Abstract
BTI-322, a rat monoclonal IgG2b directed against the CD2 antigen on T cells and natural killer (NK) cells, blocks primary and memory alloantigen proliferative responses in vitro. We have evaluated the pharmacokinetics and safety of BTI-322 during treatment of 20 transplant recipients with steroid-refractory acute graft-versus-host disease (GVHD). Treatment consisted of BTI-322 by intravenous (IV) bolus or 30-minute infusion at approximately 0.1 mg/kg/d for 10 days in addition to continuing high-dose steroids and tacrolimus or cyclosporine. Pharmacokinetic sampling was performed in 10 patients; the t1/2 ± SE was 9.1 ± 1.3 hours, the Cmaxwas 2,549 ± 291 ng/mL, the Vd was 3.97 ± 0.95 L, and the Vd/kg was 0.05 ± 0.01 L/kg. Ten patients experienced transient dyspnea sometimes accompanied by nausea, vomiting, diarrhea, and tachycardia shortly after the initial bolus dose of drug, but serious drug-related adverse events were not seen during the remainder of the infusions. At the end of treatment (day 11), there were six patients with complete responses and five with a reduction in grade of GVHD for a total response rate of 55% (95% confidence interval [CI], 32% to 77%). Antibodies targeting CD2 may be active in the treatment of acute GVHD, and evaluation of a humanized form of BTI-322 is warranted.
THE CD2 MOLECULE (T11, LFA-2) is a 55-kD glycoprotein expressed on thymocytes, mature T lymphocytes, and natural killer (NK) cells.1 It was first identified due to its ability to act as a receptor for sheep erythrocytes,2,3 but its natural ligand was subsequently shown to be LFA-3, a widely-distributed adhesion molecule.4CD2 acts as a costimulatory molecule for antigen-specific lymphocyte activation.5 Stabilization of the cell-cell interaction via the extracellular domain of CD2 on T cells and its ligand on antigen-presenting cells is necessary for this costimulatory function,6 but signaling through the cytoplasmic portion of the molecule is required as well.7 Stimulation of CD2 alone is not sufficient for T-cell activation; signaling through CD2 during the afferent phase of the T-cell response only serves to enhance the response of the T-cell receptor to antigen stimulation.8During the efferent phase of the immune response, CD2 also plays a role in adhesion between activated T cells or NK cells with target cells,9,10 and signaling through CD2 on activated cytotoxic cells stimulates exocytosis of cytotoxic granules.11 12 The participation of CD2 in both the afferent and efferent phases of the immune response makes it an attractive target in development of immunosuppressive drugs.
The extracellular portion of the CD2 molecule can be divided into three regions by epitope mapping.13 Monoclonal antibodies directed against CD2 inhibited adhesion and/or modulated T-cell activation, depending on the region of the cognate epitope. In vitro, anti-CD2 blocking antibodies inhibited T-cell activation when added to the mixed lymphocyte reaction5,9,14 and inhibited cell-mediated cytolysis when added to cytotoxicity assays.4,9 In animal models, when administered in vivo, such antibodies blunted contact sensitivity reactions, inhibited development of both helper and cytotoxic responses to antigen, and prolonged the survival of cardiac and pancreatic islet cell allografts.14-17
BTI-322 (LO-CD2a) is an anti-human CD2 rat monoclonal IgG2b described by Xia et al.18 The antibody recognized ≥90% of E-rosette–forming peripheral blood lymphocytes and T-cell leukemias, but it had no reactivity with B lymphocytes, B-cell leukemia cells, or cells of myeloid origin. The amount of bound BTI-322 was greater on phytohemagglutinin (PHA)-activated lymphocytes than on resting lymphocytes, and it did not inhibit binding of OKT11, Leu5, or MP910, suggesting that BTI-322 recognizes a partially cryptic epitope on CD2 that is expressed more highly after activation.18 BTI-322 itself is not mitogenic for human lymphocytes. In vitro, it inhibited the proliferative response to tetanus toxoid, alloantigen, and stimulation by OKT3, and the proliferative response to alloantigen was blunted even when the antibody was added to the mixed lymphocyte reaction as late as day 4 of culture.18,19 The presence of BTI-322 in primary cultures led to hyporesponsiveness in secondary cultures, suggesting that anergy was induced during culture with BTI-322.20 In clinical studies, BTI-322 was shown to be active for prevention or treatment of renal allograft rejection.21 22 To further evaluate its immunosuppressive activity, we have conducted a Phase II study of BTI-322 for treatment of steroid-refractory acute graft-versus-host disease (GVHD) in allogeneic marrow or blood stem cell transplant recipients.
MATERIALS AND METHODS
Patients.
Allogeneic marrow or blood stem cell recipients with steroid-refractory acute GVHD from five participating transplant programs were enrolled in the study. The protocol was reviewed and approved by the Institutional Review Board at each center, and written informed consent was obtained from each patient. Eligibility criteria included age ≥18 years, evidence of hematopoietic recovery (absolute neutrophil count >0.5 × 109/L), and histologically documented persistent or worsening GVHD of grade ≥2 after 3 to 10 sequential days of treatment with methylprednisolone ≥2 mg/kg/d or its equivalent. Patients were not eligible if they were more than 60 days posttransplant, had more than one allogeneic transplant, were unlikely to survive more than 10 days, were pregnant or nursing, were human immunodeficiency virus (HIV)-1 seropositive, had evidence of active malignancy, required hemodialysis or use of a ventilator, had ascites detectable by physical examination, or had a known hypersensitivity to mouse, rat, or rabbit protein.
Treatment with BTI-322.
BTI-322 0.1 mg/kg rounded up to the nearest milligram was administered intravenously (IV) daily for 10 consecutive days. This dose-schedule was found to be safe and active in phase I/II studies for renal allograft rejection.21 22 Dosing was based on actual body weight. The dose was capped at 10 mg, the highest dose known to be safe for humans. The drug was supplied in phosphate-buffered saline, and the individual daily dose was diluted to a total volume of 50 mL in normal saline (USP). The first dose of BTI-322 was administered by rapid push over 5 minutes for the first nine patients and as a 30-minute infusion for the next 11 patients. The remainder of the doses were administered as 30-minute infusions. Diphenhydramine 50 mg IV or orally and acetaminophen 650 mg orally were required premedications for the first dose only.
Safety monitoring.
Vital signs were recorded hourly through 4 hours from the start of each infusion for the first three doses and thereafter only as needed. Patients were monitored through 30 days after completion of therapy, and adverse events were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria. Blood cell counts and serum chemistries were performed before treatment and on study days 2, 4, 6, 8, 10, 11, 20, 30, 45, and 100. Urinalyses were performed before treatment and on study days 2, 4, 6, 8, and 10.
GVHD grading and response criteria.
GVHD was graded by the consensus criteria23 modified such that poor performance status was not used to upstage an organ for assignment of grade 4. Response was scored on study day 11. For the overall assessment, a complete response (CR) was defined as stage 0 in all organ systems, and a partial response (PR) was defined as a decrease in at least one grade, but less than a CR and no worsening in any organ system. For assessment of an organ system, a CR required resolution of all manifestations of GVHD in that organ system, and a PR required a decrease in stage in that organ system, but less than a CR. CR and PR together constituted the total response rate.
Measurement of anti–BTI-322 activity.
Anti–BTI-322 by enzyme-linked immunosorbent assay (ELISA) activity was evaluated before the first dose and at 11 and 45 days after the first dose of BTI-322 (BioTransplant Inc, Charlestown, MA). For assessment of anti–BTI-322 antibodies, 96-well plates (MaxiSorp Nunc Immuno Plates, Nalge Nunc International, Rochester, NY) were coated with 50 μL BTI-322 5 μg/mL, serial dilutions of patient or hyperimmune control sera were loaded into the wells, and bound Ig was detected using horseradish peroxidase-conjugated donkey anti-human IgG (Accurate Scientific, Westbury, NY) or rabbit anti-human IgM (Dako, Carpenteria, CA). Color development was achieved using o-phenylenediamine dihydrochloride (Sigma, St Louis, MO), and absorbance was measured at 490 nm using a THERMOmax plate reader (Molecular Devices, Sunnyvale, CA). Samples with antibody detected at ≥1:50 dilution were considered positive.
For assessment of serum neutralizing activity, serial dilutions of patient or hyperimmune control sera were spiked with BTI-322 40 ng/mL and loaded into 96-well plates coated with 100 μL recombinant human CD2 (sCD2; BioTransplant Inc) 2.5 μg/mL. Bound BTI-322 was detected using horseradish peroxidase–conjugated rabbit antirat Ig (Dako). Percent inhibition of binding was calculated as [(SB-C)/SB] × 100 where SB is the mean of the true absorbance of the spiked buffer and C is the true absorbance of the serum dilution. Samples with neutralizing activity detectable at ≥1:50 dilution were considered positive.
Pharmacokinetics.
Disposition parameters were determined in six patients with and four patients without gut GVHD. Blood samples were collected before and at 1, 4, 8, 12, and 24 hours after the first and fourth doses of BTI-322. Serum BTI-322 concentration was measured by ELISA (BioTransplant Incorporated). A 96-well plate was coated with a murine monoclonal antirat IgG2b (MARG2b, IMEX, Brussels, Belgium) and blocked with bovine serum albumin. The plate was loaded with patient sera, and bound BTI-322 was detected using horseradish peroxidase-conjugated mouse antirat Ig (MARK-1 and MARK-3, IMEX) as described above. BTI-322 concentrations were determined by interpolation of sample absorbance against a standard curve constructed using known graded concentrations of BTI-322 in phosphate-buffered saline-Tween. PKAnalyst (MicroMath Software, Salt Lake City, UT) was used to calculate the pharmacokinetic parameters, including volume of distribution (Vd), clearance (Cl), and half-life (t1/2). The data were fitted to a noncompartmental exponential model. Results for doses 1 and 4 were calculated separately. Elimination function was estimated by least squares. Vd was calculated using the concentration at time 0 extrapolated from the elimination function, and plasma Cl was determined. The Vd was also normalized for patient weight. Cmax, the maximal concentration, was the actual value measured at 1-hour postdose.
Lymphocyte immunophenotyping.
Immunophenotyping was performed by flow cytometry using standard techniques at Specialty Laboratories, Inc (Santa Monica, CA). Peripheral blood samples were collected before and 4, 11, 20, 30, 45, and 100 days after the first dose of BTI-322. The absolute lymphocyte count and the absolute numbers of lymphocytes expressing CD2 (OKT11), CD3, CD3/CD4, CD3/CD8, CD16/CD56, and CD19/CD20 were determined for each sample.
Statistical considerations.
The protocol was designed as an open-label phase II study. Treatment of 20 patients allowed detection of at least 15% activity with a beta error of 5%. Analyses were descriptive in nature and no formal tests of hypotheses were performed. Continuous variables were reported as medians or means and standard errors (SE), and categorical data were enumerated. Response rates were reported, and 95% confidence intervals (CI) were calculated using the exact binomial distribution. Categorical data were compared using Fisher’s exact test.
RESULTS
Patient characteristics.
Twenty patients with steroid-resistant acute GVHD were enrolled in the study. Patient characteristics are shown in Table 1. Diagnoses included 8 chronic myelogenous leukemia (5 in first chronic phase), 3 acute lymphoblastic leukemia, 2 acute myelogenous leukemia, 1 myelodysplastic syndrome, 3 multiple myeloma, 2 malignant lymphoma, and 1 breast cancer. All patients had received standard GVHD prophylaxis, and 7 (35%) had T-cell–depleted transplants. For 5 patients, BTI-322 was the second salvage therapy. At baseline, all patients had at least grade 2 GVHD, and 15 (75%) had grades 3-4 GVHD.
Response to therapy.
Response to therapy was assessed on study day 11 (Table 2). Overall, 6 (30%; 95% CI, 12% to 54%) patients had a CR and 5 (25%; 95% CI, 9% to 49%) had a PR for a total response rate of 55% (95% CI, 32% to 77%). High response rates were seen in skin (87%) and gut (54%), but liver GVHD was unaffected by treatment with BTI-322. The total response rate was significantly higher for patients with grade 2 GVHD (5 of 5 v 6 of 15, P = .04), but there was no difference in total response when patients were categorized by transplant processing (7 of 13 with T-cell depletion v 4 of 7 without T-cell depletion), or type of donor (4 of 10 with an HLA-identical donor v 7 of 10 with an alternative donor).
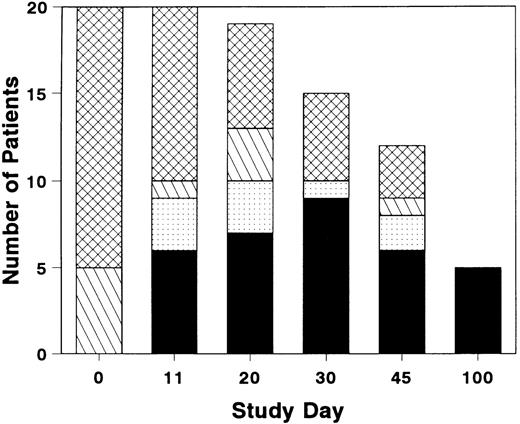
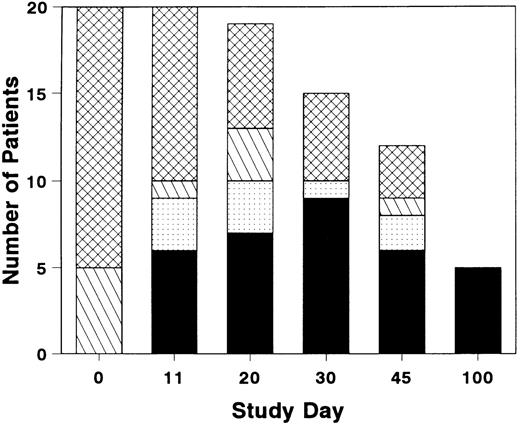
The total number of patients with grade 0 or 1 GVHD was near maximal by study day 11, although GVHD resolved in a few additional patients after study day 11 (Fig 1). Three of the six patients who achieved a CR by study day 11 had a later recurrence of acute GVHD. Five patients who completed the study day 100 assessment were in CR; the assessment was performed on day 99 for one patient who then expired on day 100. Four patients developed chronic GVHD within the study period.
Distribution of grades of GVHD during and after treatment with BTI-322. Height of bar represents the number of patients with grade 0 (▪), grade 1 (▧), grade 2 (), or grade 3-4 (▩). Total number of patients represents evaluable survivors at each evaluation timepoint.
Distribution of grades of GVHD during and after treatment with BTI-322. Height of bar represents the number of patients with grade 0 (▪), grade 1 (▧), grade 2 (), or grade 3-4 (▩). Total number of patients represents evaluable survivors at each evaluation timepoint.
Laboratory correlates.
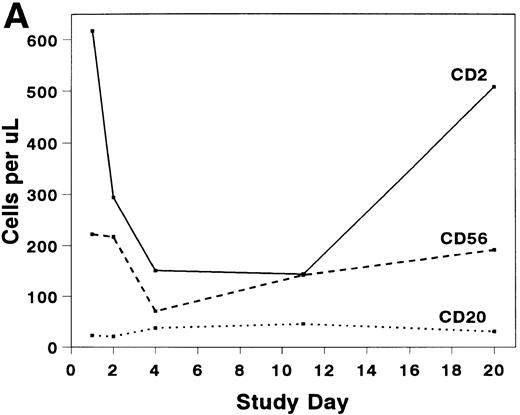
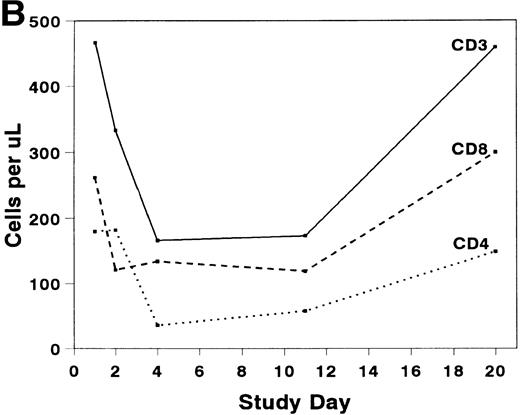
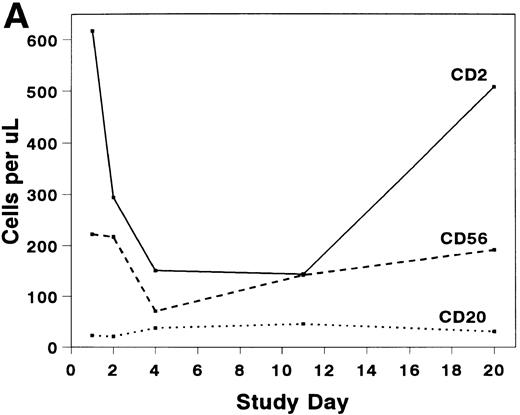
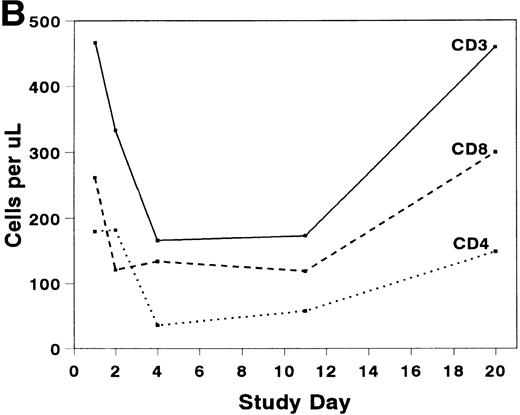
The mean absolute lymphocyte count fell from 0.765 (±0.147) × 109/L pretreatment to 0.48 (±0.138) × 109/L on study day 11 and rose to 0.743 (± 0.220) × 109/L by study day 20. The absolute CD2+ cell count fell rapidly after infusion of BTI-322, remained low throughout the treatment period, and returned to pretreatment levels by study day 20 (Fig2). The decrease in CD2+ cells was not due to inhibition of detection by blockade of CD2 by bound BTI-322, as a similar change in T-cell subsets as measured by other antibodies (CD3, CD4, and CD8) was observed (Fig 2). The absolute CD56+ cell count was affected less, and the number of B cells was relatively unaltered by treatment with BTI-322. There was no correlation between response to therapy and the absolute CD2 count on study day 11.
Absolute numbers of (A) CD2, CD16/56, and CD19/20 or (B) CD3, CD3/4, and CD3/8 lymphocytes in peripheral blood during and after treatment with BTI-322.
Absolute numbers of (A) CD2, CD16/56, and CD19/20 or (B) CD3, CD3/4, and CD3/8 lymphocytes in peripheral blood during and after treatment with BTI-322.
Pharmacokinetics of BTI-322.
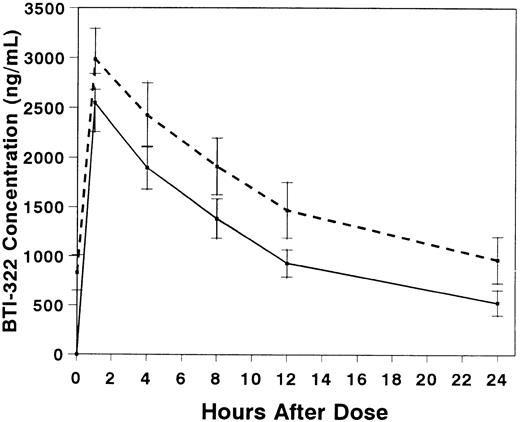
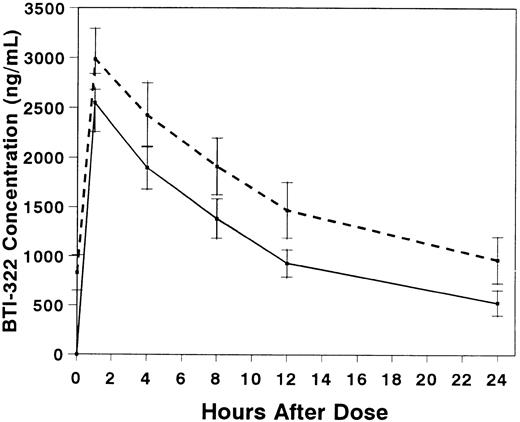
Pharmacokinetic studies were performed in 10 patients, 6 with and 4 without gut GVHD (Table 3). The presence of gut GVHD did not appear to affect the pharmacokinetics of BTI-322. Serum concentrations of BTI-322 increased rapidly after infusion, and the concentration curves essentially decreased in parallel after the first and fourth doses (Fig 3). The t1/2 was 9.1 hours with the first dose and 11.2 hours with the fourth dose (P = not significant [NS]). The mean Vd exceeded ideal plasma volume with the first dose and approached ideal plasma volume with the fourth dose.
Serum concentrations (mean ± SE) of BTI-322 after infusion of the first (solid line) or fourth (dashed line) dose of drug. Data are displayed for all 10 patients who had pharmacokinetic sampling.
Serum concentrations (mean ± SE) of BTI-322 after infusion of the first (solid line) or fourth (dashed line) dose of drug. Data are displayed for all 10 patients who had pharmacokinetic sampling.
Safety evaluation.
One patient received only four doses of BTI-322; treatment was discontinued because of progressive GVHD. The remainder of the patients received all 10 doses according to the treatment schedule. A total of 930 adverse events were reported; 156 were considered severe or life-threatening (Table 4). No patient manifested a posttransplant lymphoproliferative disorder through study day 100.
Eleven (55%) patients had an adverse event related to BTI-322. Of the total 930 adverse events, there were 44 (5%) considered related to BTI-322. Twenty-two (2.4%) adverse events related to BTI-322 were mild, 19 (2.0%) were moderate, three (0.3%) were severe, and none was life-threatening. Eighty-nine percent of the adverse events related to BTI-322 occurred with the first dose in 10 patients. These patients experienced dyspnea sometimes accompanied by nausea, vomiting, diarrhea, hypertension, and tachycardia after infusion of the first dose of BTI-322; four had chills, and two had fever. The intensity of the reaction was less with 30-minute infusion than with bolus dosing. The reaction was transient and did not preclude subsequent dosing. A typical cytokine release syndrome was not seen.
One patient developed graft failure. This patient had an absolute neutrophil count of 0.7 × 109/L before treatment with BTI-322 and >1.0 × 109/L after completion of therapy. However, the absolute neutrophil count had been persistently <0.5 × 109/L when the patient died of progressive GVHD and sepsis on study day 38. The only other hematologic abnormality noted in the study patients was lymphopenia (see above).
There were no abnormalities in serum chemistries that could be ascribed to BTI-322 treatment. No IgG or IgM anti-BTI-322 antibodies were found in the 26 samples tested after therapy. Neutralizing activity of undetermined nature was detected in one patient’s pretreatment sample. This patient died on study day 19 of progressive liver GVHD and sepsis. Neutralizing activity was not found in any of the posttreatment samples.
Causes of death.
Four patients were alive on study day 100 for a survival rate of 20% (95% CI, 6% to 44%). Sixteen patients died; 7 had persistent or recurrent GVHD, 3 had GVHD with infection, and 6 died with infection after resolution of GVHD.
DISCUSSION
Although antithymocyte globulin (ATG) is widely accepted as primary treatment of steroid-refractory acute GVHD, overall response rates are only 29% to 54%,24-26 and new strategies are required. Use of BTI-322, a monoclonal antibody directed against CD2, for treatment of GVHD was an attractive therapeutic approach, as in vitro experiments demonstrated that anti-CD2 antibodies blocked both primary and memory alloantigen responses and may also inhibit activated cytotoxic cells. In preliminary clinical studies using anti-CD2 monoclonal antibodies 9.6, 35.1, or B-E2 alone or in combination with other antibodies, improvement in GVHD was reported for 11 (48%) of 23 patients.27 28 We have treated 20 patients with BTI-322 for steroid-refractory GVHD. The complete response rate was 30%, and the overall response rate was 55%. These results confirm that targeting CD2 is a viable treatment strategy for patients with GVHD.
Treatment with BTI-322 was most successful in patients with skin or gut GVHD. None of the five patients with hepatic GVHD achieved a CR by day 11 in this study. With most therapies for GVHD, it is common to see no or minimal response in hepatic GVHD. If the bile ducts are injured severely, it may not be possible to see a reduction in serum bilirubin in the short-term.
Determining the means by which BTI-322 induces remission of GVHD might help design treatment schedules with a greater response rate. Ayroldi et al29 reported that CD2 engagement reduces anti-CD3–induced apoptosis of T lymphocytes. If blockade of CD2 engagement released the apoptotic mechanism, the prolonged hyporesponsiveness seen after treatment with anti-CD2 might be due to clonal deletion. This does not appear to be the mechanism of action of anti-CD2 antibodies in vivo, however, because the hyporesponsive state is of limited duration,15-17 and recurrence of GVHD was seen in this study after completion of therapy with BTI-322.
In our patients, there was a rapid and marked loss of CD2+and CD56+ cells from the peripheral blood after treatment with BTI-322, and all T-cell subsets were involved. Because trafficking of lymphocytes may depend in part on the adhesive property of CD2,30 downregulation of CD2 expression on lymphocytes may impede return of these cells to the intravascular space and to target organs for GVHD. The rapid return of CD2+ and CD3+ cells to pretreatment numbers after cessation of therapy with BTI-322 is consistent with this hypothesis. Further evidence is derived from rodent models in which treatment with anti-CD2 antibodies led to downregulation of expression of CD2 without altering expression of CD3, and normal numbers of CD3+ cells were seen in lymphoid organs.15-17
An alternative mechanism for the hyporesponsiveness induced by anti-CD2 is blockade of the CD2-transduced costimulatory signal required for T-cell activation. In vitro, CD2 antibodies can inhibit both primary and memory alloantigen responses,5,9,14 but it is noteworthy that anergy is induced only with the primary response.20 Consequently, although the GVH reaction could be blunted by treatment with BTI-322, a long- lasting state of tolerance may not be induced if alloreactivity involved memory T cells directed against cross-reactive epitopes on target tissues. Longer or repeated courses of therapy with BTI-322 may be required for successful treatment of GVHD.
Administration of mitogenic T-cell antibodies such as OKT3 frequently results in high fever, chills, bronchospasm, and hypotension due to release of cytokines. BTI-322 is not mitogenic and, in fact, inhibits cytokine production in lymphocytes stimulated through the T-cell receptor,19 so a classic “first dose reaction” was not expected for our patients. However, rapid infusion of BTI-322 was associated with transient dyspnea, gastrointestinal reactions, hypertension, and tachycardia that were self-limiting, and this response was abrogated by slow infusion of the drug. This was not a reaction unique to the marrow transplant patients, as it was also reported to occur in the renal allograft recipients treated with BTI-322.21,22 Similar reactions reported with other rodent monoclonal antilymphocyte antibodies have been attributed to histamine release.31 Overall, treatment with BTI-322 was otherwise well-tolerated.
The immunosuppression induced by anti-CD2 antibodies may be sufficient to abrogate antitumor immunity,16 and this raises a concern when using BTI-322 in patients with malignancies. However, neither relapse nor posttransplant lymphoproliferative disorders (PTLPD) were noted in our patients, albeit the follow-up is short. An increased risk of PTLPD was also not seen in renal transplant recipients who received BTI-322.21 Infections were the major complication seen in our patients. Given the severity of GVHD and the use of multiple immunosuppressive drugs, it is difficult to determine if BTI-322 altered the risk of infection.
Acute GVHD of the gut is frequently manifested as a protein-losing enteropathy, and the protein-losing state may alter the clearance of therapeutic antibodies. However, pharmacokinetic studies in our patients demonstrated that GVHD of the gut did not significantly alter clearance of BTI-322, so dose modification is not required for patients with gut GVHD. The half-life of BTI-322 in our patients was 9 to 11 hours, which suggests that immunosuppressive concentrations of antibody (>500 ng/mL in vitro) could be maintained with daily or every-other-day dosing. Incorporating the epitope binding regions of the rodent antibody onto a human background generally results in a therapeutic antibody with a much longer half-life than for the rodent form. Our results show that BTI-322 has activity in the treatment of GVHD with little toxicity. Pursuit of a humanized form of the antibody, which could be administered on a more convenient dosing schedule, is therefore warranted.
ACKNOWLEDGMENT
We are grateful to Dr D. Latinne (IMEX, Brussels, Belgium), who generously provided the murine antirat antibodies for the immunoassays.
Supported in part by grants from MedImmune, Inc and by Grant No. CA16672 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Donna Przepiorka, MD, PhD, Baylor College of Medicine, Center for Cell and Gene Therapy, 6621 Fannin St, MC3-3320, Houston, TX 77030; e-mail: donnap@bcm.tmc.edu.