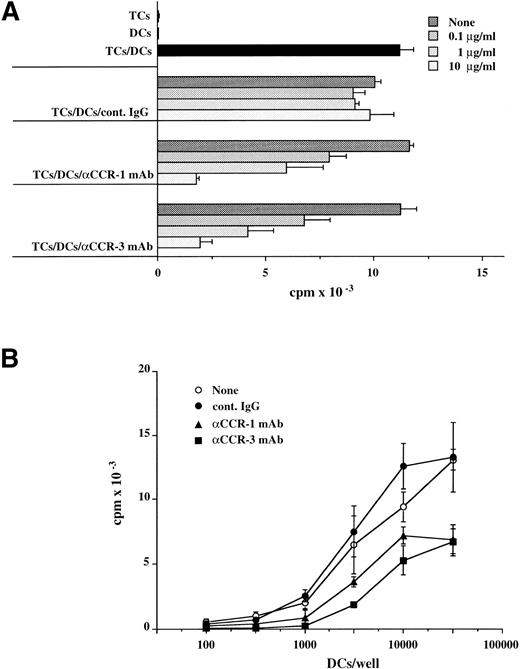
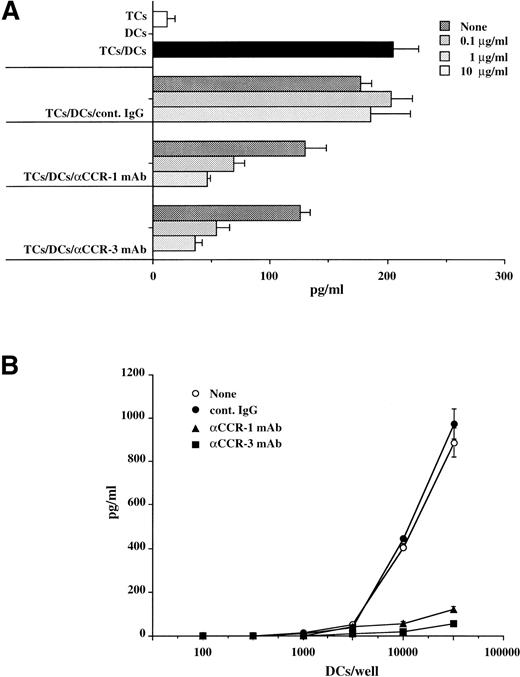
The interaction of DCs with primed TCs via chemokines and their respective receptors as well as adhesion/costimulatory molecules is believed to induce TC activation via APC function. To investigate the role of chemotactic migration between TCs and DCs, we examined the effects of MoAbs to CCR-1 and CCR-3 on the capacity of allogeneic TCs to stimulate monocyte-derived DCs. TCs (105) were cocultured with various numbers of monocyte-derived DCs (102 to 5 × 104) in the presence or absence of indicated concentrations of the anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG ranging from 0.1 to 10 μg/mL, and the proliferative responses of TCs were measured on day 5. MoAbs to CCR-1 and CCR-3 significantly abolished the allostimulatory potential of monocyte-derived DCs (Fig 3A and B), and these results were concomitant with the capacity of DCs to stimulate TC-derived IFN-γ production (Fig 4A and B). We also observed that these MoAbs had little or no effect on cell surface expression levels of CD1a, CD11c, CD40, CD80, CD86, and HLA-DR in these monocyte-derived DCs (data not shown). These results indicate that CCR-1 and CCR-3 expressed on TCs and DCs are potentially involved in DC-mediated TC activation.
MoAbs to CCR-1 and CCR-3 suppress capacity of monocyte-derived DCs to stimulate allogeneic TCs proliferation. TCs purified from PBMCs (105) were cultured (A) with monocyte-derived DCs (104) in the presence of the indicated concentrations of anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG or (B) with different numbers of monocyte-derived DCs in the presence of 10 μg/mL anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG. The proliferative response was measured on day 5. Values are the mean ± SD obtained for triplicate cultures and are representative of those obtained in two individual experiments.
MoAbs to CCR-1 and CCR-3 suppress capacity of monocyte-derived DCs to stimulate allogeneic TCs proliferation. TCs purified from PBMCs (105) were cultured (A) with monocyte-derived DCs (104) in the presence of the indicated concentrations of anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG or (B) with different numbers of monocyte-derived DCs in the presence of 10 μg/mL anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG. The proliferative response was measured on day 5. Values are the mean ± SD obtained for triplicate cultures and are representative of those obtained in two individual experiments.
MoAbs to CCR-1 and CCR-3 suppress capacity of monocyte-derived DCs to stimulate allogeneic TCs-derived IFN-γ secretion. TCs purified from PBMCs (105) were cultured (A) with monocyte-derived DCs (104) in the presence of the indicated concentrations of anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG or (B) with different numbers of monocyte-derived DCs in the presence of 10 μg/mL anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG. The IFN-γ secretion in the culture supernatants was measured by ELISA on day 5. Values are the mean ± SD obtained for triplicate cultures and are representative of those obtained in two individual experiments.
MoAbs to CCR-1 and CCR-3 suppress capacity of monocyte-derived DCs to stimulate allogeneic TCs-derived IFN-γ secretion. TCs purified from PBMCs (105) were cultured (A) with monocyte-derived DCs (104) in the presence of the indicated concentrations of anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG or (B) with different numbers of monocyte-derived DCs in the presence of 10 μg/mL anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG. The IFN-γ secretion in the culture supernatants was measured by ELISA on day 5. Values are the mean ± SD obtained for triplicate cultures and are representative of those obtained in two individual experiments.
CCR-1 and CCR-3 are directly involved in interaction of monocyte-derived DCs with TCs.
To address role of chemokines and their receptors in DC aggregations, we cultured monocyte-derived DCs with allogeneic TCs in the presence or absence of the anti–CCR-1 MoAb, anti–CCR-3 MoAb, or cont. IgG (Fig 5). Using light microscopy, treatment of the cells with these MoAbs was observed to result in suppression of their homotypic aggregation. To further examine the consequences of inhibiting the homotypic aggregation of DCs via CCR-1 and CCR-3, we evaluated the effects of MoAbs to CCR-1 and CCR-3 on the migratory capacity of monocyte-derived DCs to CM derived from DC cultures. Figure2C shows that CM of DCs induced a chemotactic migration of monocyte-derived DCs. On the other hand, treatment of monocyte-derived DCs with MoAbs to CCR-1 and CCR-3 inhibited their migratory capacities. These results indicate that interaction of chemokines derived from DCs with CCR-1 and CCR-3 expressed on DCs in part contributes to DC aggregations.
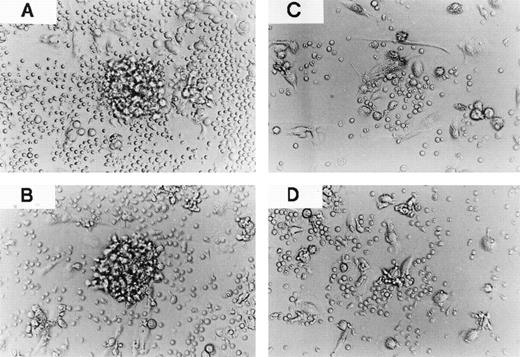
CCR-1 and CCR-3 are involved in clustering of monocyte-derived DCs with TCs. TCs purified from PBMCs (105) were cultured with monocyte-derived DCs (104) in the absence (A) or presence of 10 μg/mL cont. IgG (B), anti–CCR-1 MoAb (C), or anti–CCR-3 MoAb (D). The coculture of monocyte-derived DCs with TCs was measured on day 5. Original magnification × 200. The results are representative of two experiments performed with similar results.
CCR-1 and CCR-3 are involved in clustering of monocyte-derived DCs with TCs. TCs purified from PBMCs (105) were cultured with monocyte-derived DCs (104) in the absence (A) or presence of 10 μg/mL cont. IgG (B), anti–CCR-1 MoAb (C), or anti–CCR-3 MoAb (D). The coculture of monocyte-derived DCs with TCs was measured on day 5. Original magnification × 200. The results are representative of two experiments performed with similar results.
The priming of TC activation by DCs is thought to be mediated by clustering of DCs and TCs. We further evaluated the effect of MoAbs to CCR-1 and CCR-3 on cluster formation between TCs and DCs. As shown in Fig 5, coculture of monocyte-derived DCs with TCs in the presence of the anti–CCR-1 MoAb and anti–CCR-3 MoAb, but not cont. IgG, resulted in heterotypic aggregation of monocyte-derived DCs with TCs. To clarify the CCR-1– and CCR-3–mediated mechanisms underlying formation of TC-DC aggregation, we performed a chemotactic migration assay of monocyte-derived DCs to CM derived from coculture of monocyte-derived DCs with TCs (Fig 2D). We found that CM derived from coculture of monocyte-derived DCs with TCs promoted the migration of DCs, and MoAbs to CCR-1 and CCR-3 significantly inhibited their mobility in a dose-dependent manner. These results indicate that CCR-1 and CCR-3 are critically involved in APC function of monocyte-derived DCs.
DISCUSSION
Trafficking of DCs from local nonlymphoid areas to lymphoid tissue is believed to be a crucial event in the process of presentation of antigenic materials to naive or memory TCs. However, little is known about the precise role of chemotactic responses in APC functions of DCs. To clarify the importance of chemotactic events in interaction of DCs with TCs, we generated MoAbs to CCR-1 and CCR-3 (Kawasaki et al, manuscript submitted). The present study has demonstrated that monocyte-derived DCs expressed CCR-1 and CCR-3 as well as CCR-5 and CXCR-4 on the cell surface, treatment of DCs with anti–CCR-1 MoAb or anti–CCR-3 MoAb, but not anti–CCR-5 MoAb or anti-CXCR-4 MoAb, markedly inhibited allogeneic T-cell responses, including proliferation and IFN-γ secretion via suppression of chemotactic migration between TCs and DCs.
A series of recent studies showed that several CCRs or CXCRs were constitutively expressed on a group of DCs at the transcriptional level.27-29 To the best of our knowledge, we are the first to have detected CCR-1, CCR-3, CCR-5, and CXCR-4 on the cell surface of monocyte-derived DCs by flow cytometric analysis using MoAbs to respective CCRs or CXCR (Fig 1). On the other hand, peripheral blood TCs also constitutively expressed CCR-1, although the cells possessed relatively little expression levels of CCR-3 (data not shown). A previous report showed that CCR-1 was expressed on the surface of peripheral blood TCs,32 whereas CCR-3 was selectively expressed on the subset of Th2 cells.35 These data suggest that CCR-1 and CCR-3 expressed on subset of TCs and DCs may be involved in Ag presentation.
Previous studies have shown that subsets of DCs migrate in response to various sets of chemokines.28 35-37 We showed that monocyte-derived DCs exhibited a migratory response to RANTES and MoAbs to CCR-1 or CCR-3 significantly suppressed it, although not completely (Fig 2A). Furthermore, MoAb to CCR-5 slightly inhibited chemotactic migratory actions of monocyte-derived DCs, whereas MoAb to CXCR-4 did not suppress it (Table 1). These results indicate that monocyte-derived DCs migrated to RANTES via CCR-1, CCR-3, and CCR-5. We also showed that the combinations of these two or three MoAbs to CC chemokine receptors did not completely suppress the RANTES-mediated chemotactic migration of monocyte-derived DCs, although these combinations elicited greater inhibition on it than those of each MoAb. These results imply that other chemokine receptor(s) may also act as a receptor for RANTES on monocyte-derived DCs.
We also observed that these MoAbs inhibited chemotactic migration of monocyte-derived DCs to CM-derived PMA plus IoM-stimulated TCs (Fig2B). These results imply that chemokines secreted by TCs and their respective receptors, including CCR-1 and CCR-3 expressed on DCs, may directly regulate chemotactic migration of DCs to TCs.
Recent studies have shown that TCs and DCs can secrete various sets of CXC- or CC-chemokines.28 35-37 We observed that CM-derived coculture of TCs and DCs caused DCs to migrate, and both the anti–CCR-1 MoAb and anti–CCR-3 MoAb suppressed these migratory events (Fig 2C). These results suggest that chemokines produced by TCs and DCs and CCR-1 and CCR-3 expressed on these cells may contribute to chemotactic migratory events in the process of Ag presentation.
DCs found in TC-dependent areas have been implicated to interact with TCs via Ag presentation, followed by activation of TCs to proliferate in secondary lymphoid tissue. We showed that MoAbs to CCR-1 or CCR-3 significantly suppressed the capacity of DCs to stimulate proliferation of allogeneic TCs and producion of IFN-γ (Figs 3 and4). These phenomena led us to hypothesize that CCR-1 and CCR-3 were crucially involved in the capacity of monocyte-derived DCs to activate TCs. Indeed, we observed that heterotypic aggregation of monocyte-derived Dcs with TCs was mostly inhibited by MoAbs to CCR-1 or CCR-3 (Fig 5). Our results imply that suppression of chemotaxis of DCs and TCs by the anti–CCR-1 MoAb or anti–CCR-3 MoAb may subsequently lead to eliminate specific interaction of TCs and DCs, resulting in inhibition of activation of TCs.
In summary, our results indicate that MoAbs to CCR-1 or CCR-3 negatively regulated activation of TCs by monocyte-derived DCs via suppression of their migratory capacity. The capacity of MoAbs to CCR-1 or CCR-3 to inhibit functions of monocyte-derived DCs was markedly, but not completely, suppressed, indicating that other CC or CXC chemokine receptors may also be involved in the functions of DCs. To further characterize the roles of other CC or CXC chemokine receptors, MoAbs to these molecules have been prepared and are now under investigation in our laboratories. The chemokines and their respective receptors play important roles in inflammatory and allergic diseases as well as immune responses. On the other hand, DCs are critically involved in autoimmune diseases, graft rejection, and virus infection.1-4 Thus, the availability of MoAbs to CCR-1 or CCR-3 may be potentially useful for prevention of immune-related diseases.
ACKNOWLEDGMENT
The authors thank H. Takahashi for excellent assistance.
K.S. and H.K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Tsuneo A. Takahashi, DSc, Department of Cell Processing, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan; e-mail: takahasi@ims.u.-tokyo.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal