Abstract
The efficacy of allografting in acute lymphoblastic leukemia (ALL) is heavily influenced by remission status at the time of transplant. Using polymerase chain reaction (PCR)-based minimal residual disease (MRD) analysis, we have investigated retrospectively the impact of submicroscopic leukemia on outcome in 64 patients receiving allogeneic bone marrow transplantation (BMT) for childhood ALL. Remission BM specimens were taken 6 to 81 days (median, 23) before transplant. All patients received similar conditioning therapy; 50 received grafts from unrelated donors and 14 from related donors. Nineteen patients were transplanted in first complete remission (CR1) and 45 in second or subsequent CR. MRD was analyzed by PCR of Ig or T-cell receptor δ or γ rearrangements, electrophoresis, and allele-specific oligoprobing. Samples were rated high-level positive (clonal band evident after electrophoresis; sensitivity 10−2 to 10−3), low-level positive (MRD detected only after oligoprobing; sensitivity 10−3 to 10−5), or negative. Excluding 8 patients transplanted in CR2 for isolated extramedullary relapse (all MRD−), MRD was detected at high level in 12 patients, low level in 11, and was undetectable in 33. Two-year event-free survival for these groups was 0%, 36%, and 73%, respectively (P < .001). Follow-up in patients remaining in continuing remission is 20 to 96 months (median, 35). These results suggest that MRD analysis could be used routinely in this setting. This would allow identification of patients with resistant leukemia (who may benefit from innovative BMT protocols) and of those with more responsive disease (who may be candidates for randomized trials of BMT versus modern intensive relapse chemotherapy).
ALLOGENEIC BONE marrow transplantation (allo-BMT) provides a survival advantage over chemotherapy for patients with acute lymphoblastic leukemia (ALL) who sustain early BM relapse or present with poor-risk features at diagnosis.1-6 However, 30% to 40% of transplant recipients will still relapse after the procedure.4,7-15 Although a number of recent therapeutic interventions could potentially improve this situation, eg, intensification of conditioning or posttransplant immunotherapy,16 such measures may increase toxicity and should ideally only be targeted toward those at highest risk of further relapse.
As far as allo-BMT is concerned, outcome is poor for patients who enter transplant with a high leukemia cell burden. This is illustrated by the results from patients transplanted for resistant disease or in relapse.13,17-22 By extrapolation, because many patients transplanted in remission still relapse, it seems likely that presence of disease persisting at levels just below the remission threshold might worsen outcome. This has already been shown in patients undergoing autologous BMT where several groups have shown that the submicroscopic level of leukemia in the marrow graft, reflecting the leukemia cell burden in the patient before transplant, has a significant bearing on outcome.23-26
Submicroscopic disease is otherwise termed minimal residual disease (MRD) and can be assessed by several techniques in ALL, the most widely applicable of which involves amplification of Ig heavy chain (IgH) or T-cell receptor (TCR) gene rearrangements by polymerase chain reaction (PCR).27-30 Using combinations of IgH, TCRδ, and TCRγ primer pairs, a molecular marker of the leukemic clone can be identified in more than 95% of B-lineage and more than 90% T-lineage ALL. This can be used to track MRD with a sensitivity of 0.01% in at least 90% of patients.31
MRD analysis (using either IgH PCR or bcr-abl reverse transcriptase [RT]-PCR) to identify early signs of relapse after allo-BMT has been reported previously.32-35 However, delaying the tracking of MRD to the post–allo-BMT period inevitably restricts the therapeutic modalities available for the eradication of residual leukemia cells detected at this late stage. We decided to study MRD before allo-BMT to investigate whether useful prognostic information could be acquired on which to base earlier, more comprehensive decisions about the use of different conditioning regimens, T-cell depletion strategies, and post-BMT immunomodulation, or possibly to delineate patients worthy of comparative trials of modern intensive chemotherapy versus allo-BMT. Using a method described previously,31 we assessed pretransplant MRD retrospectively in a cohort of 64 patients undergoing allo-BMT in remission for relapsed or high-risk ALL from either sibling or unrelated donors. This study shows the profound impact of submicroscopic disease load on event-free survival (EFS).
PATIENTS, MATERIALS, AND METHODS
Patients.
Those eligible for study were children and adolescents with ALL aged less than 18 years at diagnosis who underwent allogeneic BMT between January 1, 1990 and August 1, 1996. All patients were in remission and less than 20 years of age at the time of BMT. Of 145 such patients identified, 74 were excluded due to a lack of adequate diagnostic material (39 patients) or suitably archived pre-BMT material (35 patients). This left 71 patients open to study, but MRD was not evaluable in a further 7 who lacked an amplifiable gene rearrangement.
Of the 64 patients open to study, 40 were male and 24 female. Ages at diagnosis ranged from 1.3 to 16.9 years (median, 4.4), white blood cell (WBC) counts at diagnosis from 0.8 to 606 × 109/L (median, 28) and ages at BMT from 2.0 to 19.8 years (median, 7.2). The diagnosis of ALL was confirmed by morphological and immunophenotypic analysis using standard criteria.36 37 Thirty-three patients had common ALL, 14 pre-B ALL, 2 null-B ALL, 1 mature B ALL, and 14 T-lineage ALL.
Patient cytogenetic results and remission status at BMT are summarized in Table 1. Nineteen patients were transplanted in first complete remission (CR1) for high-risk disease [t(4;11), t(9;22), presentation WBC count >100 × 109/L, high Oxford hazard score38and/or failing to remit after 4 weeks of remission induction therapy] and the remainder were transplanted in CR2 or subsequent remissions. The only patient who had experienced isolated extramedullary relapse (central nervous system [CNS]) more than 6 months after the end of first-line treatment had already received radiotherapy to the CNS. All other patients transplanted for isolated extramedullary relapse had relapsed within 6 months of completion of conventional therapy. Remission induction and consolidation therapy was administered according to the Medical Research Council (MRC) UKALL X or XI protocols for 2.9 to 8.7 months (median, 4.8) in all patients transplanted in CR1. In those patients who relapsed before BMT, the time from last relapse to BMT ranged from 2.8 to 12.1 months (median, 5.1) and all had received intensive remission reinduction and consolidation therapy according to the MRC UKALL R1 or R2 protocols (40 patients) or the BFM ALL 90 protocol (5 patients). One patient had received an autologous BMT conditioned with cyclophosphamide and etoposide as consolidation therapy after remission reinduction for first relapse.
Patient Characteristics and MRD Results
| . | No. of Patients . | TRM . | No. of Patients Relapsing/No. With Given MRD Result . | ||
|---|---|---|---|---|---|
| neg . | + . | ++ . | |||
| All patients | 64 | 5 | 8/38 | 5/9 | 12/12 |
| Cytogenetics | |||||
| Normal | 16 | 1 | 6/13 | 0/1 | 1/1 |
| t(9;22) | 10 | 1 | 0/2 | 1/3 | 4/4 |
| t(4;11) | 1 | 1 | 0/0 | 0/0 | 0/0 |
| Other abnormal | 30 | 2 | 2/17 | 4/5 | 6/6 |
| Failed | 7 | 0 | 0/6 | 0/0 | 1/1 |
| Status at BMT | |||||
| CR1 | 19 | 1 | 2/10 | 2/5 | 3/3 |
| CR2 | 39 | 3 | 6/24 | 2/3 | 9/9 |
| On treatment relapse | 13 | 0 | 1/4 | 2/2 | 7/7 |
| BM relapse only | 7 | 0 | 1/1 | 1/1 | 5/5 |
| Combined BM + EM relapse | 4 | 0 | 0/1 | 1/1 | 2/2 |
| IEMR | 2 | 0 | 0/2 | 0/0 | 0/0 |
| Relapse <6 mo off treatment | 14 | 2 | 5/11 | 0/0 | 1/1 |
| BM relapse only | 5 | 1 | 2/3 | 0/0 | 1/1 |
| Combined BM + EM relapse | 4 | 0 | 2/4 | 0/0 | 0/0 |
| IEMR | 5 | 1 | 1/4 | 0/0 | 0/0 |
| Relapse >6 mo off treatment | 12 | 1 | 0/9 | 0/1 | 1/1 |
| BM relapse only | 5 | 1 | 0/4 | 0/0 | 0/0 |
| Combined BM + EM relapse | 6 | 0 | 0/4 | 0/1 | 1/1 |
| IEMR | 1 | 0 | 0/1 | 0/0 | 0/0 |
| CR3 | 5 | 1 | 0/3 | 1/1 | 0/0 |
| CR4 | 1 | 0 | 0/1 | 0/0 | 0/0 |
| Donor | |||||
| Unrelated* | 50 | 5 | 6/27 | 5/9 | 9/9 |
| Sibling | 12 | 0 | 1/10 | 0/0 | 2/2 |
| Syngeneic twin | 1 | 0 | 0/0 | 0/0 | 1/1 |
| Parent (full HLA match) | 1 | 0 | 1/1 | 0/0 | 0/0 |
| aGVHD | |||||
| None | 30 | 1 | 4/16 | 4/7 | 6/6 |
| Grades I-II | 32 | 3 | 4/22 | 1/1 | 6/6 |
| Grades III-IV | 2 | 1 | 0/0 | 0/1 | 0/0 |
| . | No. of Patients . | TRM . | No. of Patients Relapsing/No. With Given MRD Result . | ||
|---|---|---|---|---|---|
| neg . | + . | ++ . | |||
| All patients | 64 | 5 | 8/38 | 5/9 | 12/12 |
| Cytogenetics | |||||
| Normal | 16 | 1 | 6/13 | 0/1 | 1/1 |
| t(9;22) | 10 | 1 | 0/2 | 1/3 | 4/4 |
| t(4;11) | 1 | 1 | 0/0 | 0/0 | 0/0 |
| Other abnormal | 30 | 2 | 2/17 | 4/5 | 6/6 |
| Failed | 7 | 0 | 0/6 | 0/0 | 1/1 |
| Status at BMT | |||||
| CR1 | 19 | 1 | 2/10 | 2/5 | 3/3 |
| CR2 | 39 | 3 | 6/24 | 2/3 | 9/9 |
| On treatment relapse | 13 | 0 | 1/4 | 2/2 | 7/7 |
| BM relapse only | 7 | 0 | 1/1 | 1/1 | 5/5 |
| Combined BM + EM relapse | 4 | 0 | 0/1 | 1/1 | 2/2 |
| IEMR | 2 | 0 | 0/2 | 0/0 | 0/0 |
| Relapse <6 mo off treatment | 14 | 2 | 5/11 | 0/0 | 1/1 |
| BM relapse only | 5 | 1 | 2/3 | 0/0 | 1/1 |
| Combined BM + EM relapse | 4 | 0 | 2/4 | 0/0 | 0/0 |
| IEMR | 5 | 1 | 1/4 | 0/0 | 0/0 |
| Relapse >6 mo off treatment | 12 | 1 | 0/9 | 0/1 | 1/1 |
| BM relapse only | 5 | 1 | 0/4 | 0/0 | 0/0 |
| Combined BM + EM relapse | 6 | 0 | 0/4 | 0/1 | 1/1 |
| IEMR | 1 | 0 | 0/1 | 0/0 | 0/0 |
| CR3 | 5 | 1 | 0/3 | 1/1 | 0/0 |
| CR4 | 1 | 0 | 0/1 | 0/0 | 0/0 |
| Donor | |||||
| Unrelated* | 50 | 5 | 6/27 | 5/9 | 9/9 |
| Sibling | 12 | 0 | 1/10 | 0/0 | 2/2 |
| Syngeneic twin | 1 | 0 | 0/0 | 0/0 | 1/1 |
| Parent (full HLA match) | 1 | 0 | 1/1 | 0/0 | 0/0 |
| aGVHD | |||||
| None | 30 | 1 | 4/16 | 4/7 | 6/6 |
| Grades I-II | 32 | 3 | 4/22 | 1/1 | 6/6 |
| Grades III-IV | 2 | 1 | 0/0 | 0/1 | 0/0 |
MRD results are presented, excluding patients dying from transplant-related causes, as the number of patients who relapsed divided by the number of patients studied for each possible MRD result.
Abbreviations: EM, extramedullary; IEMR, isolated extramedullary relapse; neg, MRD not detected; +, low-level MRD detected (sensitivity 10−3 to 10−5); ++, high-level MRD detected (sensitivity 10−2 to 10−3).
28 fully HLA-matched, 22 HLA-mismatched.
BMT protocol.
All transplants were performed at the Royal Hospital for Sick Children, Bristol, UK. The approach to HLA typing, BM graft processing, and supportive care was as described previously.14 Donor characteristics are given in Table 1. All patients were conditioned with cyclophosphamide 60 mg/kg for 2 days and total body irradiation (60 received 14.4 Gy fractionated into 8 doses and 4 under the age of 3 years received 10 Gy as a single fraction at low-dose rate). Twenty-three patients who had relapsed in an extramedullary site received additional radiotherapy to the affected area as part of the conditioning. Intravenous CAMPATH-1G was administered to all recipients of grafts from unrelated donors and to 6 recipients of grafts from related donors. T-cell depletion with CAMPATH-1M or -1G was performed on all grafts from unrelated donors (except 3 patients who received CellPro [CellPro Inc, Bothell, WA] CD34-selected cells) and on 3 grafts from related donors, 1 of whom had a T-cell add back infused at the time of BMT.
For graft-versus-host disease (GVHD) prophylaxis, all patients received cyclosporin A (CSA) and, in addition, short-course methotrexate (MTX) was administered to the recipients of non–T-cell–depleted related and mismatched unrelated grafts. Acute GVHD (aGVHD) was graded 0-IV and chronic GVHD (cGVHD) as none, limited, or extensive according to criteria described previously.39 40 Details on the occurrence of aGVHD are given in Table 1. Two patients in continuing remission had limited cGVHD and 1 patient died with uncontrolled extensive GVHD affecting the skin and liver.
Samples.
Samples from 64 children were analyzed for the presence of MRD in specimens taken at a median of 23 days (range, 6 to 81) before BMT. The median time from the start of remission induction to sampling was 111 days (range, 70 to 227) for those transplanted in CR1 and from the start of remission reinduction after the last pre-BMT relapse was 132 days (range, 74 to 296) for those transplanted in CR2 and beyond. All samples were analyzed morphologically to ensure remission status. Local ethical approval was obtained for the study.
DNA preparation.
DNA was extracted from BM mononuclear cells (BM MNCs) using QIAmp kits according to the manufacturer’s instructions (Qiagen GmbH, Hilden, Germany) or by conventional phenol-chloroform extraction and ethanol precipitation.41 In 26 cases stored presentation mononuclear cells were not available and DNA was obtained from archival BM aspirate slides as described previously.42 Because of concerns over the variable quality of DNA obtained from archival slides, these were not used as a source of DNA for the analysis of pre-BMT samples.
Characterization of clone-specific rearrangements and investigation of remission specimens.
A more complete description of the methodology can be found elsewhere.31 In essence, leukemic material from the time of last relapse (or from the time of diagnosis if the patient was transplanted in first remission), was screened for IgH, TCRδ, or TCRγ rearrangements using PCR amplification with FR3-JH, Vδ2-Dδ3, Vγ1/9-JγI/II, and, in two cases of T-ALL, Vδ1-Jδ1 primer pairs. Clonal bands were sequenced either directly or via a cloning step. Twenty base oligonucleotides were synthesized to map to the DNJ (IgH), VND (Vδ2-Dδ3), or VNJ (Vγ1/9-JγI/II and Vδ1-Jδ1) junctions.
Having ascertained that the BM MNC DNA from each pre-BMT specimen was amplifiable by control PCR,31 1 μg was amplified in a 100-μL reaction using the same conditions as above but an outer downstream primer was used for FR3 and Vδ2-Dδ3 PCR as a maneuver against contamination.31 A non–DNA-containing negative control and two samples each containing 1 μg of normal BM MNC DNA as well as 10-fold dilutions of leukemia cell DNA in normal BM MNC DNA were amplified in parallel as controls. The resultant PCR products were size-resolved by 8% polyacrylamide gel electrophoresis (PAGE) and transferred to a nylon support by semidry electroblotting. The membranes were then probed with the leukemia-specific oligonucleotide end-labeled with γ32P-dATP followed by autoradiography. High-level MRD was defined as that evident as a clonal band after PAGE only (ie, before allele-specific oligoprobing; sensitivity 10−2 to 10−3) and low-level MRD as that identified after PAGE and oligoprobing (sensitivity 10−3 to 10−5).
Statistical analysis.
An overall χ2 test with partition43 was used to compare relapse rates between the three MRD groups. Actuarial probabilities of EFS were calculated using the method of Kaplan and Meier44 where an event was defined as relapse or death. Univariate and multivariate analysis using the Cox proportional hazards model was performed to assess the independence of MRD as a risk factor for relapse. The results have been analyzed up to November 1, 1997, which allows a minimum follow-up of 20 months for patients in continuing complete remission (CCR).
RESULTS
Clone-specific rearrangements.
Including the 7 patients in whom a clone-specific rearrangement could not be identified, 121 clonal rearrangements were identified for the 55 patients with B-lineage ALL (73%, 31%, and 44% had at least one clonal IgH, Vδ2, and TCRγ rearrangement, respectively) and 18 for the 16 with T-lineage ALL (75% had at least one TCRγ rearrangement and a Vδ1-Jδ1 rearrangement was identified for the 2 patients negative by TCRγ PCR).
Clone-specific probes.
Eighty-five oligonucleotide (41 IgH, 9 Vδ2-Dδ3, 33 Vγ1/9-JγI/II, and 2 Vδ1-Jδ1) probes were used in the study. One probe only was used to investigate the 27 patients with only one rearrangement and 22 of the patients with more than one rearrangement available for study. Eleven patients were studied with 2 probes, 2 with 3 probes, and 2 with 4 probes: in 10 of these cases probes were designed to rearrangements at different loci. Discrepant results from probes for different loci in the same patient were found in 3 cases: 2 patients were negative with 1 probe and low-level positive with the other, and the other patient was negative with 2 probes and low-level positive with the third. These cases were deemed low-level positive for the purpose of analysis.
The sensitivity of 7 probes was not evaluable because of the poor quality or small amount of diagnostic material available. A sensitivity equivalent to the detection of one leukemic cell in at least 10,000 normal cells was shown in 71 (92%) of the remaining 77 probes as assessed by 10-fold dilutions of leukemic DNA in normal BM MNC DNA.
Patients.
Thirty-four (53%) patients remain in CCR with a median follow-up of 35 months (range, 20 to 96) from BMT. Twenty-five (39%) patients have relapsed after BMT with a median time to relapse of 5 months (range, 2.5 to 19). Twenty-two patients relapsed in the marrow only and 3 suffered a combined medullary and extramedullary relapse. Five (8%) patients, all with unrelated donors, died of complications unrelated to relapse (aGVHD and respiratory syncytial virus pneumonitis; adenovirus pneumonitis; hemolytic uremic syndrome and transfusion-associated GVHD; thrombotic thrombocytopenic purpura and cardiac failure; and pneumonitis of unknown cause). These figures compare with a CCR rate of 48%, relapse rate of 36%, and transplant-related mortality rate of 16% in the overall group of 145 patients available for study.
Six patients failed to engraft. Stored autologous marrow, obtained immediately before BMT conditioning, was returned to 4 patients between days 28 and 35 post-BMT. One patient developed autologous reconstitution. The remaining patient was reconditioned with in vivo CAMPATH 1G and cyclophosphamide 60 mg/kg for 2 days with CSA and MTX for GVHD prophylaxis before being administered peripheral blood progenitor cells from the original donor 91 days after the first BMT.
Patterns of MRD.
Results of MRD analysis from pre-BMT samples for each patient subgroup, not including those from patients dying of transplant-related causes, are given in Table 1. All 8 patients transplanted in CR2 for isolated extramedullary relapse were found to be MRD− (1 relapsed and 1 died from transplant-related mortality [TRM]) and have been excluded from the following statistical analyses (see Discussion). A statistically significant difference in relapse rate was found between the patients tested as high-level MRD+, low-level MRD+, or MRD− (overall χ2 = 21.25, P < .001). The incidence of relapse was also statistically significant when comparing patients with high-level MRD and those with low-level or negative MRD (partitioned χ2= 18.20, P < .001) but not on comparison of patients with low-level positive MRD and negative MRD (partitioned χ2 = 3.05, P = .081). Relapse rates, including patients dying of TRM, were 74% for patients found to be MRD+ (100% for high-level MRD+ and 45% for low-level MRD+) and 20% for patients MRD− (see also Table1).
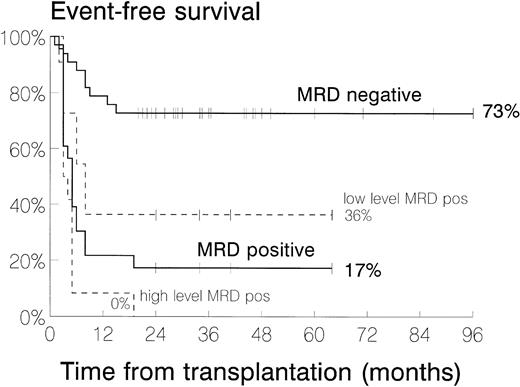
Kaplan-Meier plots of EFS, inclusive of patients dying from TRM (n = 4) but exclusive of patients relapsing before allo-BMT in an isolated extramedullary site (n = 8), are shown in Fig 1. The 2-year EFS for patients who were MRD+ was 17% compared with 73% for the group that was MRD−. Subdivision of the MRD+ group gave the following results for 2-year EFS: high-level MRD+, 0%; low-level MRD+, 36%; low-level MRD+ or MRD−, 64%.
Kaplan-Meier plots comparing event-free survival of patients with positive MRD (n = 23), divided into high level (n = 12) and low level (n = 11), and negative MRD (n = 33), but excluding those who had relapsed in an isolated extramedullary site before BMT. Two-year EFS is given for each MRD category at the end of each curve.
Kaplan-Meier plots comparing event-free survival of patients with positive MRD (n = 23), divided into high level (n = 12) and low level (n = 11), and negative MRD (n = 33), but excluding those who had relapsed in an isolated extramedullary site before BMT. Two-year EFS is given for each MRD category at the end of each curve.
Only MRD was significantly related to EFS (P < .001) out of all the prognostic variables examined by univariate analysis (Table 2A). Limited multivariate analysis confirmed the significance of MRD (P < .001) after separate adjustment for pre-BMT CR status, presence of Philadelphia chromosome, type of donor (related v unrelated), and for all of these factors (Table 2A). The other variables remained nonsignificant in all of these models. Similar analysis was performed on the subgroup of patients transplanted in CR2 after medullary relapse (Table 2B). Pre-BMT MRD and whether pre-BMT relapse occurred on first-line chemotherapy were significantly related to EFS (P < .001 in both cases) on univariate analysis. The effect of MRD remained significant after adjustment for Philadelphia chromosome positivity (P < .001) and was of borderline significance when adjustment was made for pre-BMT relapse occurring on treatment (effect of MRDP = .055). MRD remained of borderline significance after adjustment for both of these variables (P = .058) where the effect of pre-BMT relapse occurring on treatment was significant (P = .031) and that of Philadelphia chromosome positivity was not significant.
Cox Proportional Hazards Analysis for All Patients (n = 56) (A) and Patients Transplanted in CR2 (n = 31) (B) but Excluding Patients Relapsing Before BMT in an Isolated Extramedullary Site
| . | Hazard Rate Ratio . | [95% CI] . | Significance* . |
|---|---|---|---|
| A. (1) Univariate analysis | |||
| MRD | |||
| Negative | 1 | ||
| + | 3.14 | [0.99-9.96] | |
| ++ | 15.28 | [5.52-42.32] | P < .001 |
| Sex | |||
| M | 1 | ||
| F | 0.75 | [0.31-1.80] | NS (P = .509) |
| ALL subtype | |||
| B-lineage | 1 | ||
| T-lineage | 0.65 | [0.22-1.90] | NS (P = .410) |
| Ph′ | |||
| No | 1 | ||
| Yes | 1.35 | [0.50-3.62] | NS (P = .561) |
| Donor | |||
| Non-UD | 1 | ||
| UD | 1.20 | [0.45-3.22] | NS (P = .708) |
| aGVHD | |||
| 0 | 1 | ||
| 1 | 0.77 | [0.35-1.72] | NS (P = .523) |
| CR status | |||
| CR1 | 1 | ||
| CR2/3/4 | 1.48 | [0.61-3.56] | NS (P = .375) |
| Age (yr) at diagnosis | †0.0117 (SE 0.0454) | NS (P = .798) | |
| Age (yr) at BMT | †−0.0336 (SE 0.0490) | NS (P = .486) | |
| WCC (×109/L) at diagnosis | †−0.0007 (SE 0.0018) | NS (P = .669) | |
| (2) Multivariate analysis | |||
| MRD—adjusting for CR status, Ph′ and donor | |||
| Negative | 1 | ||
| + | 4.21 | [1.24-14.30] | |
| ++ | 23.65 | [7.05-79.33] | P < .001 |
| B. (1) Univariate analysis | |||
| MRD | |||
| Negative | 1 | ||
| + | 3.79 | [0.71-20.25] | |
| ++ | 13.91 | [3.21-60.30] | P < .001 |
| Ph′ | |||
| No | 1 | ||
| Yes | 3.99 | [0.49-32.47] | P = .275 |
| Donor | |||
| Non-UD | 1 | ||
| UD | 0.66 | [0.23-1.93] | P = .464 |
| Pre-BMT relapse during treatment | |||
| No | 1 | ||
| Yes | 8.38 | [2.83-24.83] | P < .001 |
| (2) Multivariate analysis | |||
| MRD—adjusted for on-treatment pre-BMT relapse and for Ph′ | |||
| Negative | 1 | ||
| + | 3.41 | [0.60-19.52] | |
| ++ | 6.50 | [1.22-34.68] | P = .058 |
| . | Hazard Rate Ratio . | [95% CI] . | Significance* . |
|---|---|---|---|
| A. (1) Univariate analysis | |||
| MRD | |||
| Negative | 1 | ||
| + | 3.14 | [0.99-9.96] | |
| ++ | 15.28 | [5.52-42.32] | P < .001 |
| Sex | |||
| M | 1 | ||
| F | 0.75 | [0.31-1.80] | NS (P = .509) |
| ALL subtype | |||
| B-lineage | 1 | ||
| T-lineage | 0.65 | [0.22-1.90] | NS (P = .410) |
| Ph′ | |||
| No | 1 | ||
| Yes | 1.35 | [0.50-3.62] | NS (P = .561) |
| Donor | |||
| Non-UD | 1 | ||
| UD | 1.20 | [0.45-3.22] | NS (P = .708) |
| aGVHD | |||
| 0 | 1 | ||
| 1 | 0.77 | [0.35-1.72] | NS (P = .523) |
| CR status | |||
| CR1 | 1 | ||
| CR2/3/4 | 1.48 | [0.61-3.56] | NS (P = .375) |
| Age (yr) at diagnosis | †0.0117 (SE 0.0454) | NS (P = .798) | |
| Age (yr) at BMT | †−0.0336 (SE 0.0490) | NS (P = .486) | |
| WCC (×109/L) at diagnosis | †−0.0007 (SE 0.0018) | NS (P = .669) | |
| (2) Multivariate analysis | |||
| MRD—adjusting for CR status, Ph′ and donor | |||
| Negative | 1 | ||
| + | 4.21 | [1.24-14.30] | |
| ++ | 23.65 | [7.05-79.33] | P < .001 |
| B. (1) Univariate analysis | |||
| MRD | |||
| Negative | 1 | ||
| + | 3.79 | [0.71-20.25] | |
| ++ | 13.91 | [3.21-60.30] | P < .001 |
| Ph′ | |||
| No | 1 | ||
| Yes | 3.99 | [0.49-32.47] | P = .275 |
| Donor | |||
| Non-UD | 1 | ||
| UD | 0.66 | [0.23-1.93] | P = .464 |
| Pre-BMT relapse during treatment | |||
| No | 1 | ||
| Yes | 8.38 | [2.83-24.83] | P < .001 |
| (2) Multivariate analysis | |||
| MRD—adjusted for on-treatment pre-BMT relapse and for Ph′ | |||
| Negative | 1 | ||
| + | 3.41 | [0.60-19.52] | |
| ++ | 6.50 | [1.22-34.68] | P = .058 |
Abbreviations: WCC, white blood cell count; Ph′, Philadelphia chromosome positive; UD, unrelated donor; CR2, second complete remission; +, low-level MRD+; ++, high-level MRD+; NS, not significant.
Significance by likelihood ratio test.
Coefficient and SE.
DISCUSSION
Chemosensitivity of the leukemia clone is an important prerequisite for successful outcome after allo-BMT for ALL. This is well illustrated by the poor outcome in patients with disease refractory to conventional chemotherapy17,19 or with advanced disease.10,45 46 After remission induction (or reinduction) and consolidation, MRD acts as a surrogate marker of remaining chemoresistance and we reasoned that MRD analysis before allo-BMT might provide useful prognostic information.
The first notable observation is that MRD was not detected in pre-BMT marrow in any of the 8 patients transplanted in CR2 for isolated extramedullary relapse, whether this had occurred during or after conventional therapy. Because MRD is usually present in the marrow at the time of “isolated” extramedullary relapse of ALL,47,48 this implies that postrelapse chemotherapy had cleared disease to below the threshold of detection (even in the single patient from this group who relapsed after transplant). Taken together with the fact that tracking marrow MRD in patients undergoing first-line treatment for ALL is an unreliable method for predicting extramedullary relapse,31 we conclude that pretransplant MRD assessment is likely to have little value for the prediction of outcome after BMT in this minor subgroup of patients.
The remainder of this discussion will therefore concentrate on the 52 evaluable patients treated in CR1 for high-risk disease or in higher remission states after BM relapse, either in isolation or combined with extramedullary relapse. In these patients, we have shown a strong correlation between the persistence of MRD before BMT and risk of post-BMT relapse. All 12 patients with high-level MRD went on to relapse compared with only 12 of the 40 (30%) who were MRD− or found to have MRD detectable only after allele-specific probing (P < .001). The patients with high-level MRD made up half of those who relapsed in this study and are readily detectable by a clonality test that could be performed in any routine molecular biology laboratory.
To some extent, the poor outcome of some of the 12 patients with high-level MRD could have been predicted from first principles. Eight had relapsed whilst still receiving (7 cases) or within 6 months of finishing (1 case) first-line chemotherapy, and 3 had Philadelphia chromosome–positive ALL (Ph1-ALL) transplanted in CR1. Such patients are already known to be at high risk.7,15,49However, statistical analysis suggested that pre-BMT MRD level was a risk factor independent of on-treatment pre-BMT relapse and cytogenetics by multivariate analysis (Table 2B) and did yield potentially important prognostic information—the remaining patient with high level MRD had relapsed 12 months off treatment and would otherwise have been viewed as having a lower risk of relapse. In patients with Ph1-ALL as a whole, all 4 of those found to have high-level MRD relapsed whereas 4 out of the 5 patients with undetectable or low-level MRD remain free of disease.50Most notably, of the patients relapsing during conventional first-line chemotherapy, the only one to have cleared MRD continues in remission 28 months after BMT.
Four of the 9 patients with low-level MRD before BMT remain in remission, suggesting that conditioning therapy or a graft-versus-leukemia effect successfully eliminated their residual disease. Two of these patients were transplanted for Ph1-ALL in CR1 and survive in CCR 24 and 64 months post-BMT. However, only 1 of the 4 patients with low-level MRD transplanted in CR2 remains in remission, suggesting that the finding of MRD in this setting highlights those needing more innovative therapy. Conversely, 7 of the 31 patients who were MRD− went on to relapse. This “false-negative” prediction may reflect inadequate sensitivity of the assay30,51 or sampling error due to heterogeneous distribution of MRD throughout the marrow.52-54
Despite obvious limitations, including the use of retrospective analysis, the bias toward T-depleted unrelated donor (UD) BMT, and slight under-representation of patients suffering transplant-related mortality, this study constitutes the first major examination of the effect of leukemia cell burden on outcome in patients receiving allo-BMT for ALL. It is interesting to consider the impact that the straightforward detection of clonal bands after a single round of PCR might have had on clinical decision making. Twelve patients with high-level MRD could have been offered alternative treatment (eg, further cytoreduction before conditioning, intensified conditioning, T-replete grafts, and/or post-BMT immunotherapy), which might have improved upon their universally poor outcome. By contrast, allo-BMT resulted in a 2-year EFS of 64% in the remaining 40 patients who were either low-level MRD+ or MRD− and transplanted in CR1 or after medullary relapse. Allo-BMT was more successful in the subgroup of patients who were MRD− (2-year EFS: 73% overall and 67% for patients transplanted in CR2 after medullary relapse). An important question remains as to how modern intensive chemotherapy would compare with allo-BMT in this latter group of patients whose marrow disease has been cleared to undetectable levels by the chemotherapy administered either as remission induction and consolidation or after relapse.
ACKNOWLEDGMENT
We are particularly appreciative of the support for C.J.C.K. from the Ben Drewer Research Fund, and that for N.J.G. from the Leukaemia Research Fund, the COGENT Trust for providing laboratory facilities, and PG. We also thank Dr M.N. Potter for supervising C.J.C.K. during the early part of this project, and all colleagues involved in sample collection and patient care at the Royal Hospital for Sick Children, Bristol, in particular Dr H. Kershaw and the nursing staff of Oncology Day Care Unit. We also thank Prof S. Haidas (St Sophia Hospital, Athens, Greece), Dr J. Kingston (St Bartholomew’s Hospital, London, UK), Dr S. Dempsey (Royal Hospital for Sick Children, Belfast, UK), Dr M. Stevens (Hospital for Sick Children, Birmingham, UK), Drs R. Marcus, D. Williams, and V. Broadbent (Addenbrooke’s Hospital, Cambridge, UK), Dr D. Webb (Llandough Hospital, Cardiff, UK), Dr L. Evan-Wong (Queen Margaret Hospital, Dunfermline, UK), Prof O. Eden and Dr H. Wallace (Royal Hospital for Sick Children, Edinburgh, UK), Dr S. Kelly (Wycombe General Hospital, High Wycombe, UK), Prof J. Chessells and Dr F. Katz (Hospital for Sick Children, Great Ormond Street, London, UK), Prof R. Pinkerton (Royal Marsden Hospital, London, UK), Drs D. Walker and M. Hewitt (Queen’s Medical Centre, Nottingham, UK), Prof J. Lilleyman (Children’s Hospital, Sheffield, UK), Drs J. Kohler and M. Radford (General Hospital, Southampton, UK), and Dr C. Hatton (Wexham Park Hospital, Slough, UK) for the patient referrals and their help with providing bone marrow material and clinical information on some of the patients in the study. We are obliged to R. Thorne for the Kaplan-Meier plots and to Drs P. Virgo and A. McDermott (Southmead Hospital, Bristol, UK) for immunophenotyping and cytogenetic data, respectively.
Supported by the Ben Drewer Research Fund, the COGENT Trust, the Leukaemia Research Fund, and PG.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Colin G. Steward, MA, PhD, c/o Oncology Day Care Unit, Royal Hospital for Sick Children, St Michael’s Hill, Bristol BS2 8BJ, UK.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal