Mice lacking interleukin-2 (IL-2) developed a severe hematopoietic disorder characterized by the abnormal development of myeloid cells and neutropenia. Analysis of the bone marrow of IL-2–deficient (IL-2−/−) mice showed that the number of mature polymorphonuclear cells was decreased by 65% to 75%, and granulocyte/macrophage precursor cells were reduced by 50%. Bone marrow cells from IL-2−/− mice were unable to sustain myelopoiesis in lethally irradiated mice and in long-term bone marrow cultures (LTBMC). The addition of exogenous IL-2 to LTBMC of IL-2−/− cells partially restored hematopoietic progenitor activity. In the bone marrow of wild-type mice, immature (Mac-1lo) myeloid cells, including myeloblasts and promyelocytes, constitutively expressed the β-chain of the IL-2R, and the number of Mac-1loIL-2Rβ+ cells was increased by twofold to threefold in IL-2−/− mice. During culture in the presence of IL-2 and the absence of stromal cells, Mac-1loIL-2Rβ+ immature myeloid cells proliferated and gave rise to mature granulocytes and macrophages. Collectively, these observations indicate that defective myelopoiesis in IL-2−/− mice is at least in part a consequence of their direct dependency on IL-2, and by regulating the growth of immature myeloid cells, IL-2 plays an important role in the homeostatic regulation of myelocytic cell generation.
INTERLEUKIN-2 (IL-2) WAS initially described as a mitogenic signal and growth factor for T lymphocytes.1,2 However, subsequent studies have shown that IL-2 receptors (IL-2R) are widely distributed on other lymphoid3-5 and nonlymphoid hematopoietic cells including cells of the myeloid lineage,6-11 and that IL-2 can modulate the growth, survival, or function of natural killer (NK) cells, lymphokine-activated killer (LAK) cells, and B cells.3,12-14 While the role of IL-2 in T-lymphocyte development has been extensively investigated (reviewed in Carding and Reya15), there is now growing evidence that IL-2 may be able to influence the development of other lymphocytes. Recent studies describing the expression of IL-2Rα on pre-B cells16,17and our own studies identifying expression of functional IL-2Rs by pre-pro B cells18 suggest a role for IL-2 in B-cell development. Additionally the absence of NK cells in IL-2Rγ–deficient19 and IL-2Rβ–deficient20mice suggests that signaling through the IL-2R complex by IL-2 and/or IL-15 is also necessary for NK cell development.
In contrast to lymphoid cells, the function of IL-2R in cells of the myeloid lineage is largely unknown. Although the ability of IL-2 to influence the survival21 and effector function21-26 of mature myeloid cells has been extensively characterized, it is not clear if, or how, IL-2 influences their development. The differential expression of IL-2Rs during the in vitro differentiation of human myeloid cells27 and the increase in number of circulating myeloid (colony-forming unit-granulocyte/macrophage [CFU-GM]) progenitor cells after treatment of cancer patients with IL-228-31 are consistent with IL-2 having a positive effect on myelopoiesis. However, other studies have produced contradictory findings. The addition of IL-2 to long-term bone marrow cultures (LTBMC) or in vitro colony forming assays has been shown to inhibit myeloid progenitor cell growth and development.32-36 It is also not clear if the effects of IL-2 on myeloid cell progenitors are direct or indirect. For example, it has been shown that IL-2 can influence myelopoiesis by inducing cytokine production by IL-2R+ T cells.35,37-39However, the finding that the addition of recombinant IL-2 to cultures of T-cell–depleted populations of bone marrow hematopoietic progenitors33 and murine myeloid precursors32promotes their growth and development is consistent with a direct role for IL-2 in regulating hematopoietic cell activity. It is not clear, therefore, what the mechanism(s) of action of IL-2 on developing myeloid cells is, or if IL-2 does, in fact, normally play a role in promoting or regulating myelopoiesis in vivo.
To address these questions and to establish the role of IL-2 in myeloid cell development, we have determined the consequences of IL-2 deficiency on myelopoiesis in vivo. We show here that IL-2−/− mice develop a profound hematopoietic disorder characterized by the abnormal development of myeloid cells and deficiencies in mature granulocytes. Furthermore, the finding that immature myeloid cells can use IL-2 for their growth and differentiation in vitro, and exogenously provided IL-2 can restore IL-2−/− hematopoietic cell proliferation in vitro suggests that IL-2 acts directly on immature myeloid cells and that IL-2/IL-2R interactions can regulate myeloid cell development.
MATERIALS AND METHODS
Mice.
C57BL.6, C57BL.6-Ptprca Pep3b/BoyJ (CD45.1) and C57BL.6-Thy1a/Cy (Thy1.1) mouse strains were obtained from The Jackson Laboratory (Bar Harbor, ME) and used between 4 and 6 weeks of age. IL-2–deficient mice back-crossed for eight generations to C57BL.6 were obtained from R. Schwartz (National Institutes of Health [NIH], Bethesda, MD) and were bred and maintained in filter cages at the University of Pennsylvania and used between 3 and 7 weeks of age. Sentinel animals housed with the mutant mice were routinely screened for the presence of common mouse pathogens and none have been detected at any time since the animals have been at the University of Pennsylvania. The derivation and maintenance of gnotobiotic IL-2−/− mice has been described previously.40 Animals homozygous for the mutant IL-2 gene were identified from samples of tail DNA using the polymerase chain reaction (PCR)-based method described by Schorle et al.41
Cell isolation.
Blood was obtained by tail bleeding or cardiac puncture and collected in polypropylene tubes containing heparin. Blood counts were obtained using a Baker-1000 automated cell counter (Coulter, Hialeah, FL) and leukocyte differential counts were performed manually on Wright-stained blood smears. Bone marrow cells were obtained from the femurs and tibias of mice by flushing the bones with phosphate-buffered saline (PBS) and scraping the bone fragments. T cells for adoptive transfer experiments were isolated from mesenteric lymph nodes or spleen of 6 to 7 week old IL-2−/− and IL-2+/+ littermates using a negative immunomagnetic selection procedure.42Anti-B220 (clone RA3.6B2; American Type Culture Collection [ATCC], Rockville, MD), -CD11b (M1/70.15; Caltag Labs, San Francisco, CA) antibodies and mouse Ig (Sigma, St Louis, MO) in conjunction with goat antimouse Ig conjugated to immunomagnetic particles (BioMag Beads; Perseptive Diagnostics, Cambridge, MA) were used to remove non-T–cell populations. The isolated cells were routinely >98% CD3+as determined by flow cytometric analysis.
Monoclonal antibodies, flow cytometry, and cell sorting.
The following mouse and rat monoclonal antibodies were used for the immunochemical analysis of bone marrow cells: antimouse CD32/CD16/FcRγII/III (clone 2.4G2; ATCC); CD45R/B220 (RA3-6B2; Pharmingen, San Diego, CA); CD45.1 (A20; Pharmingen); CD45.2 (104; Pharmingen); Sca-1/Ly-6A/E (E13-161.7; Pharmingen); CD90.1/Thy1.1 (HIS51; Pharmingen); CD90.2/Thy1.2 (30H.12; Life Technologies, Gaithersburg, MD); CD3 (29B; Life Technologies); CD24/HSA (M1/69; Pharmingen); CD11b/Mac-1 (M1/70.15; Caltag Laboratories, San Francisco, CA); CD43/S7 (1B11; Pharmingen); Ly-51/BP-1 (6C3; Pharmingen); Gr-1 (RB6-8C5; Pharmingen); IgM (Fab2 fragments of goat antibody; Jackson Immunoresearch, Westgrove, PA); Ter-119 (Pharmingen). Strepavidin-phycoerythrin and strepavidin-red 670 (SA-PE; SA-RED670; Life Technologies) were also used to detect reactivity of biotinylated primary antibodies. All steps of the two- and three-color staining procedure were performed using 96-well, “V”-bottomed microtiter plates. All incubations were performed at 4°C. Approximately 1 × 105 cells in 50 μL of staining buffer (PBS, 5% fetal calf serum [FCS]) were incubated with 5 μg of antimouse FcRγ antibody and 10 mg/mL of mouse and rat IgG (Sigma) for 1 hour to block nonspecific binding of mouse and rat antibodies. The cells were then incubated with the appropriate biotin-conjugated antibodies for 45 minutes, washed twice, incubated for 30 minutes with SA-PE or SA-RED670 and fluorochrome (fluorescein isothiocyanate [FITC], Red 613 or PE)-conjugated antibodies, washed twice and fixed in fixation buffer (PBS, 1% paraformaldehyde). Stained cells were run on a FACScan or FACSCalibur (Becton Dickinson, San José, CA). Antibody-positive populations of cells were distinguished according to the level of staining obtained with fluorochrome-conjugated, mouse and/or rat Ig-isotype–matched, control antibodies of irrelevant specificity. Ten thousand events were collected and analyzed using CellQuest software (Becton Dickinson). For cell sorting, 15 to 30 × 106 bone marrow cells were stained with Mac-1 and anti-IL–2Rβ antibodies as described above, using antibiotic (penicillin/streptomycin and gentamycin, GIBCO-BRL, Gaithersburg, MD) containing staining buffer. Stained cells were run on a FACStar Plus cell sorter (Becton Dickinson) and the Mac-1loIL-2Rβ+ cells were collected and cultured (5 × 104 cells/mL) in Dexter Media (Iscoves Modified Dulbecco's Medium, 10% FCS, 10% horse serum, glutamine, penicillin, streptomycin, and gentamycin) in the presence or absence of 100 U/mL of recombinant IL-2 (Boehringer Mannheim, Indianapolis, IN).
Histology.
The intact spleen and liver of IL-2−/− and control animals was fixed in formalin before embedding in paraffin and sectioning. Ten micron tissue sections and cytocentrifuge preparations of bone marrow cells were counterstained with Wright-Giemsa stain (Sigma) before photomicroscopy. Myeloperoxidase and acid phosphatase activity in cytocentrifuge preparations of bone marrow and blood smears was detected using commercial cytochemical staining kits (Sigma).
In situ hybridization.
Two nonoverlapping IL-2Rβ probes corresponding to either the 176-729 or 985-1,744-bp region of the gene43 were used to synthesize 35S-labeled RNA probes and detect expression of IL-2Rβ-mRNA in cytocentrifuge preparations of sorted bone marrow cells by in situ hybridization as described by us previously.18
Hematopoietic progenitor cell assays.
Soft agar colony forming assays were performed by plating cells in 1.5% methylcellulose (Methocel; Dow, Midland, MI) containing, 30% fetal bovine serum, 1% bovine serum albumin, 40 μg/mL penicillin/streptomycin, 20 ng/mL IL-3, 20 ng/mL GM-CSF, 1 U/mL erythropoietin, and 100 ng/mL stem cell factor. All growth factors used were recombinant. Colonies were scored 11 days after culture. In some experiments, 106 purified T cells from IL-2+/+or IL-2−/− mice were mixed with 5 × 104 bone marrow cells from IL-2+/+ mice before plating in methylcellulose. LTBMC in which wild-type or IL-2−/− bone marrow cells were cultured with wild-type bone marrow stromal cell monolayers in the presence or absence of 100 U/mL recombinant murine IL-2 were performed essentially as described previously.44 Fresh IL-2 was added every 3 to 4 days and at 7, 14, and 21 days the cultures were 100% depopulated of nonadherent cells, which were subsequently evaluated for colony-forming unit (CFU) activity in methylcellulose as described above.
Adoptive T-cell transfer.
Fifty million IL-2−/− or IL-2+/+purified T cells (described above) were injected via a tail vein into sublethally-irradiated (650R) C57BL.6 mice. Six weeks after T-cell transfer, the animals were euthanized and the number and distribution of hematopoietic cells present in the bone marrow determined by flow cytometric analysis.
Bone marrow chimeras.
Recipient B6(CD45.1) mice (n = 4 to 5 per group) were lethally irradiated (1.1 Gy) using a 250-kV x-ray machine at 100 rad/minute. After irradiation, mice were maintained on antibiotic water containing 106 U/liter polymixin B sulfate and 1.1 g/liter of neomycin sulfate. Two hours postirradiation 1 × 106unmanipulated bone marrow cells were injected (200 μL/mouse) into the retro-orbital plexus of anesthetized mice. Five groups of recipient mice (n = 4 to 5) were used; radiation control mice injected with PBS alone, reconstitution control mice injected with host bone marrow cells, and three groups of mice that received IL-2−/− (CD45.2) bone marrow cells alone or IL-2−/− cells mixed with host bone marrow cells in ratios of 1:1 and 5:1 (for competitive reconstitution). Four week old gnotobiotic IL-2−/− mice were used as the source of donor cells because at this age the cellularity and composition of the bone marrow is comparable to that of wild-type littermates (Table 1). At approximately 30, 60, and 90 days postreconstitution, peripheral blood was obtained from the retro-orbital sinus or tail vein and after lysis of erythrocytes, the mononuclear cells were stained with antibodies specific for lineage markers for B cells (anti-B220), myeloid cells (anti-Gr-1 and Mac-1), or T cells (anti-CD3, CD4, and CD8) in combination with antibodies specific for donor (CD45.2) or host (CD45.1) hematopoietic cells and analyzed by flow cytometry. All (five of five) of the radiation control mice died 10 to 14 days postirradiation.
Statistical analysis.
Data are presented as mean ± standard deviation (SD). Statistical significance was assessed by Student's t-test.
RESULTS
Hematopoietic failure and abnormal myeloid cell development in IL-2−/− mice.
By approximately 6 weeks of age, IL-2−/− mice reared and housed in a specific pathogen-free (SPF) environment developed a hematopoietic disorder characterized by anemia and neutropenia (Tables 1 and2). Although the onset and severity of this hematopoietic disorder varied with age and among individual homozygous animals, it was present in all (45/45) SPF IL-2−/− mice. Gnotobiotic IL-2−/− mice also developed this hematopoietic disorder40 (Table 1), although onset was delayed by 1 to 3 weeks compared with SPF IL-2−/− animals. The reason for this delayed onset is not known, but is presumably related to the presence of environmental microbes that may exacerbate and/or accelerate disease. Importantly, since gnotobiotic IL-2−/− mice do not develop colitis,40 it can be concluded that abnormal hematopoiesis in IL-2−/− mice is not secondary to the inflammation, infection, and blood loss associated with colitis. We were unable to detect any evidence for a similar disorder in SPF or gnotobiotic heterozygous mice. SPF IL-2−/− mice of between 5 and 7 weeks of age were used for the majority of the studies described below.
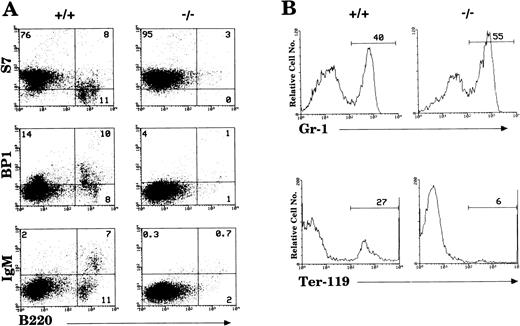
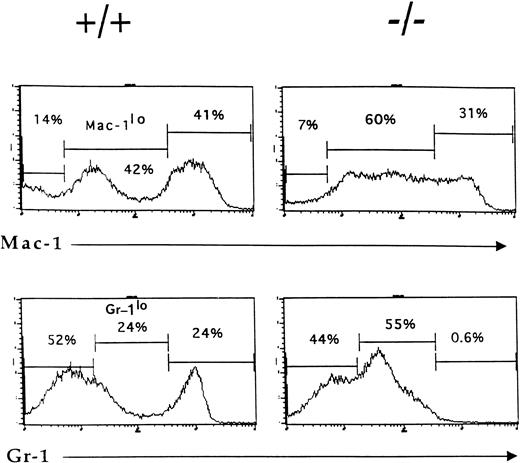
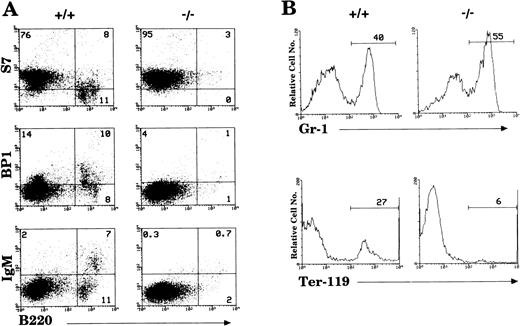
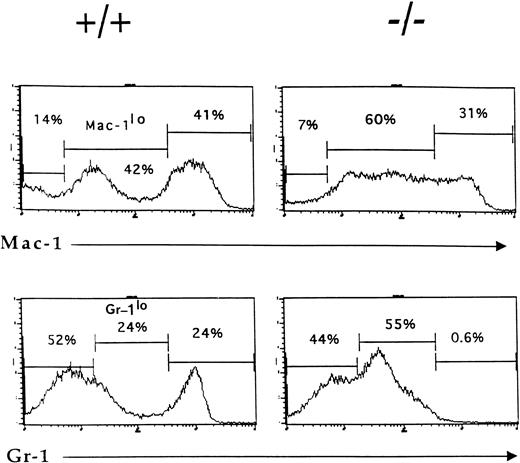
By 6 weeks of age, the bone marrow of IL-2−/−mice showed a 35% to 50% reduction in cellularity compared with the bone marrow of wild-type littermates (Table 1) due primarily to a reduction in the size of the B cell (B220+) and erythroid (Ter-119+) compartments (Fig1). Among populations of B cells, developing pro- (S7+), pre- (BP1+), and mature (IgM+) cells were all affected by the absence of IL-2 (Fig 1A). Although the proportion of granulocytic (Gr-1+) cells (Fig 1B) and myelomonocytic (Mac-1+) cells increased as a result of this loss of B and erythroid cells, the absolute number of these cells was not significantly different from that present in bone marrow of normal mice. However, morphologic analysis of viable cells recovered from the bone marrow of 5 to 7 week old IL-2−/− mice showed that there was an increase in the number of immature myeloid cells (Fig 2). On average, there were twofold to threefold more blasts and promyelocytes/metamyleocytes and a corresponding 65% to 70% decrease in the number of mature polymorphonuclear bone marrow cells in 6 week old IL-2−/− mice compared with IL-2+/+animals (Table 1). This left-shift in IL-2−/−bone marrow cells was confirmed by indirect immunoflourescent staining using biotin-labeled anti–Mac-1 and Gr-1 antibodies in combination with PE-strepavidin. The amplification of staining obtained using the indirect avidin-biotin labeling system enabled the immature (Mac-1/Gr-1lo) and mature (Mac-1/Gr-1hi) myeloid cell populations to be more easily resolved (compare Fig 3 with Fig 1B). The bone marrow of IL-2−/− mice contained increased numbers of Gr-1lo and Mac-1lo cells characteristic of immature cells of the granulocytic45 and myelomonocytic46 47 cell lineages, respectively (Fig 3).
Loss of hematopoietic cells in the bone marrow of IL-2−/− mice. (A) Antibodies reactive with surface antigens that distinguish various stages of B-cell development were used to identify pro- (S7+), pre- (BP1+), and mature (IgM+) B cells in the bone marrow of 6-week old IL-2−/− mice. (B) FITC-Gr–1 and PE-Ter–119 were used to distinguish cells of the myeloid and erythroid lineage, respectively. Stained cells were run on a flow cytometer and analyzed using CellQuest software. The level of staining obtained with isotype-matched control antibodies was used to establish quadrant settings for the dot plots and determine the frequency of cells stained with B cell, erythroid and myeloid cell-specific antibodies (numbers shown in each quadrant of dot plots in A and in histograms in B). The results shown are representative of 10 groups of mice.
Loss of hematopoietic cells in the bone marrow of IL-2−/− mice. (A) Antibodies reactive with surface antigens that distinguish various stages of B-cell development were used to identify pro- (S7+), pre- (BP1+), and mature (IgM+) B cells in the bone marrow of 6-week old IL-2−/− mice. (B) FITC-Gr–1 and PE-Ter–119 were used to distinguish cells of the myeloid and erythroid lineage, respectively. Stained cells were run on a flow cytometer and analyzed using CellQuest software. The level of staining obtained with isotype-matched control antibodies was used to establish quadrant settings for the dot plots and determine the frequency of cells stained with B cell, erythroid and myeloid cell-specific antibodies (numbers shown in each quadrant of dot plots in A and in histograms in B). The results shown are representative of 10 groups of mice.
The absence of IL-2 causes an accumulation of immature myeloid cells in the bone marrow of IL-2−/− mice. Cytocentrifuge preparations of bone marrow cells (BM) from 6-week old SPF wild-type (+/+) and IL-2−/− (×/×) mice were fixed and stained with Wright-Giemsa stain before photomicroscopy. Magnification 800X. The results shown are representative of 10 groups of mice.
The absence of IL-2 causes an accumulation of immature myeloid cells in the bone marrow of IL-2−/− mice. Cytocentrifuge preparations of bone marrow cells (BM) from 6-week old SPF wild-type (+/+) and IL-2−/− (×/×) mice were fixed and stained with Wright-Giemsa stain before photomicroscopy. Magnification 800X. The results shown are representative of 10 groups of mice.
Accumulation of phenotypically-immature myeloid cells in the bone marrow of IL-2−/− mice. Bone marrow cells from 6-week old wild-type (+/+) and IL-2−/− (−/−) littermates were stained with biotin-labeled Mac-1 or Gr-1 antibodies followed by SA-PE and analyzed by flow cytometry. Note the reduction in the size of the mature granulocytes (Gr-1hi) and myelomonocytic (Mac-1hi) cell populations in IL-2−/− mice. The level of staining obtained with isotype-matched control antibodies was used to determine the frequency of cells stained with Mac-1 and Gr-1 antibodies (% values shown in each histogram). The results shown are representative of eight independent experiments.
Accumulation of phenotypically-immature myeloid cells in the bone marrow of IL-2−/− mice. Bone marrow cells from 6-week old wild-type (+/+) and IL-2−/− (−/−) littermates were stained with biotin-labeled Mac-1 or Gr-1 antibodies followed by SA-PE and analyzed by flow cytometry. Note the reduction in the size of the mature granulocytes (Gr-1hi) and myelomonocytic (Mac-1hi) cell populations in IL-2−/− mice. The level of staining obtained with isotype-matched control antibodies was used to determine the frequency of cells stained with Mac-1 and Gr-1 antibodies (% values shown in each histogram). The results shown are representative of eight independent experiments.
The disruption of normal bone marrow hematopoiesis in the IL-2−/− mice was accompanied by extensive extramedullary hematopoiesis in the spleen and liver (data not shown). While the spleens of wild-type mice showed a mild degree of extramedullary hematopoiesis (<5% immature myeloid/erythroid cells), the spleens of IL-2−/− mice showed extensive (70% to 95%) extramedullary hematopoiesis. This increase in extramedullary hematopoiesis was attributable to an increase in immature myeloid cells resulting in enlargement of the red pulp and a relative decrease in the white pulp.
Examination of the blood of SPF IL-2−/− mice showed a progressive anemia and neutropenia (Table 2). The reduction in mature segmented granulocytes was first evident in 3- to 5-week old animals and appeared to precede anemia, which was detected at 6 weeks of age. Accompanying neutropenia was an absence or loss of macrophages in both the thymus48 and peritoneum (data not shown). Peritoneal exudate cells (PEC) from 6-week old IL-2−/−mice contained 60% to 80% less glass-adherent, peroxidase-positive macrophages than wild-type littermates. Despite this loss or absence of mature macrophages in IL-2−/− PEC, it was not possible to detect any quantitative differences in the microbicidal activity or antigen presentation capabilities of peritoneal phagocytic cells when compared with those obtained from wild-type mice (TR and SRC unpublished observations).
Decreased hematopoietic progenitor cell activity in IL-2−/− mice.
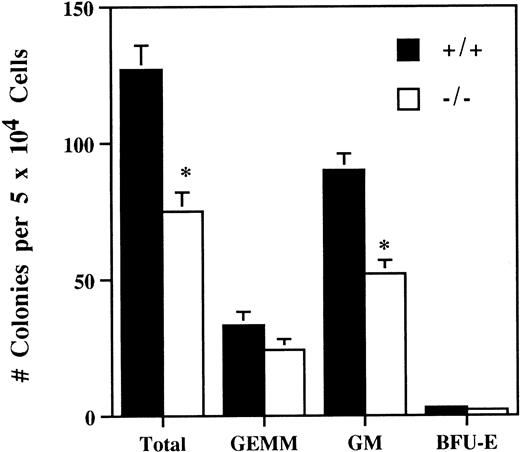
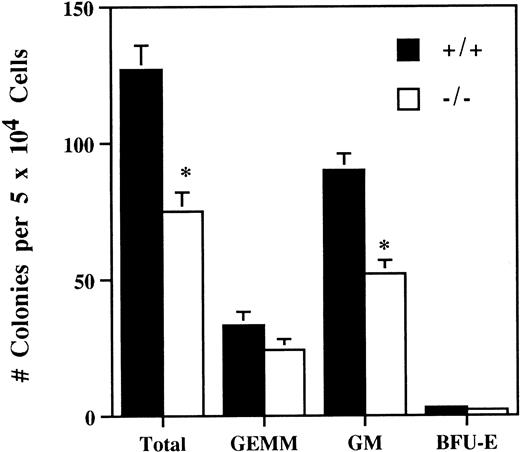
The number of bone marrow hematopoietic and myeloid progenitor cells was evaluated by comparing in vitro colony-forming ability of bone marrow cells from 5-week old SPF IL-2−/− and wild-type littermates. On average, the number of in vitro colony-forming cells in the bone marrow of IL-2−/− mice was reduced by about 50% (Fig 4). In addition, the colonies generated from IL-2−/− bone marrow were smaller and contained approximately 50% less CFU-GM (Fig 4). By contrast, the number of more primitive, multilineage progenitor cells (granulocytic/erythroid/macrophage/megakaryocyte [GEMM]) and erythroid progenitor cells (burst-forming unit-erythroid [BFU-E]) were similar in IL-2+/+ and IL-2−/− bone marrow. These findings are consistent with a proliferative defect in (myeloid) progenitor cells generated in the absence of IL-2. To investigate this possibility further, bone marrow transplant studies were undertaken.
Reduced hematopoietic progenitor cell activity in the bone marrow of IL-2−/− mice. Bone marrow mononuclear cells from 5-week old SPF wild-type (+/+) or IL-2−/− (−/−) mice were cultured in methylcellulose containing IL-3/GM-CSF/erythropoietin/stem cell factor for 11 days and the number of granulocyte/erythroid/myeloid/megakaryocytic (GEMM), granulocyte/macrophage (GM) and blast forming unit-erythroid (BFU-E) colonies counted. * P < .005 for comparison of IL-2−/− and IL-2+/+. The results shown were obtained from three independent experiments.
Reduced hematopoietic progenitor cell activity in the bone marrow of IL-2−/− mice. Bone marrow mononuclear cells from 5-week old SPF wild-type (+/+) or IL-2−/− (−/−) mice were cultured in methylcellulose containing IL-3/GM-CSF/erythropoietin/stem cell factor for 11 days and the number of granulocyte/erythroid/myeloid/megakaryocytic (GEMM), granulocyte/macrophage (GM) and blast forming unit-erythroid (BFU-E) colonies counted. * P < .005 for comparison of IL-2−/− and IL-2+/+. The results shown were obtained from three independent experiments.
IL-2−/− bone marrow cells cannot sustain myelopoiesis upon transfer to lethally irradiated wild-type animals.
Whole bone marrow (106 cells) from IL-2−/− (CD45.2+) mice was injected into lethally irradiated C57BL.6-CD45.1+ hosts; enabling all donor cells and their progeny in chimeric animals to be distinguished by a monoclonal antibody specific for CD45.2. Host animals were divided into five groups (n = 4 to 5 animals per group) depending on the source and composition of donor cells injected; those receiving no cells (radiation control), host bone marrow (reconstitution control), IL-2−/− bone marrow or, for competitive reconstitution experiments, a 1:1 or 1:5 mixture of IL-2−/−:host bone marrow cells. Five-week old gnotobiotic IL-2−/− animals were used as bone marrow donors because at this age, the bone marrow is similar in cellularity and composition to wild-type bone marrow (Table 1). In particular, the number of Sca-1+Thy1loLin- multipotential stem cells in IL-2−/− and IL-2+/+bone marrow was comparable (data not shown). At approximately 30, 60, and 90 days postreconstitution, blood was drawn from surviving animals and the contribution made by donor-derived cells to the myeloid (Gr-1+/Mac-1+), B (B220+), and T (CD3+/CD4+/CD8+) cell lineages determined using anti-CD45.2 or CD45.1 antibodies in conjunction with lineage specific antibodies and flow cytometry.
All of the lethally irradiated mice receiving no cells (PBS alone) died within 10 to 14 days after irradiation. By contrast, all (four of four) of the animals that received IL-2+/+ bone marrow and the majority (four of five) of mice injected with IL-2−/− bone marrow survived until euthanized at 95 days postreconstitution. At necropsy, the lymphoid tissues and intestines of the animals reconstituted with IL-2−/− cells were grossly and histologically normal.
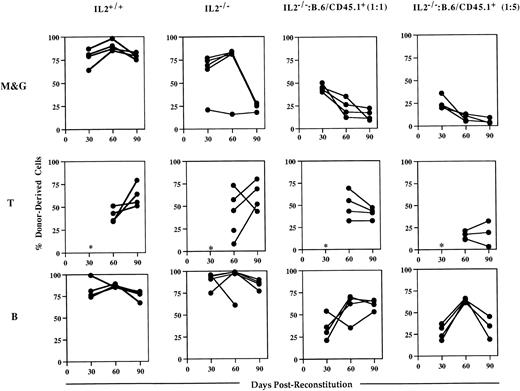
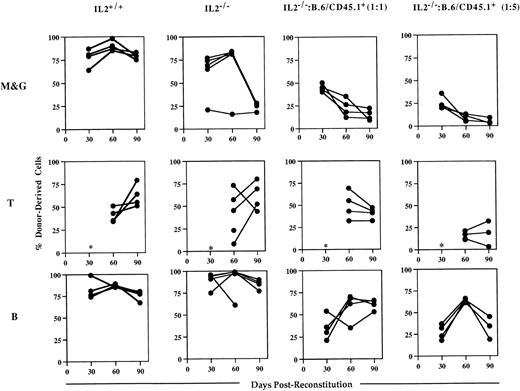
Analysis of the blood of host animals injected with IL-2−/− cells showed that while they could contribute to short-term engraftment and multilineage reconstitution, they were unable to sustain myelopoiesis (Fig 5). Whereas myeloid (Mac-1+/Gr-1+) cell reconstitution by IL-2+/+ bone marrow was almost complete (>80%) in all of the chimeras at 90 days postreconstitution, IL-2−/−-derived cells represented 25% or less of Mac-1+/Gr-1+ cells present in the blood of surviving chimeras. In contrast, the level of T- and B-cell reconstitution in animals receiving IL-2−/−bone marrow was high and similar to that seen in animals reconstituted with IL-2+/+ bone marrow; consistent with the presence of lymphocyte progenitor activity in IL-2−/− bone marrow. These findings were confirmed by the results of competitive reconstitution experiments (Fig 5). Although at 30 days postreconstitution, IL-2−/−–derived cells made up the expected frequency of myeloid cells (approximately 50% and 20% in animals receiving host and IL-2−/− cells in a 1:1 and 5:1 ratio, respectively), their contribution declined thereafter. By 90 days postreconstitution, IL-2−/−–derived cells contributed less than 20% and 10% to the myeloid cells present in animals receiving host and IL-2−/− cells in a 1:1 and 5:1 ratio, respectively. In contrast, IL-2−/−–derived cells made up the expected frequency of B cells in the majority of these chimeras (Fig 5). Together, the data from both the in vitro and in vivo progenitor cell assays suggest that myeloid progenitor cells in IL-2−/− mice have an intrinsic proliferative and/or developmental defect.
IL-2−/− bone marrow cells cannot sustain myelopoiesis in lethally irradiated host animals. Groups (n = 4 to 5) of irradiated host (B.6/CD45.1+) mice were injected with autologous (IL-2+/+) bone marrow or with bone marrow from 5-week old gnotobiotic IL-2−/− mice (IL-2−/−), or IL-2−/− bone marrow mixed with host bone marrow cells in 1:1 or 1:5 ratios. Approximately 30, 60, and 90 days postreconstitution, animals were bled and the mononuclear cells stained with either antibodies specific for the host or donor CD45 allele in combination with antibodies that identify the myeloid (Mac-1 and Gr-1; M&G), T (CD3/CD4/CD8) and B (B220) lineages and analyzed by flow cytometry. The results shown represent the levels (%) of donor-derived cells in each lineage at each time point for individual mice. The asterisk indicates that at 30 days postreconstitution the number of T cells was too low (<1%) to allow accurate quantitation.
IL-2−/− bone marrow cells cannot sustain myelopoiesis in lethally irradiated host animals. Groups (n = 4 to 5) of irradiated host (B.6/CD45.1+) mice were injected with autologous (IL-2+/+) bone marrow or with bone marrow from 5-week old gnotobiotic IL-2−/− mice (IL-2−/−), or IL-2−/− bone marrow mixed with host bone marrow cells in 1:1 or 1:5 ratios. Approximately 30, 60, and 90 days postreconstitution, animals were bled and the mononuclear cells stained with either antibodies specific for the host or donor CD45 allele in combination with antibodies that identify the myeloid (Mac-1 and Gr-1; M&G), T (CD3/CD4/CD8) and B (B220) lineages and analyzed by flow cytometry. The results shown represent the levels (%) of donor-derived cells in each lineage at each time point for individual mice. The asterisk indicates that at 30 days postreconstitution the number of T cells was too low (<1%) to allow accurate quantitation.
Exogenous IL-2 can restore IL-2−/−hematopoietic progenitor cell activity in LTBMC.
To determine if exogenous IL-2 can overcome the intrinsic proliferative and/or developmental defect in IL-2−/−myeloid progenitor cells, the effects of adding IL-2 in vitro to LTBMC of IL-2−/− bone marrow cells was evaluated. This in vitro approach was chosen in preference to administering IL-2 to intact IL-2−/− animals due to the large amounts of IL-2 required to treat animals, the uncertainty of the dose and duration of treatment required, and the potential toxicity of IL-2. Monolayers of bone marrow stromal cells were established from wild-type bone marrow and seeded with either bone marrow cells from 3-week old, healthy SPF IL-2−/− mice or wild-type littermates in the presence or absence of 100 U/mL IL-2. At 7, 14, and 21 days, the cultures were 100% depopulated of nonadherent cells, which were subsequently evaluated for colony-forming ability in soft agar colony-forming assays.
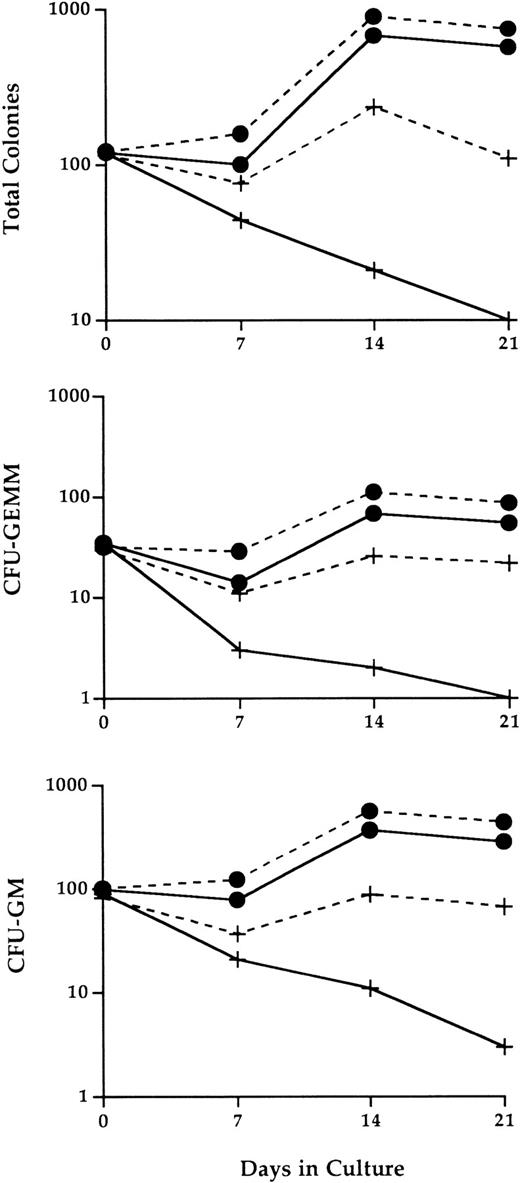
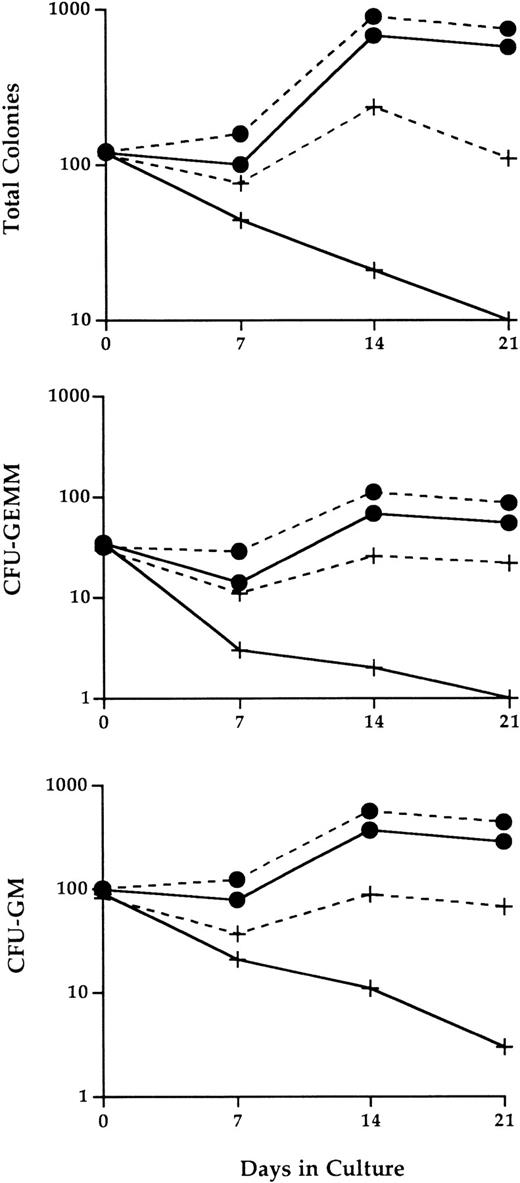
Compared with bone marrow cultures seeded with wild-type hematopoietic cells, the number of progenitor cells continuously declined in cultures seeded with IL-2−/− cells (Fig 6). By 3 weeks of culture, the number of progenitor cells that could be recovered from these cultures was reduced by more than 90%. The progenitor cell loss was particularly apparent for both CFU-GEMM mixed-lineage progenitor population and grnaulocyte/macrophage precursors (CFU-GM).
Abnormal IL-2−/− bone marrow hematopoietic progenitor cell activity in LTBMC. Adherent stromal cell layers established from bone marrow cells of normal adult C57BL.6 mice were seeded at day 0 with 1.2 × 107 bone marrow cells from 3-week old SPF wild-type (•) or IL-2−/− (+) mice in the presence (dashed lines) or absence (solid lines) of 100 U/mL recombinant IL-2. At the times indicated, the cultures were 100% depopulated and the number and type of progenitor cells present identified by methylcellulose CFU assays as described in Materials and Methods. The results shown are representative of three independent experiments. CFU-GM, colony forming unit-granulocyte/macrophage; CFU-GEMM, colony forming unit-granulocyte/erythroid/myeloid/megakaryocyte.
Abnormal IL-2−/− bone marrow hematopoietic progenitor cell activity in LTBMC. Adherent stromal cell layers established from bone marrow cells of normal adult C57BL.6 mice were seeded at day 0 with 1.2 × 107 bone marrow cells from 3-week old SPF wild-type (•) or IL-2−/− (+) mice in the presence (dashed lines) or absence (solid lines) of 100 U/mL recombinant IL-2. At the times indicated, the cultures were 100% depopulated and the number and type of progenitor cells present identified by methylcellulose CFU assays as described in Materials and Methods. The results shown are representative of three independent experiments. CFU-GM, colony forming unit-granulocyte/macrophage; CFU-GEMM, colony forming unit-granulocyte/erythroid/myeloid/megakaryocyte.
This loss of hematopoietic progenitor cell activity was at least partially restored by the addition of exogenous IL-2 to long-term cultures of IL-2−/− bone marrow cells. At 14 and 21 days of culture, the number of colony-forming cells generated from IL-2−/− cells in the presence of IL-2, although still less than that obtained from cultures of IL-2+/+ cells, was between eightfold and 10-fold higher than the number obtained in the absence of IL-2. Similar levels of restored progenitor cell activity were seen for both CFU-GEMM and CFU-GM populations. In contrast, the effect of IL-2 on wild-type progenitor cell activity was less dramatic, resulting in a twofold to threefold increase in the number of progenitor cells. This may be attributable to lower levels of IL-2R–responsive immature myeloid cells in wild-type versus IL-2−/− bone marrow (see below and Fig 7).
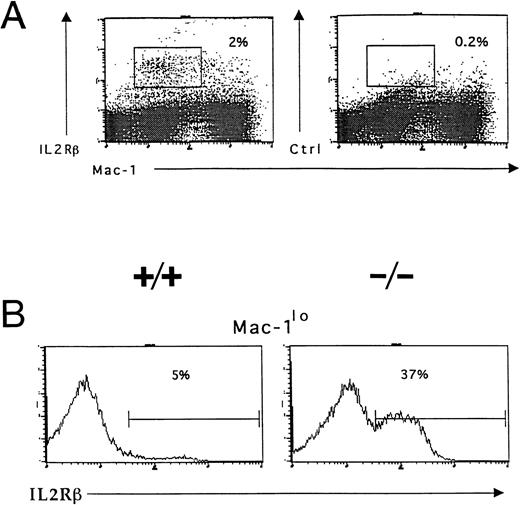
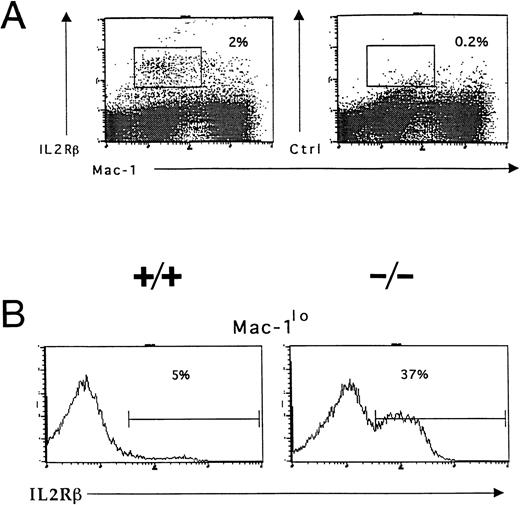
Accumulation of IL-2R–expressing immature myeloid cells in the bone marrow of IL-2−/− mice. Bone marrow cells were isolated from 5-week old wild-type (+/+) and IL-2−/− (−/−) littermates and stained with Mac-1 and IL-2Rβ or isotype-matched control antibodies before analysis using flow cytometry as described in the legend to Fig 3. (A) IL-2Rβ–expressing myeloid cells in the bone marrow of 6-week old C57BL.6 mice. The boxed region represents the frequency of IL-2Rβ+Mac-1lo cells. Isotype-matched control antibodies (Ctrl, right panel) were used to determine the level of nonspecific staining (boxed region). The results shown are representative of five independent experiments. (B) C57BL.6 (+/+) and IL-2−/− (−/−) bone marrow cells expressing low levels of Mac-1 (Mac-1lo) were reanalyzed for expression of IL-2Rβ; the % values represent the frequency of Mac-1locells that are IL-2Rβ+. Note the accumulation of IL-2Rβ–expressing Mac-1lo cells in IL-2−/− mice. The results shown are representative of six groups of mice.
Accumulation of IL-2R–expressing immature myeloid cells in the bone marrow of IL-2−/− mice. Bone marrow cells were isolated from 5-week old wild-type (+/+) and IL-2−/− (−/−) littermates and stained with Mac-1 and IL-2Rβ or isotype-matched control antibodies before analysis using flow cytometry as described in the legend to Fig 3. (A) IL-2Rβ–expressing myeloid cells in the bone marrow of 6-week old C57BL.6 mice. The boxed region represents the frequency of IL-2Rβ+Mac-1lo cells. Isotype-matched control antibodies (Ctrl, right panel) were used to determine the level of nonspecific staining (boxed region). The results shown are representative of five independent experiments. (B) C57BL.6 (+/+) and IL-2−/− (−/−) bone marrow cells expressing low levels of Mac-1 (Mac-1lo) were reanalyzed for expression of IL-2Rβ; the % values represent the frequency of Mac-1locells that are IL-2Rβ+. Note the accumulation of IL-2Rβ–expressing Mac-1lo cells in IL-2−/− mice. The results shown are representative of six groups of mice.
These findings are consistent with the results obtained from the in vivo bone marrow transplant studies and suggest that the abnormal myelopoiesis in IL-2−/− mice is a consequence of an intrinsic developmental or proliferative defect in a myeloid progenitor cell(s). They also show that in vitro, IL-2 can partially overcome this defect and promote myeloid progenitor cell growth. However, due to the inherent complexity and cellular heterogeneity of LTBMC, there are many potential mechanisms by which IL-2 could enhance myelopoiesis in LTBMC. Additional experiments were designed, therefore, to determine if immature myeloid cells normally express functional IL-2Rs and if IL-2 can directly influence the growth and development of myeloid progenitors.
Immature myeloid cells express IL-2Rs.
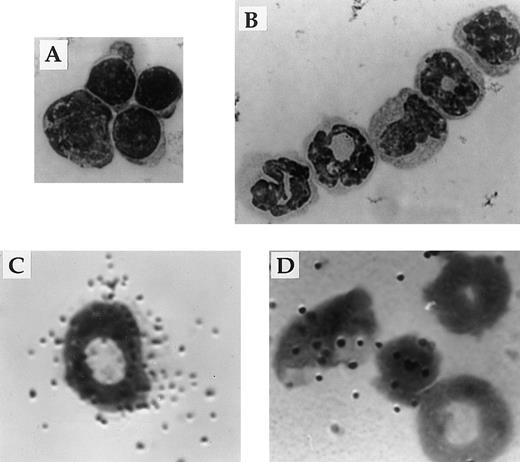
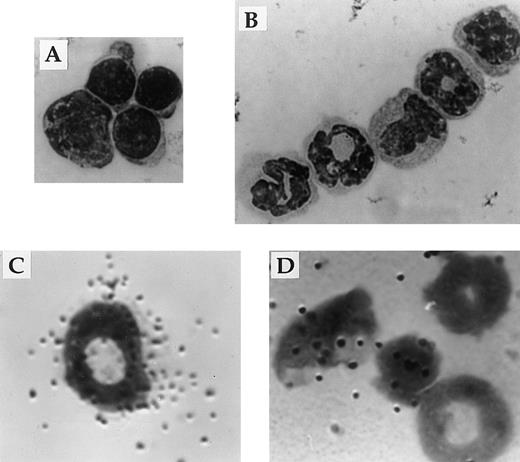
Responsiveness to IL-2 depends on expression of a receptor for IL-2 on the surface of the cell. Flow cytometric and in situ hybridization techniques were used to examine expression of CD122 (IL-2Rβ), the signaling component of the IL-2R complex, by immature myeloid cells in the bone marrow of 6-week old IL-2+/+ mice. Flow cytometric analysis showed that approximately 5% of myeloid (Mac-1+) cells in the adult bone marrow expressed IL-2Rβ (Fig 7A). The Mac-1loIL-2Rβ+ cells made up approximately 50% of IL-2Rβ-expressing Mac-1+ cells (boxed region in Fig 7A). The specificity of anti–IL-2Rβ antibody staining was confirmed by the lack of staining by these cells with isotype-matched control antibodies (Fig 7A). The majority of IL-2Rβ+cells also expressed low levels of the IL-2Rα-chain (data not shown). Analysis of IL-2−/− bone marrow showed that the number of Mac-1loIL-2Rβ+ immature myeloid cells was increased by more than sevenfold (Fig 7B) in agreement with the increased number of blasts and promyelocytes/metamyelocytes detected by histomorphologic analysis (Table 1). Morphologic analysis of fluorescence-activated cell sorting (FACS) isolated IL-2Rβ+ Mac-1lo cells confirmed that this population of cells contained myeloid progenitors (myeloblasts and promyelocytes; Fig 8A). In contrast, the Mac-1hiIL-2Rβ+ population of bone marrow cells (Fig 6) comprised more mature cells including metamyelocytes, band cells, and mature granulocytes (Fig 8B). This distribution of Mac-1 expression is consistent with it being a myeloid differentiation marker that is upregulated during myeloid cell development.46 47 To substantiate the results of the phenotypic analysis and exclude the possibility that the anti–IL-2R antibody staining was an artifact, in situ hybridization was used to identify IL-2Rβ-mRNA expression among bone marrow cells from 6-week old severe combined immunodeficient (SCID) mice. As seen in Fig 8C, IL-2Rβ-mRNA was readily detected in populations of myeloid cells that were morphologically similar to FACS-isolated IL-2Rβ-expressing Mac-1+ cells.
Expression of IL-2Rβ mRNA and protein by immature cells of the myeloid lineage. The appearance of Mac-1loIL-2Rβ+ (A) and Mac-1hiIL-2Rβ+ (B) bone marrow cells isolated by FACS. The identity of bone marrow cells expressing the gene encoding the β-chain of the IL-2R (C and D) was determined by hybridization in situ of 35S-labeled probes synthesized in the antisense (C) or sense (D) orientation to cytocentrifuge preparations of total bone marrow from adult SCID mice. All cytocentrifuge preparations were fixed and stained with Wright-Giemsa stain before photomicroscopy. Magnification in A 800X and B through D 1000X. The results shown are representative of four independent experiments.
Expression of IL-2Rβ mRNA and protein by immature cells of the myeloid lineage. The appearance of Mac-1loIL-2Rβ+ (A) and Mac-1hiIL-2Rβ+ (B) bone marrow cells isolated by FACS. The identity of bone marrow cells expressing the gene encoding the β-chain of the IL-2R (C and D) was determined by hybridization in situ of 35S-labeled probes synthesized in the antisense (C) or sense (D) orientation to cytocentrifuge preparations of total bone marrow from adult SCID mice. All cytocentrifuge preparations were fixed and stained with Wright-Giemsa stain before photomicroscopy. Magnification in A 800X and B through D 1000X. The results shown are representative of four independent experiments.
IL-2Rs expressed by immature myeloid cells are functional.
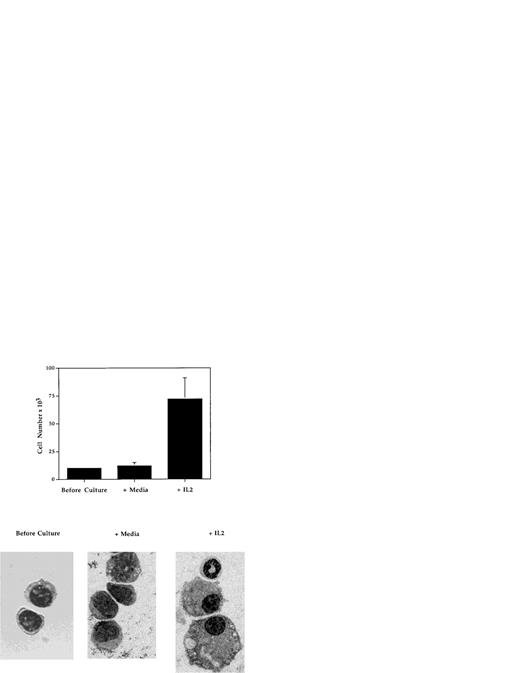
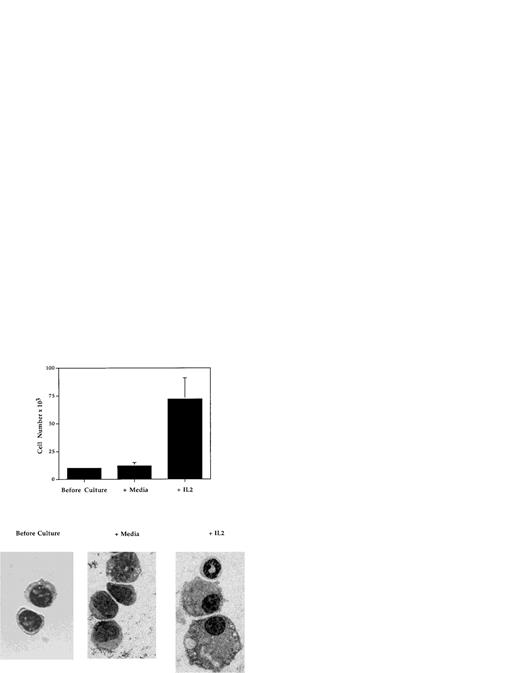
To determine whether IL-2 is capable of influencing the development of myeloid progenitor cells, the effects of IL-2 on the growth and development in vitro of highly purified Mac-1loIL-2Rβ+ cells (Fig 9) were evaluated. Cells were isolated from the bone marrow of adult C57BL.6 or SCID mice by FACS and cultured in 96-well tissue culture plates for 5 to 7 days in the absence or presence of IL-2 (100 U/mL), without any additional growth factors or stromal cells. In three independent experiments (Fig 9), the number of myeloid cells recovered from cultures containing IL-2 (mean, 70 × 103; range, 60 to 88 × 103) was on average sixfold to sevenfold greater than that obtained from cultures containing media alone (mean = 10.6 × 103; range, 9 to 13 × 103). Histologic analysis of cells before and after culture showed that the addition of IL-2 resulted in the generation of morphologically mature myeloid cells including band cells and segmented granulocytes, as well as macrophages (Fig 9). In contrast, in cultures containing media alone (Fig 9) or IL-15 (data not shown), the only other known ligand for IL-2Rβ, the viable cells remaining contained few if any mature granulocytes or macrophages. These findings demonstrate that, at least in vitro, IL-2 can directly induce the growth and development of immature (Mac-1loIL-2Rβ+) myeloid cells.
IL-2 promotes the growth and differentiation of immature myeloid cells. FACS-isolated Mac-1loIL-2Rβ+bone marrow cells (before culture) were cultured for 7 days in complete media in the absence (media) or presence (+IL-2) of 100 U/mL of IL-2 after which the cells were harvested, counted, and cytocentrifuge preparations made. Upper panel: addition of IL-2 resulted in significant increase in the number of cells recovered at the end of culture (P < .001). Lower panel: cytocentrifuge preparations of Mac-1loIL-2Rβ+ cells before (before culture) and 7 days after culture in the absence (media) or presence (+IL-2) of IL-2. In the presence of IL-2, Mac-1loIL-2Rβ+ cells give rise to macrophages as well as mature granulocytes. Magnification 1,000X. The results are representative of three independent experiments.
IL-2 promotes the growth and differentiation of immature myeloid cells. FACS-isolated Mac-1loIL-2Rβ+bone marrow cells (before culture) were cultured for 7 days in complete media in the absence (media) or presence (+IL-2) of 100 U/mL of IL-2 after which the cells were harvested, counted, and cytocentrifuge preparations made. Upper panel: addition of IL-2 resulted in significant increase in the number of cells recovered at the end of culture (P < .001). Lower panel: cytocentrifuge preparations of Mac-1loIL-2Rβ+ cells before (before culture) and 7 days after culture in the absence (media) or presence (+IL-2) of IL-2. In the presence of IL-2, Mac-1loIL-2Rβ+ cells give rise to macrophages as well as mature granulocytes. Magnification 1,000X. The results are representative of three independent experiments.
Dysfunctional IL-2−/− T cells do not disrupt normal hematopoietic progenitor cell activity.
Several studies have suggested that the hematopoietic and immune system disorders that develop in IL-2−/− mice are caused by lymphoid hyperplasia and T-cell infiltration into various tissues including the bone marrow.49 50 We determined, therefore, if hematopoietic failure in IL-2−/−mice could be the result of indirect effects of the absence of IL-2 and the presence of dysfunctional T cells.
The ability of IL-2−/− T cells to disrupt the activity of normal hematopoietic progenitor cells in in vivo and in vitro assays was determined. Approximately 5 × 107 purified lymph node T cells from 6- to 7-week old IL-2−/− or wild-type C57BL.6 mice (Thy1.2+) were injected intravenously into sublethally irradiated Thy1.1+ C57BL.6 mice. Six weeks later animals were euthanized and their bone marrow analyzed to determine the affects of the transferred IL-2−/− T cells. As shown in Table 3, donor (Thy1.2+) T cells were the predominant T-cell population in the bone marrow of host animals. However, despite their presence, it was not possible to detect any significant changes in the number of B (B220+), erythroid (Ter-119+), or myeloid (Mac-1+/Gr-1+) cells. Importantly, the proportion of immature (Mac-1lo/Gr-1lo) and mature (Mac-1hi/Gr-1hi) myeloid cells was also similar to that seen in animals transfused with wild-type T cells.
The possibility that factors produced by IL-2−/− T cells were inhibitory to hematopoietic progenitor cell growth was also investigated. One million purified lymph node or splenic IL-2−/− or IL-2+/+ T cells were added to 5 × 104wild-type bone marrow cells in methylcellulose and the number of colonies generated after 12 days counted. The number of colonies produced in the presence of IL-2−/− T cells (68 ± 10) was not significantly different from the number generated in the absence of any T cells (77 ± 6), or IL-2+/+ T cells (81 ± 11). In addition, the type of colonies generated was also comparable in cultures containing IL-2+/+ or IL-2−/− T cells (data not shown). Together these results show that dysfunctional IL-2−/− T cells do not adversely affect hematopoietic cell development and are unlikely to be the cause of abnormal myelocytic cell generation in IL-2−/− mice.
DISCUSSION
Because myeloid cells form a vital and integral part of the immune system, it is important to understand the regulation of their generation. A variety of experimental approaches have established that granulocytes and macrophages are derived from CFU-GM in the presence of hematopoietic growth factors or colony-stimulating factors (CSF).51 Among these CSFs, G-, M-, and GM-CSF have been shown to influence the survival, proliferation, differentiation, and function of mature granulocytes and macrophages, as well as their precursors. However, in view of the overlapping activities of these factors and the absence of any overt effects on myelopoiesis in some CSF-deficient animals,52,53 it is possible that there may be considerable redundancy among these factors,54 and other unrelated factors may be required for myeloid cell development in vivo. The results of our studies presented here demonstrate that IL-2, generally considered a lymphocyte growth factor, plays a role in myelopoiesis.
In the absence of IL-2, mice develop a profound hematopoietic disorder characterized by defective myelopoiesis and decreased numbers of mature granulocytes. The reduced number or absence of tissue-specific macrophages (eg, peritoneal and thymic48 macrophages) in neonatal and adult IL-2−/− mice also suggests that IL-2 can influence macrophage development. Although we have not been able to detect any significant difference in the microbicidal, cytotoxicity or antigen presentation capabilities of residual peritoneal phagocytic cells in IL-2−/− mice (T.R. and S.R.C., unpublished observations), the loss or absence of thymic macrophages in SPF and gnotobiotic IL-2−/− mice is accompanied by abnormalities in intrathymic T-cell progenitor development.48 The ability to prolong the life span of neutropenic IL-2−/−mice when reared and maintained under gnotobiotic conditions40 suggests that the rapid deterioration in health status and premature death of SPF IL-2−/− mice may be attributable, at least in part, to the loss of mature, functional granulocytes and increased susceptibility to (opportunistic) environmental pathogens.
Based on the inability of IL-2−/− bone marrow cells to sustain myelopoiesis in lethally irradiated mice and in LTBMC, this hematopoietic disorder could be due to an intrinsic proliferative and developmental defect in a hematopoietic stem or myeloid progenitor cell population. Because inactive stem cells would prevent multilineage reconstitution,55 56 the normal number of stem cells and multilineage (GEMM) progenitors, and the presence of long-term lymphoid progenitor activity in IL-2−/− bone marrow suggests that stem cells are intact and that the defective hematopoietic cell is a myeloid progenitor cell(s). Our inability to detect any differences in the ability of IL-2−/− bone marrow cells to generate erythroid (BFU-E) or lymphoid progenitor cells in in vitro or in vivo differentiation assays when compared with IL-2+/+ cells is consistent with this interpretation.
Several lines of evidence suggest that the myeloid progenitor cell defect in IL-2−/− mice is a consequence of their direct dependency on IL-2 for their growth and possibly development: (1) expression of functional IL-2Rs by myeloblasts and promyelocytes, (2) the generation of mature granulocytes (as well as macrophages) from Mac-1loIL-2R+ immature myeloid cells in the presence of IL-2, and (3) the accumulation of IL-2Rβ+ immature myeloid (Mac-1lo) cells in IL-2−/− mice. Together, these findings demonstrate that the IL-2/IL-2R signaling pathway can directly influence myeloid cell development in vitro. The demonstration that IL-2 can directly promote myeloid progenitor cell growth is consistent with, and now provides an explanation for, the observed differential expression of IL-2Rs by myeloid cells during in vitro differentiation27 and the ability of IL-2 to increase the number of circulating myeloid progenitor cells in vivo.28-31 Importantly, the requirement by myeloid progenitor cells for IL-2 also provides an explanation for the accumulation of IL-2R–bearing immature myeloid cells and the concomitant loss of mature granulocytes in IL-2−/− mice. Finally, the occurrence of a granulocyte deficiency in mice that lack the IL-2Rβ-chain57 similar to that which we have described in IL-2−/− mice agrees with our conclusion that the IL-2/IL-2R signaling pathway plays an important role in regulating myelocytic cell generation.
Although our data is most consistent with this interpretation, we cannot exclude the possibility that IL-2 may influence myeloid cell development in other ways. For example, IL-2 may be a competence factor enabling immature myeloid cells to acquire responsiveness to other growth and differentiation factors. Because IL-2 is known to be able to influence the production of other hematopoietic growth factors by activated T cells, and possibly by other hematopoietic cells, it may be required to induce the synthesis of other factors essential for hematopoiesis and stromal cell growth. The finding that IL-2 can prevent apoptosis in cultured human blood-derived neutrophils21 suggests that IL-2 can act as a survival factor for myeloid cells. However, we have been unable to demonstrate a similar effect of IL-2 when included in 48-hour cultures of IL-2+/+ mouse blood or bone marrow mononuclear cells (H.B. and S.R.C., unpublished observations). Finally, the possibility that IL-2 has multiple effects during myelopoiesis and that the nature of its influences is dictated by the developmental stage of the IL-2R–bearing myeloid cell cannot be ignored. This would be consistent with the wide distribution of IL-2Rs among developing myeloid cells and the different responses (proliferation, survival, differentiation, and modulation of functional activity) to IL-2 that have been observed. This mechanism of action of IL-2 would be analogous to the multiple actions of the CSFs on CFU-GM and their progeny.51
The lymphoid hyperplasia that disrupts the bone marrow and other tissues in IL-2−/−58 and IL-2Rβ−/−57 mice may exacerbate hematopoietic failure in these animals. Indeed, the defective or abnormal B-cell development and hemolytic anemia that occurs in IL-2−/−50 and IL-2Rβ−/−57 mice has been attributed to dysfunctional T cells. However, the fact that in progenitor cell assays myeloid cell development is defective in the absence of T cells, the presence of IL-2−/− T cells does not interfere with the development of normal bone marrow hematopoietic cells either in vitro or in vivo, and the onset of the hematopoietic disorder occurs in gnotobiotic IL-2−/− mice before lymphocyte hyperplasia40 makes it unlikely that they are the cause of abnormal myelopoiesis. Defective myelopoiesis in gnotobiotic IL-2−/− that do not develop colitis40 also convincingly demonstrates that this disorder is not secondary to the inflammation, infection, and blood loss associated with colitis. Finally, the inability to abrogate or prevent the granulocyte defect and hematopoietic failure in IL-2Rβ−/− mice after antibody-mediated T-cell depletion57 is also inconsistent with the involvement of dysfunctional T cells in the disruption of myelocytic cell development that occurs in the absence of a functional IL-2/IL-2R signaling pathway.
Collectively, we interpret our findings as evidence for the involvement of IL-2 in the homeostatic regulation of myelopoiesis. We propose that through its growth promoting activity IL-2 normally serves to expand and maintain the size of the Mac-1loIL-2Rβ+myeloid progenitor cell population in the bone marrow. In its absence, this population would be progressively depleted by the successive waves of progenitor cell development necessary to continually replace short-lived (on average <24 hours55) peripheral blood granulocytes. This could explain why some level of myeloid cell maturation occurs in the absence of IL-2 in IL-2−/− mice, why IL-2−/− bone marrow cells cannot sustain long-term granulopoiesis, and why the hematopoietic defect in IL-2−/− mice is not apparent until after birth. Alternative possibilities such as the existence of IL-2–independent myeloid progenitors and granulocytes and a differential requirement for IL-2 by fetal and adult myeloid progenitor cells and/or the environments in which they develop cannot be excluded. Because the incidence of hematopoietic failure in IL-2−/−mice is coincident with, or occurs soon after, development of the bone marrow as a definitive adult hematopoietic organ has been completed,59 adult progenitor cells may be more dependent on IL-2 than their fetal counterparts.
In summary, the findings we report here show for the first time that IL-2 plays an important role in regulating myelocytic cell development in vivo. The observation that human immunodeficiency due to defective IL-2 production60 is also associated with deficient hematopoiesis in the bone marrow (K. Weinberg, personal communication, 1996) suggests that IL-2 may have a similar role in regulating hematopoiesis in humans.
ACKNOWLEDGMENT
We thank G. Trinchieri, M.I. Greene, and K. Weinberg for their critical review of the manuscript, R. Schwartz for providing the IL-2–deficient mice, and H. Pletcher for assistance with flow cytometric analyses and cell sorting.
Supported in part by grants from the American Cancer Society (to S.R.C.), the Leukemia Society of America (to S.G.E. and M.S.C.), W.W. Smith Chari Trust (to S.R.C.), and the National Institutes of Health (to S.R.C. and S.G.E.).
Address reprint requests to Simon R. Carding, PhD, Department of Microbiology, University of Pennsylvania, 303A Johnson Pavilion, Philadelphia, PA 19104-6076.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.