Adoptive transfer of Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes (CTLs) is effective prophylaxis and treatment of EBV-positive immunoblastic lymphoma in immunocompromised patients. In 50% of patients with Hodgkin's disease, the tumor cells are EBV antigen-positive and may therefore also be suitable targets for treatment with virus-specific CTLs. However, Hodgkin's disease may produce several inhibitory effects on immune induction and effector function in vivo, which may preclude the generation or effector function of CTLs reactive against EBV viral proteins, including those expressed by the tumor cells. We have investigated whether EBV-specific CTLs could be generated ex vivo from 13 patients with Hodgkin's disease: nine with active relapsed disease and four who were in clinical remission after a first or subsequent relapse. CTL lines were successfully generated from nine of 13 patients (five active disease, four remission). Although these lines had an abnormal pattern of expansion comparable to EBV-specific CTLs generated from normal donors, their phenotype was normal except for reduced expression of the zeta chain of the T-cell receptor (TCR). Their cytotoxicity was also compared to EBV-specific lines generated from normal donors and included activity against LMP2a, one of the three weakly immunogenic viral antigens expressed by Hodgkin's tumor cells. To assess the activity of the CTLs in vivo, they were gene-marked and infused into three patients with multiply relapsed disease. The CTLs persisted for more than 13 weeks postinfusion and retained their potent antiviral effects in vivo, thereby enhancing the patient immune response to EBV. This approach may therefore have value in the treatment of EBV-positive Hodgkin's disease.
ALTHOUGH THE ETIOLOGY of Hodgkin's disease remains unclear, approximately 50% of cases in North America and Europe are associated with Epstein-Barr virus (EBV).1In South America, Kenya, and parts of Asia, there is a 90% to 100% association.2,3 EBV is a ubiquitous gamma-herpes virus that has been associated with several malignant diseases in addition to Hodgkin's lymphoma. These include nasopharyngeal carcinoma, Burkitt's lymphoma, and immunoblastic lymphoma as seen in the immunocompromised host.4-6
Recent work from our group has shown that EBV-specific cytotoxic T lymphocytes (CTLs) generated from normal donors can be adoptively transferred to patients who have received T-cell–depleted allogeneic stem-cell transplants. These CTLs persist long-term in vivo, reconstitute the immune response to EBV, and are effective as prophylaxis and treatment of immunoblastic lymphoma.7,8Implementing such an approach for EBV-positive Hodgkin's disease would have considerable appeal; although 80% or more of patients are cured with conventional therapy, more than half of those who relapse fail to respond to salvage chemotherapy or relapse a second time have a poor long-term prognosis. Furthermore, the unacceptably high level of therapy-related secondary malignancies (18% at 5 years) and other serious medical complications in those who are treated successfully also underscores a need to improve current therapeutic options.9
A number of obstacles may diminish the effectiveness of EBV-specific CTLs in Hodgkin's disease. Many patients with Hodgkin's disease may have T-cell abnormalities, such as low expression of the zeta chain of the T-cell receptor (TCR).10,11 In addition, Hodgkin's cells may secrete interleukin-10 (IL-10), a cytokine inhibitory to the induction of a cytotoxic T-cell response.12 Other CTL-inhibitory mechanisms may also operate.13,14 Finally, the malignant cells of Hodgkin's disease express a restricted set of viral genes, namely, EBNA1 in the nucleus and LMP1 and LMP2 in the plasma membrane.15 EBNA1 cannot enter the human leukocyte antigen (HLA) class I processing pathway, because of its gly/ala repeat, which inhibits its binding the TAP-transported proteins. Although a minority of normal donors have CTLs against LMP1 and LMP2, these antigens are weakly immunogenic in the context of most HLA types.16
To assess the feasibility of using EBV-specific CTLs as therapy for Hodgkin's disease, we generated EBV-specific CTLs from the peripheral blood of patients with Hodgkin's disease, hoping that they could be expanded in vitro, in the absence of in vivo immunosuppressive effects. We then compared them with CTLs generated from normal donors.17 To discover whether autologous EBV-specific CTLs could persist and have antiviral activity in vivo, we genetically marked them and adoptively transferred them to three patients with relapsed disease.
MATERIALS AND METHODS
Patients and EBV status of the tumors.
Patients were enrolled onto a study that was approved by the hospital's institutional review board and by the Food and Drug Administration. All of these children and adolescents (Table1) had histologically proven EBV-positive Hodgkin's disease. Samples were collected from the patients while their disease was in clinical remission after a first or subsequent relapse, or while they had active relapsed disease provided they had received no chemotherapy for a minimum of 4 weeks.
Patient Details
| Patient No. . | Age at Diagnosis (yr) . | Histologic Subtype . | Tumor EBV Status . | Clinical Status . | |
|---|---|---|---|---|---|
| EBER . | LMP1 . | ||||
| 1 | 16 | MC | + | + | Relapse |
| 2 | 18 | NS | + | + | Relapse |
| 3 | 17 | MC | + | + | Relapse |
| 4 | 10 | MC | + | − | Remission |
| 5 | 12 | MC | + | + | Remission |
| 6 | 14 | NS | + | − | Relapse |
| 7 | 17 | MC | + | + | Relapse |
| 8 | 15 | MC | + | − | Remission |
| 9 | 12 | MC | + | + | Relapse |
| 10 | 17 | NS | + | + | Relapse |
| 11 | 7 | NS | + | + | Relapse |
| 12 | 13 | NS | + | + | Remission |
| 13 | 18 | NS | − | + | Relapse |
| Patient No. . | Age at Diagnosis (yr) . | Histologic Subtype . | Tumor EBV Status . | Clinical Status . | |
|---|---|---|---|---|---|
| EBER . | LMP1 . | ||||
| 1 | 16 | MC | + | + | Relapse |
| 2 | 18 | NS | + | + | Relapse |
| 3 | 17 | MC | + | + | Relapse |
| 4 | 10 | MC | + | − | Remission |
| 5 | 12 | MC | + | + | Remission |
| 6 | 14 | NS | + | − | Relapse |
| 7 | 17 | MC | + | + | Relapse |
| 8 | 15 | MC | + | − | Remission |
| 9 | 12 | MC | + | + | Relapse |
| 10 | 17 | NS | + | + | Relapse |
| 11 | 7 | NS | + | + | Relapse |
| 12 | 13 | NS | + | + | Remission |
| 13 | 18 | NS | − | + | Relapse |
Abbreviations: MC, mixed cellularity; NS, nodular sclerosing.
The EBV status of the tumors was determined as described elsewhere.18 In brief, dewaxed sections of paraffin-embedded tissue were hybridized to digoxygenin-labeled riboprobes specific for EBER1 (EBV-encoded small nuclear RNA) in a standard in situ hybridization assay. Bound probe was detected by an antidigoxygenin antibody–alkaline phosphatase conjugate (Boehringer Mannheim, Mannheim, Germany) as suggested by the manufacturer. For detection of latent membrane protein 1 (LMP1), the CS1-4 mouse monoclonal antibody (Dako, Carpenteria, CA) was used in an immunoperoxidase protocol recommended by the manufacturer (Vectastain Elite ABC; Vector, Burlingame, CA). Of 35 cases analyzed, 13 were positive for either EBER1 or LMP1 or both, and were considered EBV-positive (Table 1).
Normal donors.
EBV-specific CTL lines were generated from the peripheral blood of normal bone marrow donors, for the prophylaxis and treatment of EBV lymphoma in the marrow recipients.17 Data from these CTL lines have been collected over the past 4 years.
EBV-transformed B-cell lines.
Samples of peripheral blood (20 to 40 mL) were collected from patients with EBV-positive Hodgkin's disease or normal bone marrow donors and used to generate both a B-lymphoblastoid cell line (LCL) and a CTL line. To establish spontaneously transformed or B95-8–transformed LCL, peripheral blood mononuclear cells (PBMC) were plated at 106 cells per well in flat-bottomed 96-well plates containing RPMI 1640 medium (GIBCO Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum, 1%l-glutamine, 1% penicillin/streptomycin, and 1 μg/mL cyclosporine A (Sandoz Pharmaceuticals Inc, Washington, DC). Concentrated supernatant (10 μL, see later) from B95-8 cultures, a human type I EBV-transformed marmoset B-cell line, was added to parallel samples.19 20 Once B95-8–infected LCL were established, they were expanded into 25-cm2flasks. Aliquots of these long-term cultures were frozen.
Concentrated supernatant from B95-8 cells.
We used a B95-8 virus-producer cell line that was negative for mycoplasma (Gen-Probe, San Diego, CA), for squirrel monkey retrovirus (by Southern and Western blotting), and for viruses, other than EBV, detectable by electron microscopy. Virus stocks were made from a master cell bank that was grown for 7 to 9 days in supplemented RPMI 1640. Supernatants were harvested by centrifugation, filtered (0.45-μm pores), and then concentrated 50-fold by ultrafiltration through a pressurized concentrator (Cole Parmer, Niles, IL) containing a disk with a molecular weight cutoff of 500,000 kD. Aliquots of virus were frozen in liquid nitrogen, then tested for their ability to transform peripheral B cells from EBV-negative donors. Virus stocks that transformed B cells at dilutions of 10−5 or lower were used to produce patient LCL.
EBV-specific CTL lines.
EBV-specific CTLs were activated from the peripheral blood of patients and normal donors by coculturing 2 × 106 PBMC per well of a 24-well (1.5-cm diameter) plate with 5 × 104 autologous LCLs that had been irradiated with 40 Gy from a cesium source. This radiation dose entirely prevented LCL outgrowth in control cultures. After 10 days, the cells were harvested on Ficoll gradients (ICN, Irvine, CA), subcultured in 24-well plates at 5 × 105 per well, and restimulated with 1.25 × 105 irradiated LCLs per well. After 4 days, the cultures were fed with 20 U/mL of IL-2 (Proleukin; Cetus, Emeryville, CA). Thereafter, the cultures were fed three times weekly, twice with 20 U/mL of IL-2 and the third time with 10 to 15 U/mL of IL-2 supplemented with irradiated LCLs (T cell:LCL ratio, 4:1). CTL lines were successfully established from nine of the 13 patients: seven on the first attempt and two on the second attempt. CTL lines could not be established on the only attempt from four patients, all with relapsed disease. Lines from patients no. 1, 2, and 13 were infused into patients for the treatment of their relapsed disease. In contrast, CTL lines were successfully established from greater than 95% of normal EBV-seropositive donors.17
To increase the rate of expansion when large cell numbers were required or when the cell line was growing poorly, the third feeding consisted of a “superexpansion cocktail” that was a variation of one reported by Riddell and Greenberg.21 This mitogenic mixture contained 10-15 U/ml of IL-2, a submitogenic (0.01 μg/mL) dose of anti-CD3 monoclonal antibody (Ortho Diagnostics, Raritan, NJ), irradiated autologous LCLs (T cell:LCL ratio, 4:1), and irradiated PBMC from cytomegalovirus-negative donors who met our institutional requirements for blood donors (T cell:PBMC ratio, 8:1). To avoid stimulating allospecific clones, this cocktail was never used less than 21 days after initiation of the CTL line. This step was rarely required for the generation of CTLs from the normal bone marrow donor controls.
CTL reactivations.
To assess the contribution of the infused CTLs to the patient's immune response to EBV postinfusion, EBV-specific CTL lines were reactivated from patients no. 1 and 13, 1 and 2 months postinfusion using the autologous LCLs as stimulators as described earlier. After 4 weeks of culture, the cytotoxic specificity of the line was analyzed and DNA was extracted to compare the level of gene marking of the reactivated line with that of the infused line (results not shown).
Gene marking of EBV-specific CTLs.
CTLs were genetically marked by transduction with the G1Na retroviral vector, which contains the Escherichia coli–derived neomycin resistance gene (neo). Clinical grade G1Na-containing supernatant from a PA317 amphotropic packaging cell line (Genetic Therapy, Gaithersburg, MD) was incubated for 6 hours with the CTLs at a multiplicity of infection of 10:1, in the presence of IL-2 (50 U/mL) and protamine sulfate (4 μg/mL) in a 75-cm2flask.17
To determine the efficiency of transduction, DNA was extracted from an aliquot of the transduced cells by using an anion-exchange column (Qiagen, Chatsworth, CA). The DNA concentration was measured with a fluorimeter (Hoeffer, San Francisco, CA), and 1 μg was added to a polymerase chain reaction (PCR) to detect the neo gene, using primers and conditions described previously.17 The amplification products were analyzed by Southern blotting using a radiolabeled, neo probe. Positive control DNA was prepared from G1Na-transduced K-562 cells (one integrant per cell) diluted with nontransduced cells to give mixtures containing 0.1%, 1%, and 10%neo-positive cells.
Cytotoxicity assays.
The cytotoxicity of each CTL line was analyzed in a standard 4-hour chromium-51 release assay using effector:target ratios of 40:1, 20:1, 10:1, and 5:1. Target cells included autologous and HLA-class I–mismatched LCLs, and the T-cell line HSB-2, which is sensitive to killing by lymphokine-activated killer cells. To determine whether cytolysis was restricted by HLA class I or class II, target cells were preincubated for 30 minutes with 20 μg/mL of W6/32 (Dako), a monoclonal antibody that recognizes a monomorphic HLA class I determinant or CR3/43 (Dako), which recognizes HLA-DR, -DP, and -DQ and blocks HLA class II–restricted killing. In the minority of patients in whom no antibody inhibition was obtained, the cell line was depleted of CD56+ and CD16+ cells to remove nonspecific natural killer/antibody-dependent cytotoxic cell effectors, and then retested (see later).
To determine whether EBV-specific CTL lines generated in this way would be able to recognize the EBV antigens expressed by Hodgkin's cells, we depleted patient CTLs of nonspecific (CD16+ and CD56+) killer cells using a FACStar (Becton Dickinson, San Jose, CA). A dermal fibroblast line was prepared from a normal donor, who shared HLA-A,B antigens with the patient. These fibroblasts were treated for 24 hours with interferon-γ and infected for 1 hour with 5 plaque-forming units of vaccinia virus recombinant containing one of the EBV genes, EBNA1, EBNA2, EBNA3a, EBNA3b, EBNA3c, EBNA-LP, LMP1, or LMP2a, or the control gene β-galactosidase (all a gift of Elliott Kieff, Boston, MA).22 A total of 106fibroblasts was infected with each construct and the incubation took place in the presence of 0.1 mCi 51Cr. The target cells were then washed five times and plated at 5 × 103 cells per well with effector cells to give effector:target ratios of 40:1, 20:1, 5:1, and 2.5:1. After 5 hours, supernatants were harvested and chromium release measured to determine specific cytotoxicity.
Immunophenotyping.
For cell-surface phenotyping, the CTLs were incubated with combinations of fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies to CD3, CD4, CD8, CD5, CD25 (Dako), HLA-DR, TCR-γδ, CD16, CD56, CD19 (Becton Dickinson), or TCR (T-Cell Diagnostics, Cambridge, MA). Cells were analyzed on a FACScan flow cytometer.
To simultaneously stain T cells for CD3 (a cell-surface marker) and TCR zeta (an intracytoplasmic antigen), PBMC were isolated and washed in phosphate-buffered saline (PBS). The cell pellet was resuspended in 10 μL of a CD3-FITC monoclonal antibody (Dako) and incubated for 10 minutes at room temperature in the dark. The cells were then washed once in PBS and fixed by incubating them in 0.01% formaldehyde (in PBS) for 20 minutes on ice. After four washes with ice-cold PBS, the cells were resuspended at 5 × 106 cells/mL in 10 μg/mL digitonin (Sigma Chemicals, St Louis, MO; in PBS) and incubated for 5 minutes on ice. The cells were pelleted, resuspended in 10 μL of a zeta-chain antibody (IgG1 unconjugated; Dako) or an isotype-matched control, and incubated for 10 minutes at room temperature. After washing twice in 0.05% Tween-20 (in PBS), the cells were resuspended in 10 μL rat antimouse IgG1-PE (Dako) and placed in the dark for 10 minutes. The cells were then resuspended in PBS containing 1% formaldehyde and analyzed by flow cytometry. For LMP1 staining of biopsy materials, fixed sections were incubated with the CS1 to CS4 antibodies as described.18
Frequency of EBV-specific precursors.
To compare the precursor frequency of EBV-specific CTLs before and after CTL infusion, patient PBMC were seeded at limiting dilution from 5 × 105 to 5 × 102 cells per well (twofold dilutions) in 96-well plates. Each well was stimulated with 105 autologous LCLs, and supplemented with 104irradiated autologous PBMC feeder cells if the wells contained 104 or fewer PBMC. After 6 weeks in culture, the responding cells were split into two parts and assayed for cytotoxic activity against autologous and HLA-mismatched LCLs; both target populations were labeled with 51Cr. This extended duration of culture was required to grow testable numbers of CTLs from patient samples.8 Positive wells were scored on the basis of ≥10% lysis of the cells; this value exceeded the mean spontaneous release of51Cr by 3 SD. The frequency of CTL precursors was estimated from the slope of a regression plot of the log percentage of negative wells versus the number of responder lymphocytes.
Detection of EBV DNA in patient PBMC.
The level of EBV DNA in patient PBMC was assessed every 2 to 4 weeks after CTL infusion using a nested PCR to amplify a portion of the EBNA2 gene. The first pair of primers (5′ primer, 5′-AACTTCAACCCACACCATCA-3′; 3′ primer, 5′-TTCTGGACTATCTGGATCAT-3′) led to amplification of a 115-bp segment of the EBNA2 gene; the reaction volume was 100 μL and contained 1 μg PBMC DNA. The second PCR (template, 2 μL of the first-round product; total volume, 100 μL) used the same 5′ primer, but the 3′ primer was 5′-GGTGCATTGATTGGTCT-3′, leading to amplification of a 78-bp segment of the EBNA2 gene. The conditions for both rounds of amplification were 93°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes for 25 cycles. We used the BL2/B95-8 cell line, which contains two integrated copies of EBV per cell, to determine the sensitivity of the nested PCR reaction by amplifying 0.1 μg BL2/B95-8 DNA (representing ≃10 to 20 copies of EBV) diluted in 1 μg DNA from the EBV-negative cell line HSB-2.7
RESULTS
CTLs from patients with Hodgkin's disease expand slowly compared with those from healthy donors.
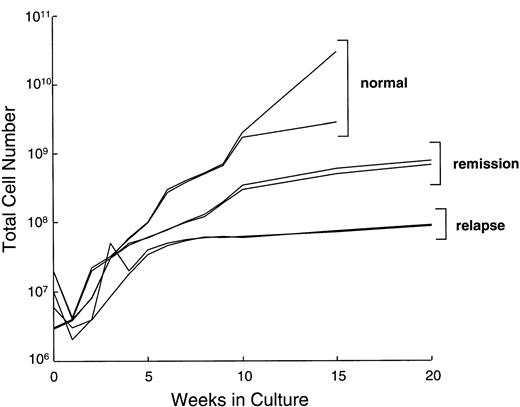
We compared the LCL-induced proliferation of T lymphocytes from Hodgkin's patients (in remission or relapse) with normal donors. During the first 10 to 14 days in culture, the total cell number did not increase significantly in lines from healthy donors or patients, presumably due to death of unresponsive T cells. During the subsequent expansion phase in the presence of IL-2, cell counts of cultures from healthy donors (n = 15) typically increased by 10-fold in the first 2 weeks and then slowed down. These kinetics are illustrated for two normal donors in Fig 1. After 16 weeks in culture, CTLs from normal donors had expanded by a median of 1,500-fold (range, 130- to 5,000-fold). During the same 16-week period, CTL cultures from patients in remission expanded by a median of only 160-fold (range, 100- to 300-fold), while those from patients with relapsed disease increased by a median of 80-fold (range, 20- to 100-fold). Despite this slower rate of expansion, addition of a mitogenic cocktail (see Methods) allowed us to generate at least 108 CTLs from four of five patients in remission and from five of nine patients with relapsed Hodgkin's disease. This number of cells exceeded that required for our in vivo study.7 8
Growth kinetics of representative EBV-specific CTL cultures from normal donors, 2 patients with Hodgkin's disease in remission, and 2 patients with relapsed disease. All CTL lines from Hodgkin's patients required weekly stimulation with the four-component cocktail of IL-2, allogeneic irradiated PBMC, LCLs, and anti-CD3 from day 28. Lines from normal donors received only IL-2. Total cell number is illustrated as a log scale on the y-axis.
Growth kinetics of representative EBV-specific CTL cultures from normal donors, 2 patients with Hodgkin's disease in remission, and 2 patients with relapsed disease. All CTL lines from Hodgkin's patients required weekly stimulation with the four-component cocktail of IL-2, allogeneic irradiated PBMC, LCLs, and anti-CD3 from day 28. Lines from normal donors received only IL-2. Total cell number is illustrated as a log scale on the y-axis.
Phenotype of CTL lines from patients with Hodgkin's disease.
To determine whether the phenotype of CTL lines from Hodgkin's patients might provide an insight into the mechanism of their low proliferative rate, we analyzed the CTL lines from patients in the two study groups and compared them with lines previously derived from normal individuals.23 CTL lines from normal individuals and patients with Hodgkin's disease exhibited considerable heterogeneity with respect to the percentages of CD56+, CD8+, and CD4+ cells and in their CD4/CD8 ratio, but were nonetheless broadly comparable. In lines from healthy individuals, CD8+ cells ranged from 3% to 99% (median, 77%), CD4+ cells ranged from 2% to 98% (median, 19%), and DR+ cells ranged from 82% to 100% (median, 98%).23 In lines from Hodgkin's patients (Table2), CD8+ cells ranged from 2% to 92% (median, 67%), CD4+ cells ranged from 2% to 86% (median, 10%), and DR+ cells ranged from 73% to 100% (median, 97.6%). The proportion of CD56+ cells was similar in all groups and no phenotypic differences were observed in lines from remission versus relapsed patients. The high proportion of γδ+ cells seen in three of the nine lines was also seen in five of 29 lines from normal individuals.23
Characteristics of CTL Lines Generated From Patients With Hodgkin's Disease
| Patient Status . | % Specific Lysis . | % Inhibition of Autologous LCL . | Phenotype . | |||||
|---|---|---|---|---|---|---|---|---|
| Autologous LCL . | MM-LCL . | HSB-2 . | Class I . | Class II . | CD4+ . | CD8+ . | γδ . | |
| 1, Relapse | 78 | 7 | 34 | 49 | 20 | 3 | 92 | 0.2 |
| 2, Relapse | 62 | 8 | 38 | 61 | 3 | 10 | 89 | 1 |
| 3, Relapse | 22 | 21 | 77 | 0 | 55 | 19 | 55 | 5 |
| 4, Remission | 65 | 8 | 24 | 57 | 8 | 5 | 84 | |
| 5, Remission | 17 | 18 | 66 | 0 | 46 | 86 | 2 | 0.4 |
| 5, Relapse | 19 | 17 | 78 | 0 | 37 | |||
| 6, Relapse | 36 | 12 | 52 | 22 | 17 | 2 | 58 | 59 |
| 8, Remission | 58 | 0 | 21 | 62 | 21 | 5 | 82 | |
| 12, Remission | 43 | 9 | 29 | 51 | 0 | 17 | 60 | 67 |
| 13, Relapse | 57 | 35 | 86 | 01 | 01 | 17 | 67 | 16 |
| Median | 43 | 9 | 38 | 22 | 17 | 10 | 67 | 5 |
| Range | 17-78 | 0-21 | 21-86 | 2-86 | 2-92 | 0.2-67 | ||
| Normal donors23 | ||||||||
| Median | 40 | 13 | 26 | 19 | 77 | 2 | ||
| Range | 12-88 | 0-67 | 21-86 | 2-98 | 3-99 | 0-34 | ||
| Patient Status . | % Specific Lysis . | % Inhibition of Autologous LCL . | Phenotype . | |||||
|---|---|---|---|---|---|---|---|---|
| Autologous LCL . | MM-LCL . | HSB-2 . | Class I . | Class II . | CD4+ . | CD8+ . | γδ . | |
| 1, Relapse | 78 | 7 | 34 | 49 | 20 | 3 | 92 | 0.2 |
| 2, Relapse | 62 | 8 | 38 | 61 | 3 | 10 | 89 | 1 |
| 3, Relapse | 22 | 21 | 77 | 0 | 55 | 19 | 55 | 5 |
| 4, Remission | 65 | 8 | 24 | 57 | 8 | 5 | 84 | |
| 5, Remission | 17 | 18 | 66 | 0 | 46 | 86 | 2 | 0.4 |
| 5, Relapse | 19 | 17 | 78 | 0 | 37 | |||
| 6, Relapse | 36 | 12 | 52 | 22 | 17 | 2 | 58 | 59 |
| 8, Remission | 58 | 0 | 21 | 62 | 21 | 5 | 82 | |
| 12, Remission | 43 | 9 | 29 | 51 | 0 | 17 | 60 | 67 |
| 13, Relapse | 57 | 35 | 86 | 01 | 01 | 17 | 67 | 16 |
| Median | 43 | 9 | 38 | 22 | 17 | 10 | 67 | 5 |
| Range | 17-78 | 0-21 | 21-86 | 2-86 | 2-92 | 0.2-67 | ||
| Normal donors23 | ||||||||
| Median | 40 | 13 | 26 | 19 | 77 | 2 | ||
| Range | 12-88 | 0-67 | 21-86 | 2-98 | 3-99 | 0-34 | ||
Inhibition seen after depletion of CD16+ and CD56+ cells: see text and Fig 7.
Abbreviation: MM, mismatched.
The level of TCR zeta chain is abnormally low in Hodgkin's patients.
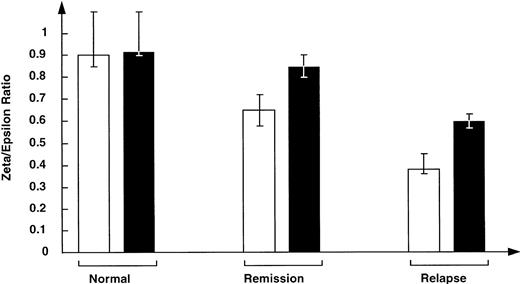
Because the cytoplasmic portion of the TCR zeta chain is involved in signal transduction and subsequent activation and proliferation of T cells, downregulation of this chain may result in the decreased ability of patient T cells to make a proliferative response to autologous LCLs. By comparison with unstimulated T cells from normal donors, those from patients with relapsed or remission status Hodgkin's disease expressed less TCR zeta chain (Fig 2). Normal donors had a zeta:epsilon ratio of 0.90. In contrast, patients in remission had a ratio of 0.75, and relapse patients had a greatly reduced ratio, 0.38 (P < .03 for comparisons with normals), which corresponded to their poor proliferative response. Evaluation of TCR zeta-chain expression in T cells cultured for 10 days in the presence of IL-2 showed expression levels of CTLs from remission patients had increased versus normal levels. While zeta-chain expression also increased in CTLs from patients with relapsed disease, the ratio remained subnormal (Fig 2).
Level of TCR zeta chain is abnormally low in fresh, unstimulated T cells from patients either in remission of Hodgkin's disease or with relapsed disease v that in T cells from normal donors (□). Stimulation of T cells with IL-2, anti-CD3, and HLA-mismatched PBMC resulted in upregulation of the TCR zeta-chain expression as assessed by FACs analysis (▪).
Level of TCR zeta chain is abnormally low in fresh, unstimulated T cells from patients either in remission of Hodgkin's disease or with relapsed disease v that in T cells from normal donors (□). Stimulation of T cells with IL-2, anti-CD3, and HLA-mismatched PBMC resulted in upregulation of the TCR zeta-chain expression as assessed by FACs analysis (▪).
CTLs from Hodgkin's patients are HLA-restricted.
To verify the cytotoxic specificity of the CTL lines generated, we tested them against autologous and HLA class I–mismatched LCLs and HSB-2 (the LAK-sensitive EBV-negative T-cell lymphoma). Table 2 shows the cytotoxicity of CTLs generated from patients with Hodgkin's disease. The results are not different from CTLs from normal donors.23 Thus, CTLs from Hodgkin's patients contained HLA-restricted components, as killing of autologous LCLs was higher than that of HLA-mismatched LCLs. In six of 10 experiments, killing of autologous LCLs was inhibited by an anti-HLA class I monoclonal antibody, thereby confirming the presence of HLA class I–restricted EBV-specific CTL. Significant killing through class II also occurred, since inhibition was seen with antibody to class II antigens (Table 2).
The level of LAK-activated killing varied from 0% to 77% (median, 26%) in CTL lines generated from healthy individuals and from 21% to 86% (median, 38%) in CTL lines from Hodgkin's patients (Table 2). Lines containing high levels of LAK activity were often not sensitive to HLA-class I blocking antibody. However, removal of CD56+and CD16+ nonspecific cytotoxic effector cells was able to reveal class I/II–restricted killing of autologous EBV LCLs, even when bulk cultures were apparently major histocompatibility complex (MHC)-unrestricted (Fig3). This phenomenon has been reported previously in EBV-specific CTLs generated from normal donors.23
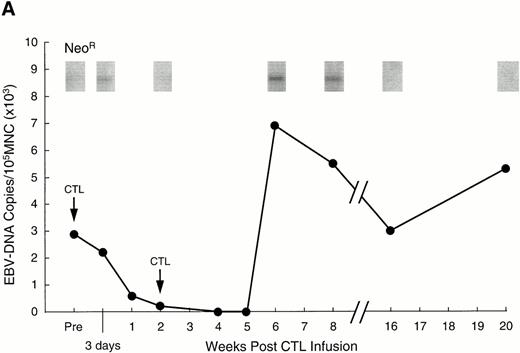
Analysis of PBMC for evidence of the neo marker and for EBV DNA. (A) Levels of neo DNA are shown in the upper portion. They were measured by semiquantitative PCR analysis of 1 μg total DNA at various time points after the second CTL infusion. Lower portion shows EBV DNA in PBMC for the corresponding time points. Levels of EBNA 2 DNA were measured by nested semiquantitative PCR analysis of 1 μg total DNA. (B) Detection of marker gene in pleural fluid of patient no. 13, 3 weeks after CTL infusion. Detection in the infused CTL line and blood obtained on the same day are shown for comparison.
Analysis of PBMC for evidence of the neo marker and for EBV DNA. (A) Levels of neo DNA are shown in the upper portion. They were measured by semiquantitative PCR analysis of 1 μg total DNA at various time points after the second CTL infusion. Lower portion shows EBV DNA in PBMC for the corresponding time points. Levels of EBNA 2 DNA were measured by nested semiquantitative PCR analysis of 1 μg total DNA. (B) Detection of marker gene in pleural fluid of patient no. 13, 3 weeks after CTL infusion. Detection in the infused CTL line and blood obtained on the same day are shown for comparison.
Hence, EBV-specific CTLs generated from patients with Hodgkin's disease in relapse or remission were similar in activity to those generated from normal individuals, even though the lines from patients had been in culture longer and had required exposure to the mitogenic cocktail for expansion. Having shown that we could generate EBV-specific CTLs with normal phenotype and cytotoxicity from Hodgkin's patients, we tested the function of these CTLs in vivo by gene modifying them and infusing them into three patients with multiply relapsed disease.
Infused CTLs persist in vivo for more than 13 weeks.
To track the persistence of infused CTLs, they were genetically marked with a retrovirus containing the neomycin resistance gene (neo). The efficiency of gene marking of CTLs generated from Hodgkin's patients was 0.5% to 10%, identical to that obtained in normal donor CTLs and sufficient for detection in vivo in stem-cell transplant recipients.7 8 In patient no. 1, the neogene was detectable in PBMC for 12 weeks after the initial dose of 2 × 107/m2 EBV-specific CTLs (Fig3A). As the marking efficiency of the CTL line was 2% and 0.002% of the PBMC were positive for neo, approximately one of every 1,000 circulating PBMC was derived from the infused cell line. Patient no. 2 had a similar level of neosignal in PBMC, persisting in this case for 10 weeks after the initial dose, while in patient no. 13, marked cells were detected in peripheral blood for more than 13 weeks (the duration of the study). Moreover, in patient no. 13, who had extensive pleural involvement with Reed Sternberg cells, marker signal was detected in the pleural fluid at a 10-fold higher level than in peripheral blood, indicating that the infused CTLs can traffic to sites of active disease (Fig 3B).
We next looked for evidence that these CTLs were functional.
EBV-specific CTLs activity increases after infusion of in vitro cultured CTLs.
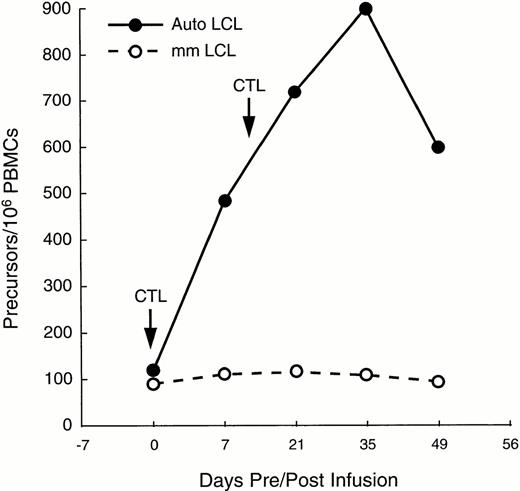
In patient no. 1, sufficient PBMC were available at multiple time points after infusion to determine whether CTL infusion increased the proportion of circulating EBV-specific cytotoxic precursor cells (CTLp), showing an enhanced cell-mediated immune response to EBV. Figure 4 shows that following two doses of 2 × 107/m2 EBV-specific CTLs 2 weeks apart, the CTLp frequency increased from one in 10,000 PBMC to one in 1,000 within 3 weeks of the second CTL infusion, thereby reaching the range found in healthy donors.24 The number of MHC-unrestricted cytotoxic effector precursors remained constant, as shown by the minimal change in the proportion of CTLp that killed HLA-mismatched LCL (Fig 4).
Increase in EBV-specific CTL precursors after two infusions of CTL in a patient with relapsed Hodgkin's disease (—). CTL precursor frequencies (per 106 PBMC) were measured immediately before and for as long as 49 days after the infusion of the T cell lines. Frequency of precursors specific for autologous LCL increased by 10-fold. However, proportion of precursors able to kill allogeneic LCL changed only minimally, indicating that the observed increment in cytotoxicity was due to activity of “classical” CTL, rather than to MHC-unrestricted killer cells (---).
Increase in EBV-specific CTL precursors after two infusions of CTL in a patient with relapsed Hodgkin's disease (—). CTL precursor frequencies (per 106 PBMC) were measured immediately before and for as long as 49 days after the infusion of the T cell lines. Frequency of precursors specific for autologous LCL increased by 10-fold. However, proportion of precursors able to kill allogeneic LCL changed only minimally, indicating that the observed increment in cytotoxicity was due to activity of “classical” CTL, rather than to MHC-unrestricted killer cells (---).
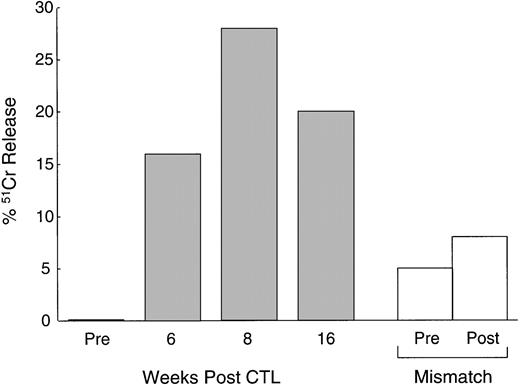
In addition to the increase in CTLp frequency, Fig5 shows that the CTL activity, tested after culture with autologous LCLs, also increased in vivo. PBMC from patient no. 1 cultured before CTL infusion showed a low level of cytotoxic activity (1% killing of autologous LCLs at an effector:target ratio of 40:1). This level of killing increased substantially in PBMC drawn and cultured 4 weeks and 8 weeks after infusion (28% killing of autologous LCLs at the same effector:target ratio). There was little effect on the activity of MHC-unrestricted cytotoxic effector cells, suggesting that an increase in classical MHC-restricted CTL activity was responsible for the observed increase in autologous LCL target killing.
Increased anti-EBV activity after CTL infusion. Immediately before and at 6, 8, and 16 weeks after the first infusion of CTL, cytotoxic T-cell lines were generated from the PBMC of a patient with relapsed Hodgkin's disease by culturing the cells with autologous LCLs (see Methods). The data, presented as percentage of51Cr release from autologous LCLs at an effector:target ratio of 20:1, demonstrate a maximum 28-fold rise in anti-EBV activity following CTL infusion (▪). There was no significant increase in MHC-unrestricted activated killer activity as demonstrated by there being no increase in the percentage of 51Cr release from HLA-mismatched LCL targets (□).
Increased anti-EBV activity after CTL infusion. Immediately before and at 6, 8, and 16 weeks after the first infusion of CTL, cytotoxic T-cell lines were generated from the PBMC of a patient with relapsed Hodgkin's disease by culturing the cells with autologous LCLs (see Methods). The data, presented as percentage of51Cr release from autologous LCLs at an effector:target ratio of 20:1, demonstrate a maximum 28-fold rise in anti-EBV activity following CTL infusion (▪). There was no significant increase in MHC-unrestricted activated killer activity as demonstrated by there being no increase in the percentage of 51Cr release from HLA-mismatched LCL targets (□).
It is possible that the increase in the EBV-specific precursor frequency and CTL activity could be accounted for by a general improvement in immune function after cessation of chemotherapy at least 1 month previously. To determine whether the infused CTLs contributed to this immunologic improvement, the frequency of marked CTLs in lines reactivated from patients no. 1 and 13, 1 and 2 months after infusion, was compared with the frequency of marked cells in the infused CTL lines. Semiquantitative PCR analysis showed that the marking efficiency of the infused lines was between 5% and 10% and that of the reactivated CTL lines was between 2% and 10%. Thus, a significant portion of the immune response to EBV was derived from the infused CTL line, suggesting that a major portion of the immunologic improvement and antiviral effects was due to CTL infusion.
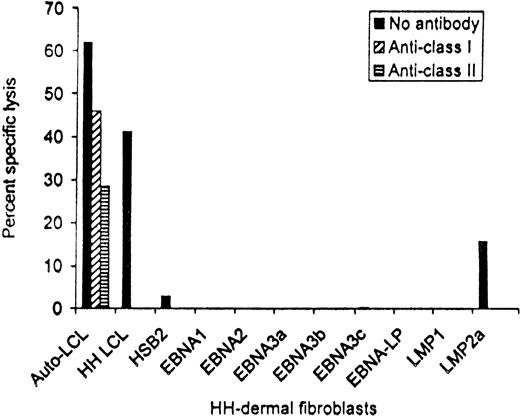
EBV antigen-specificity of patient no. 13 CTL line after depletion of CD56+ and CD16+ cells. Targets were the autologous LCLs, HH-LCL that was HLA-matched at HLA-A2, -A3, -B35, and -DR1, and HH-derived dermal fibroblasts that had been infected with vaccinia recombinants expressing each of the EBV latency-associated proteins. Killing at an effector:target ratio of 20:1 is shown.
EBV antigen-specificity of patient no. 13 CTL line after depletion of CD56+ and CD16+ cells. Targets were the autologous LCLs, HH-LCL that was HLA-matched at HLA-A2, -A3, -B35, and -DR1, and HH-derived dermal fibroblasts that had been infected with vaccinia recombinants expressing each of the EBV latency-associated proteins. Killing at an effector:target ratio of 20:1 is shown.
Infused EBV-specific CTLs have antiviral activity in vivo.
To demonstrate the in vivo antiviral activity of these circulating cytotoxic cells, we used semiquantitative nested PCR analysis to calculate the viral burden in peripheral blood before and after infusion. In more than 50 healthy individuals, the number of EBV DNA genomes ranged from fewer than 20 to 2,000 copies per 106PBMC.8,25 Before CTL infusion, the level of EBV DNA in patient no. 1 was consistent with a genome number of 30,000 per 106 PBMC (Fig 3A). This 15-fold higher than normal level was within the range seen for stem-cell transplant recipients with immunoblastic lymphoma.7,8 25 As shown in Fig 3A, the level of EBV DNA decreased dramatically after CTL infusion and was undetectable after 4 weeks. Associated with this drop in EBV DNA, the patient showed improvement of stage B symptoms, with increased appetite and resolution of fever and sweats; there was also stabilization of his pulmonary disease. These effects were not due to concomitant chemotherapy, as the patient had received no other treatment for 4 weeks before or 6 weeks postinfusion. In fact, the viral load in the peripheral blood of this patient subsequently increased coincident with readministration of chemotherapy, at 6 weeks postinfusion (the end of the evaluation period) (Fig 3A). Patient no. 2 had insufficient blood counts to allow measurement of EBV DNA levels, but experienced improvement in stage B symptoms before the institution of further chemotherapy and disease progression. In patient no. 13, EBV burden was at an initial level of 400 EBV genomes per 106 PBMC, but fell to undetectable levels by 19 days after the first infusion. These effects were again produced in the absence of intensive chemotherapy (>4 weeks before and after).
CTLs from patients with Hodgkin's disease contain LMP2a-specific clones.
These data, showing reduction in viral burden, suggest that the EBV-specific CTL lines derived from patients with Hodgkin's disease have efficacy against circulating EBV-infected cells. To discover whether they may have activity against the EBV proteins expressed by the tumor cells, the CTLs infused into patient no. 13 were tested for their ability to kill HLA-matched fibroblasts that had been infected with vaccinia recombinants and expressed each of the EBV latent cycle proteins individually. Figure 6 shows that following depletion of nonspecific CD56+ and CD16+cytotoxic cells, the line contained both HLA class I and class II–restricted CTLs that killed not only the autologous and an HLA-A2, -A3, -B35, and -DR1–matched LCL from donor HH, but also HH fibroblasts that were expressing LMP2a, confirming the presence of effector cells able to recognize at least one of the antigens present on Hodgkin's tumor cells.
DISCUSSION
The results presented here show the feasibility of generating and infusing EBV-specific CTLs in patients with advanced Hodgkin's disease. Global unresponsiveness to EBV antigens clearly cannot account for the persistence of EBV-positive tumor cells in Hodgkin's patients. Frisan et al26 previously showed that EBV-specific CTL lines can be generated from patients with EBV-positive Hodgkin's disease at the time of their diagnosis, and Sing et al27showed that such lines may contain clones with specificity for LMP1 and LMP2. We have now shown that EBV-specific cellular immunity can be detected and expanded in vitro even from patients with active disease who have had multiple relapses, that these cell lines may be LMP2a-specific, and that they have antiviral function in vivo.
Although the EBV-specific CTL lines generated from patients with relapsed Hodgkin's disease are phenotypically similar to those generated from normal donors, the expansion rate and proliferative potential of the lines from patients is much lower. This may result in part from their suboptimal expression of the TCR zeta chain.11 The TCR zeta chain contributes to the assembly of the CD3 complex, and its cytoplasmic domain is involved in signal transduction and subsequent activation and proliferation of T cells.28 Hence, decreased expression of the TCR zeta chain results in abnormal activation through the TCR/CD3 complex and in depressed T-cell responses.11 Our results, showing decreased expression of the TCR zeta chain in peripheral blood lymphocytes from Hodgkin's patients, agree with results from Rubin et al29 and also show that the severity of the impairment correlates with the disease status. The TCR zeta chain defect can be corrected in vitro by allostimulation,29 and our results show that it may also be reversed by IL-2 when the EBV-specific CTL lines are generated from patients in remission. However, the defect is only partially correctable in patients with relapsed disease.
Despite this relatively limited proliferative potential, we have shown that it is feasible to obtain substantial ex vivo expansion of EBV-specific T cells from nine of 13 patients with advanced Hodgkin's disease, and that these cells have normal cytotoxic activity against autologous EBV-infected B-cell lines. Using gene marking, we were able to compare the in vivo performance of autologous CTLs in Hodgkin's patients with that of normal donor-derived CTLs in allogeneic stem-cell recipients,7,8 monitoring their survival in vivo and assessing their function directly. If EBV-positive Hodgkin's disease resists endogenous cytotoxic T-cell activity because of a systemic effect on CTL survival or activity, then this in vivo study of patients with Hodgkin's disease should have shown reduced survival and diminished antiviral activity of CTLs compared with that of CTLs in our previous studies of bone marrow transplant patients.7,8 In fact the marker signal in the peripheral blood of the three patients with Hodgkin's disease persisted for 10, 12, and greater than 13 weeks in the first three patients, which is within the range of the duration of the marker signal observed in the peripheral blood of stem-cell transplant recipients in our previous study.7 8 Marked cells were also found in fluid obtained from involved pleura at levels 10-fold higher than peripheral blood, implying infused CTLs are able to traffic to sites of disease. An alternative explanation for the observed improvements in immune responses to EBV and antiviral effects is that patient endogenous immune responses recovered after cessation of chemotherapy, which had occurred at least 4 weeks before CTL infusion. However, the high level of gene-marked CTLs in the lines reactivated from two patients postinfusion suggested that a major part of the immune response was derived from the infused CTLs.
The cells transferred during the present study functioned normally, producing an increase in the EBV-specific cytotoxic activity of peripheral blood lymphocytes and a decline in the viral burden in the peripheral blood. While it is uncertain whether supranormal levels of EBV DNA seen in many patients with EBV-positive Hodgkin's disease is a measure of circulating tumor cells or reflects poor control of EBV-infected normal B cells, it is evident from our data that the virally infected cells can be controlled by infused EBV-specific CTLs. The infused cells must therefore be able to recognize one or more of the EBV antigens expressed by the tumor (EBNA1, LMP1, LMP2) or by circulating normal B cells (LMP2 only).30,31 These data demonstrated that the EBV-infected Hodgkin's tumor cells did not decrease survival or inhibit the function of infused EBV-specific CTLs during the time period studied.12-14 Because the first two patients received cytotoxic drugs after the T-cell infusion, we do not know whether an antitumor effect was produced in addition to the antiviral effect. However, both individuals had resolution of type B symptoms after infusion. Patient no. 13 received no additional intensive chemotherapy and has stable disease greater than 4 months after CTL infusion.
Even though EBV-specific T cells can be generated from patients with advanced Hodgkin's disease, and even though these cells survive and have antiviral function in vivo can we anticipate that this approach will successfully treat EBV-positive Hodgkin's disease? While we are actively exploring this possibility, there remain a number of mechanisms by which Hodgkin's cells may evade even a high-level, adoptively transferred anti-EBV response. EBV-positive Hodgkin's tumor cells express only three EBV latency proteins, EBNA1, LMP1, and LMP2. In contrast, immunoblastic lymphoma cells, which are highly susceptible to CTL activity, express five additional antigens, LP and EBNA2, 3A, 3B, and 3C.22,32 In the majority of cases, memory CTL responses are preferentially directed against the highly immunogenic EBNA3A, 3B, and 3C antigens, irrespective of the patient's HLA type. By comparison, EBNA1 and LMP1 and LMP2 are usually only weakly immunogenic. EBNA1 contains a repeating gly-ala amino acid sequence that inhibits processing and presentation of the EBNA1 antigen by HLA class I antigens.33 The reason for the limited immunogenicity of LMP1 and LMP2 is less clear, since both contain CTL epitopes that are presented, at least in the context of the HLA-A2.1, -B24, -B40, -B51, and -B55 alleles.22,34 An EBV-specific immune response may thus simply fail to recognize the EBV antigens expressed by Hodgkin's cells. While this possibility remains a concern, three lines of evidence suggest that CTLs from patients with Hodgkin's disease can exhibit activity against at least one of the EBV antigens expressed by the tumor cells. First, we demonstrated LMP2a-specific activity in the CTL line from patient no. 13. Second, the infused CTL lines reduced the virus burden, suggesting activity either against the tumor cells or against EBV-infected normal B cells in which only the LMP2 gene product can be detected.30,31Finally LMP1- and LPMP2-specific CTLs may be isolated from patients with Hodgkin's disease.27
While these results offer promise for CTL therapy of Hodgkin's disease, the tumor may evade killing by other mechanisms. Many Hodgkin's tumors have mutations within the 3′ region of the LMP1 oncogene, including a 30-bp deletion and a high frequency of nonrandom point mutations.35,36 These mutations may further reduce the antigenicity of LMP1 or create epitopes that are not recognized by CTLs specific for wild-type LMP1. Other factors, such as impaired antigen presentation37-39 or downregulation of HLA class I molecules, may also protect Hodgkin's cells. Of note, most of the patient-derived CTL lines described here contained HLA class II–restricted CTLs, which may remain active against class I–negative cells.
Many barriers may remain to prevent the successful therapy of EBV-positive Hodgkin's disease using virus-specific CTLs. Nonetheless, our demonstration that it is possible to generate EBV-specific CTLs from patients with advanced Hodgkin's disease, that these cells survive and function in vivo, and that may be directed to the subdominant viral epitopes expressed by Hodgkin's cells, provides reason to hope that such cytotoxic T-cell therapy will become a useful adjunct to conventional treatments for EBV-positive Hodgkin's disease.
ACKNOWLEDGMENT
We acknowledge Nancy Parnell for word processing.
Supported in part by National Institutes of Health Cancer Center Support Core Grants No. CA 21765 and CA 71426, the Assisi Foundation, and the American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Cliona M. Rooney, PhD, Texas Children's Hospital, 6621 Fannin St, MC3-3320, Houston, TX 77030-2399.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1784 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal