Abstract
In this study, we investigated whether (1) collagen-induced platelet aggregation is associated with a burst of H2O2, (2) this oxidant species is involved in the activation of platelets, and (3) the pathways of platelet activation are stimulated by H2O2. Collagen-induced platelet aggregation was associated with production of H2O2, which was abolished by catalase, an enzyme that destroys H2O2. H2O2 production was not observed when ADP or thrombin were used as agonists. Catalase inhibited dose-dependently thromboxane A2 production, release of arachidonic acid from platelet membrane, and Inositol 1,4,5P3 (IP3) formation. In aspirin-treated platelets stimulated with high concentrations of collagen, catalase inhibited platelet aggregation, calcium mobilization, and IP3 production. This study suggests that collagen-induced platelet aggregation is associated with a burst of H2O2 that acts as a second messenger by stimulating the arachidonic acid metabolism and phospholipase C pathway.
COLLAGEN IS AN important platelet agonist that is thought to be involved in the early stages of platelet activation during both hemostasis and thrombosis. The first step in the interaction of platelets to collagen is adhesion through specific platelet receptors, most probably the integrin glycoprotein GPIa/IIa.1-3 After adhesion, a signal is transmitted into the cells that produces other signaling events that eventually lead to increases in cytosolic Ca2+, activation of protein kinase C, activation of phospholipase A2, and platelet secretion. The products of these events, ADP, and thromboxane A2(TxA2) act to recruit more platelets by a process of aggregation. The exact mechanism of the signal transduction by collagen is not completely clear. However, several investigators4-6have shown that collagen activates platelets via a tyrosine kinase-dependent phosphorylation of PLCγ2. Current evidence indicates that the receptor for signal transduction is a 62-kD protein named GP VI.7 8
H2O2 generated by superoxide-dismutase (SOD) seems to be directly involved in the collagen-induced activation of platelets in experiments in which subthreshold concentrations of collagen and SOD caused platelet aggregation9; catalase, which destroys H2O2, fully prevented SOD-dependent aggregation.9
The role of H2O2 and oxygen free radicals in the mechanism of collagen-induced platelet aggregation was also outlined by stimulation of platelets with either threshold or high concentrations of collagen. Del Principe et al10 showed that collagen-induced platelet aggregation is associated with H2O2 release and that catalase inhibits both H2O2 formation and platelet aggregation. Furthermore, in aspirin (ASA)-treated platelets, aggregation induced by high concentrations of collagen was inhibited by several antioxidants such as salicylic acid, vitamin E, and dipyridamole.11These data suggest that H2O2 and oxygen free radicals have a role in the activation of platelets mediated by collagen, but the underlying mechanism has not been fully understood. It has been suggested that oxidant species may behave as second messengers in stimulating cyclooxygenase-dependent and independent pathways of platelet activation, but experimental evidence in support of this suggestion is still lacking.11 The aim of the present study was to investigate the role of H2O2 on the mechanism of platelet activation by collagen. To this purpose, we analyzed the separate effects of catalase and ASA plus catalase on collagen-induced platelet responses such as H2O2 production, aggregation, secretion, phospholipase C (PLC) activation, calcium mobilization, TxA2 production, and arachidonic acid release. The results support the suggestion that H2O2 formation may have a role in stimulating cyclooxygenase-dependent and independent pathways of platelet aggregation.
MATERIALS AND METHODS
Materials.
32Pi was from Amersham (Arlington Heights, IL). [3H]-Ins 1,3,4 P3 and [3H]-arachidonic acid (3H-AA) were from NEN Life Science Products (Boston, MA). Fura 2-AM and 2′7′-dichlorofluorescein diacetate (DCFH-DA) were from Molecular Probes (Eugene, OR). Sepharose 2B was from Pharmacia (Uppsala, Sweden). Collagen, type 1, was from Semmelweis (Mascia Brunelli, Milan, Italy). High-performance liquid chromatography (HPLC) columns, Partisil 10 SAX were from Whatman (Haverhill, MA). Luciferin and Luciferase were from Chrono-Log (Havertown, PA). Bovine serum albumin, HEPES, ASA, catalase (bovine liver, tymol-free), fibrinogen, inorganic-pyrophosphatase, acid/citrate/dextrose, digitonin, EGTA, EDTA, Tris, perchloric acid, formaldehyde, CaCl2, indomethacin, ammoniun formate, maleic acid, Lucigenin (bis-methylacridinium nitrate), bovine erythrocyte SOD, creatine phosphate (CP), creatine phosphokinase (CPK), and ferricytochrome c (c-7752) were from Sigma Chemicals Co (St Louis, MO).
Inactive catalase was denaturated by treatment with 3-amino-1,2,4-triazole according to Darr and Fridrovich.12
Diphenylene iodonium (DPI) was purchased from Aldrich (Milwaukee, WI).
Platelet preparation.
Human blood was obtained from drug-free, healthy volunteers and anticoagulated with acid/citrate/dextrose.13 Platelet-rich plasma (PRP), which was obtained by centrifugation (15 minutes at 180g), was recentrifuged (800g for 20 minutes) to concentrate the platelets, and the pellet was resuspended in 0.5 vol of autologous platelet-poor plasma.
The platelet suspensions were incubated for 1 hour at 37°C with 3 μmol/L Fura 2-AM or with 40 μmol/L DCFH-DA, or 32Pi 2 mCi/mL of cell suspension, or 3H-AA 2 μCi/mL of cell suspension.
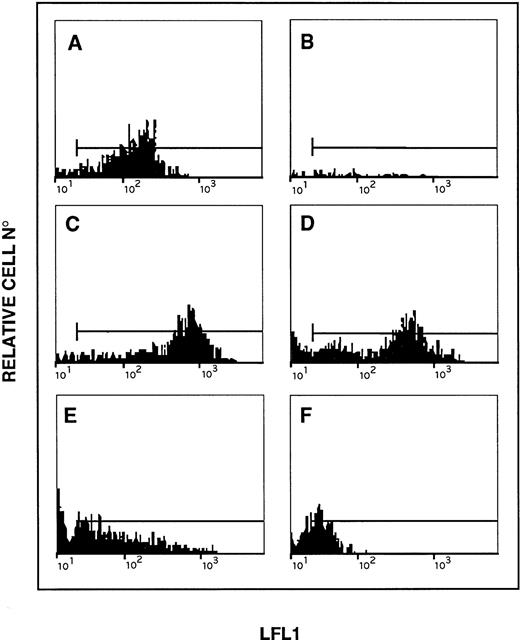
Cytofluorimetric evaluation of H2O2 production in DCFH-DA–loaded platelets. (A) Fluorescence peak of unstimulated platelets. (B) Fluorescence peak of unstimulated platelets with 250 U/mL of catalase (absence of fluorescence). (C) Shift of fluorescence after incubation with H2O2 (1 mmol/L). (D) Shift induced by collagen 50 μg/mL. (E) Effects of the same dose of collagen in GFP treated with catalase (500 U/mL). (F) Effects of the same dose of collagen in GFP treated with catalase (1,000 U/mL). Results are representative of five separate experiments.
Cytofluorimetric evaluation of H2O2 production in DCFH-DA–loaded platelets. (A) Fluorescence peak of unstimulated platelets. (B) Fluorescence peak of unstimulated platelets with 250 U/mL of catalase (absence of fluorescence). (C) Shift of fluorescence after incubation with H2O2 (1 mmol/L). (D) Shift induced by collagen 50 μg/mL. (E) Effects of the same dose of collagen in GFP treated with catalase (500 U/mL). (F) Effects of the same dose of collagen in GFP treated with catalase (1,000 U/mL). Results are representative of five separate experiments.
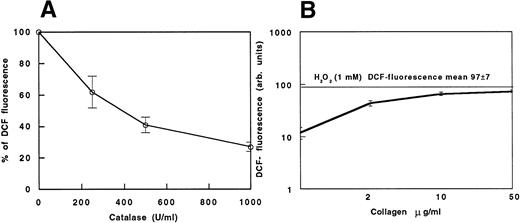
(A) DCF fluorescence in collagen-stimulated platelets (100%) and in collagen-stimulated platelets added with scalar concentrations (250, 500, and 1,000 U/mL) of catalase. (B) Cytofluorimetric evaluation of oxidation of DCFH-DA–loaded platelets to fluorescent DCF induced by scalar doses of collagen. Data are expressed as the mean fluorescence channel ± SEM of five separate experiments.
(A) DCF fluorescence in collagen-stimulated platelets (100%) and in collagen-stimulated platelets added with scalar concentrations (250, 500, and 1,000 U/mL) of catalase. (B) Cytofluorimetric evaluation of oxidation of DCFH-DA–loaded platelets to fluorescent DCF induced by scalar doses of collagen. Data are expressed as the mean fluorescence channel ± SEM of five separate experiments.
Platelets were separated from plasma proteins and from excess of Fura 2-AM and 32Pi by gel filtration on Sepharose 2B using Ca2+ free Tyrode's buffer containing 0.2% bovine serum albumin, 5 mmol/L glucose, and 10 mmol/L HEPES, pH 7.35. After gel filtration, the cell suspension (gel-filtered platelets [GFP]) was adjusted to a final concentration of 2 × 108cells/mL.
The ASA-treated platelets were obtained by incubating GFP with ASA (100 μmol/L for 10 minutes at 37°C). Catalase was added in different concentrations (250 to 1,000 U/mL) 5 minutes before the activation of GFP with collagen. The ratio between the volumes of catalase and platelet suspension was 5 to 20 μL/mL.
CP/CPK (20 mmol/L and 50 U/mL, respectively) was added 1 minute before platelet stimulation.
As control, in all experiments, GFP were incubated with the dilution medium.
Platelet aggregation.
In vitro platelet aggregation was evaluated according to Born14 in a four-sample Aggrecorder II Menarini (Florence, Italy) at 37°C using siliconized glass cuvets under continuous stirring at 1,000 rpm. Fibrinogen (1 mg/mL) was added before the agonist.
The threshold concentration (TC) of agonist was defined as the lowest dose that induced more than 50% of aggregation.
ATP release.
Collagen-induced platelet secretion was evaluated by measuring the release of ATP. The activation of platelets (see above) was stopped after 2 minutes with formaldehyde/EDTA, as according to Costa and Murphy.15 After centrifugation at 10,000g for 30 seconds, the ATP concentration in the supernatant was measured using an LKB 1251 luminometer (Pharmacia) after the addition of Luciferin (40 mg/mL) and Luciferase (880 U/mL). The results were expressed as the percentage of ATP released relative to the total ATP present in lysed cells.16
Production of TXB2.
The activation of platelets (see above) was stopped after 3 minutes with indomethacin (14 μmol/L). TXA2 production was determined using TXB2 enzyme-linked immunosorbent assay kits (Boehringer Mannheim GmbH, Mannheim, Germany).
Platelet cytosolic Ca2+ concentrations.
Calcium measurements were made using the fluorescent indicator dye Fura-2. The fluorescence changes were then monitored with an SFM 25 fluorimeter (Kontron Instruments AG, Zurig) set at 340 nm excitation and 510 nm emission. To convert fluorescence measurements into Ca2+ concentrations, the Fmin was determined after the addition of digitonin (50 μmol/L) in the presence of EGTA (2 mmol/L) and Tris base (20 mmol/L); the Fmax was measured by the addition of excess CaCl2 (10 mmol/L). The calcium concentration was calculated using these values and a kd of 224 nmol/L, according to Grynkiewicz et al,17 after correction for extracellular dye.
PLC activation.
Because the activation of PLC produces inositol-1,4,5 P3from phosphatidyl-inositol-4,5-bis-phosphate (PIP2) and Ins 1,4,5 P3 is converted within 30 to 60 seconds into inositol-1,3,4,5 P4 (Ins 1,3,4,5 P4) that is rapidly degraded into the more stable inositol-1,3,4 P3(Ins 1,3,4 P3),18 we have studied the Ins 1,3,4 P3 production 1 minute after the platelet stimulation with collagen or thrombin. The collagen activation of the [32P]-labeled platelets resuspended in phosphate free Tyrode's buffer was stopped by means of perchloric acid (0.44 N). The neutralized platelet extracts (1 × 109 cells/mL) were treated overnight with Zn2+-pyrophosphatase at 20 U/mL in the presence of Tris-maleic buffer 0.1 mol/L, pH 6.5, and then passed on an HPLC column that was eluted with a 50-minute linear gradient of water as first buffer and ammonium formate at 1.5 mol/L, pH 3.75, as final buffer. Inositol peaks were detected using a dual-channel (3H-32P) HPLC radioactivity detector FLO-ONE A100 (Radiomatic; Camberra Co, Tampa, FL) using [3H]-Ins 1,3,4 P3 as pure standard.18
Tritiated AA release.
The tritiated AA release was studied by prelabeling platelets with tritiated AA. Platelets resuspended in plasma were incubated with 2 μCi/mL tritiated AA for 1 hour at 37°C, then washed twice to remove the remaining free arachidonate, and finally resuspended in Tyrode's buffer. Samples of tritiated AA-labeled platelets were preincubated for 3 minutes at 37°C before stimulation. After 1 minute, the reaction was stopped by adding a solution containing 5 mmol/L EDTA, 5 mmol/L theophylline, and 0.2 μg/mL prostaglandin E1 (PGE1). After centrifugation for 3 minutes at 5,000g, the percentage of 3H-AA released into the supernatant was determined by liquid scintillation counting of 100 μL aliquots in 2 mL of aqueous fluid. Each experiment was performed in duplicate.
Detection of superoxide anion.
The chemiluminescence (CL) of lucigenin was detected with an LKB 1251 luminometer (see above), as previously described.19
Briefly, CL was detected in GFP at a fixed concentration of 3 × 108 cells/mL at 37°C. Each sample, added with 0.25 mmol/L lucigenin, 1 mmol/L CaCl2, and 150 mg/dL fibrinogen, was stimulated with 1 μg/mL collagen and the CL, obtained at intervals of 1 to 3 minutes, was measured. Samples containing lucigenin plus components (with the exception of platelets) were counted, and these blank values were subtracted from the CL signals obtained from collagen-stimulated platelets. CL was expressed as nanomoles of O2− per milliliter per minute. In some experiments, 300 U/mL SOD, or 50 μmol/L DPI, or 100 μmol/L ASA was added to the platelet suspension before collagen stimulation.
Flow cytometric analysis.
PRP, which was obtained by centrifugation (15 minutes at 180g), was recentrifuged (800g for 20 minutes) to concentrate the platelets. The pellet was resuspended in phosphate-buffered saline, 8 μL/mL of 5 mmol/L DCFH-DA was added, and, after 15 minutes of incubation, the platelet suspension was washed twice and resuspended in Tyrode's buffer at the final concentration of 2 × 108 cells/mL. Platelet preparation was activated with collagen alone or in the presence of catalase and stopped with 2 mmol/L EGTA after 1 minute of reaction.
All samples were analyzed on a Coulter Epics (Hialeah, FL) flow cytometer equipped with an argon laser (480 nm emission). The instrument was set up to measure logarithmic forward light scatter (LFS), which is a measure of particle size; logarithmic 90° light scatter (LSS), which is a measure of cell granularity; and green (DCF) 510 to 550 nm fluorescence (LFL1). Fluorescent parameters (in arbitrary units [AU]) were collected using three-decade logarithmic amplification.
Statistical analysis.
Data are reported as the mean ± SEM. The comparison between variables was analyzed using the Student's t-test for unpaired data. Significance was accepted as the P < .05 level.
RESULTS
Flow cytometric analysis.
This method uses the properties of DCFH-DA,20-22 which rapidly diffuses across cell membranes and is then trapped within the cell by a deacetylation reaction. In the presence of hydrogen peroxide, this compound is oxidized to DCF, which is highly fluorescent.23
Figure 1 shows the DCF green fluorescence distribution of unactivated platelets (Fig 1A; mean fluorescence, 20 ± 2), which was fully prevented by 250 U/mL of catalase (Fig 1B). The largest increase of DCF fluorescence occurred with 1 mmol/L H2O2, which caused a mean fluorescence channel shift of 5 times (mean fluorescence, 97 ± 7; P < .02v control; Fig 1C). Collagen-induced platelet activation doubled the shift of DCF fluorescence in comparison to control (mean fluorescence, 44 ± 16; P < .04 v control; Fig1D). Catalase inhibited the shift of DCF fluorescence induced by collagen dependently upon its concentration (mean fluorescence with 500 U/mL = 18 ± 3, P < .05 v collagen; mean fluorescence with 1,000 U/mL = 12 ± 2, P < .05v collagen; Fig 1E and F and Fig 2A).
Figure 2B reports a dose-response curve showing that the increase of H2O2 is dependent on collagen concentration. Comparing the curve with the mean fluorescence observed while adding 1 mmol/L H2O2 to the platelet suspension, the amount of H2O2 released by collagen-stimulated platelets seems to be lower than 1 mmol/L. When using ADP or thrombin as platelet agonists, no increase in DCF fluorescence was observed (not shown).
Platelet aggregation.
In every GFP preparation, we searched for the lowest collagen concentration (TC) able to induce more than 50% of aggregation. In all cases, the effect of this concentration was completely inhibited by preincubation of GFP with ASA; to restore aggregation, ASA-treated platelets were stimulated with fourfold to eightfold TC of collagen (Fig 3B).
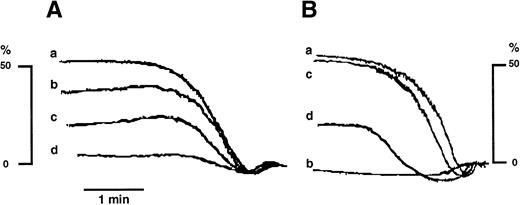
Catalase inhibits collagen-induced platelet aggregation both in untreated and ASA-treated platelets. (A): (a) TC of collagen; (b, c, and d) TC collagen plus catalase (250, 500, and 1,000 U/mL respectively). (B): (a) same as in (A); (b) ASA (100 μmol/L)-treated platelets stimulated with TC collagen; (c) ASA-treated platelets stimulated by eightfold TC collagen; (d) same as in (c) plus catalase at 250 U/mL. Similar results have been obtained in five separate experiments. The threshold concentration (TC) in this experiment was 2 μg/mL.
Catalase inhibits collagen-induced platelet aggregation both in untreated and ASA-treated platelets. (A): (a) TC of collagen; (b, c, and d) TC collagen plus catalase (250, 500, and 1,000 U/mL respectively). (B): (a) same as in (A); (b) ASA (100 μmol/L)-treated platelets stimulated with TC collagen; (c) ASA-treated platelets stimulated by eightfold TC collagen; (d) same as in (c) plus catalase at 250 U/mL. Similar results have been obtained in five separate experiments. The threshold concentration (TC) in this experiment was 2 μg/mL.
Catalase inhibited dose-dependently the aggregation induced by TC of collagen (Fig 3A); moreover, it inhibited the aggregation of ASA-treated GFP induced by fourfold to eightfold TC of collagen (Fig 3B).
Catalase had no effect when other agonists such as ADP (2 μmol/L) or thrombin (0.1 U/mL) were used (Table 1).
No effect was observed when denatured catalase was added to GFP stimulated by collagen (data not shown).
ATP release.
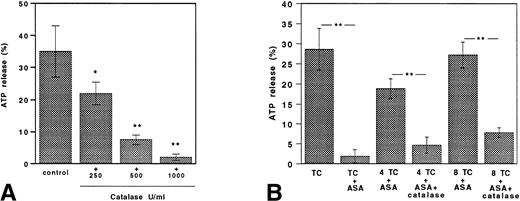
In degranulation studies, we used the same TC of collagen tested for aggregation studies. ATP release induced by TC collagen was inhibited by catalase in a dose-dependent fashion (Fig 4A).
Catalase inhibits collagen-induced ATP secretion both in untreated and aspirin-treated platelets. (A) TC of collagen; TC collagen plus catalase (250, 500, and 1,000 U/mL). (B) ATP release in platelets stimulated with TC of collagen in ASA (100 μmol/L)-treated platelets stimulated with TC and fourfold to eightfold TC of collagen added with or without catalase (250 U/mL). Data are expressed as the mean ± SEM of five separate experiments (*P < .01; **P < .001).
Catalase inhibits collagen-induced ATP secretion both in untreated and aspirin-treated platelets. (A) TC of collagen; TC collagen plus catalase (250, 500, and 1,000 U/mL). (B) ATP release in platelets stimulated with TC of collagen in ASA (100 μmol/L)-treated platelets stimulated with TC and fourfold to eightfold TC of collagen added with or without catalase (250 U/mL). Data are expressed as the mean ± SEM of five separate experiments (*P < .01; **P < .001).
ATP release induced by TC collagen was completely inhibited by preincubation of GFP with ASA. Fourfold to eightfold TC of collagen was able to restore platelet degranulation to levels similar to those obtained in control platelets (Fig 4B).
Catalase was able to inhibit the secretion of ASA-treated platelets stimulated by fourfold to eightfold TC of collagen.
Platelet TXB2 formation.
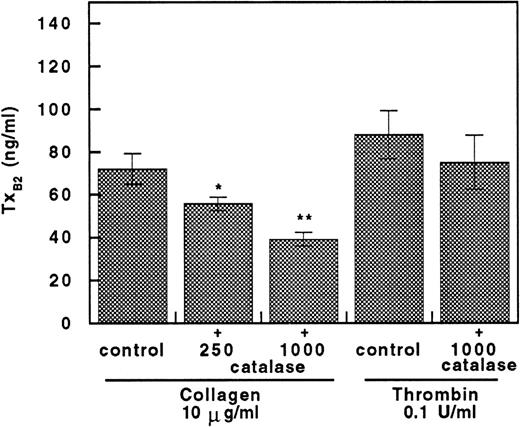
Collagen-induced TXB2 formation was dose-dependently inhibited by catalase. This effect seems to be specific for collagen, because no change was observed with 0.1 U/mL of thrombin (Fig 5). Thus, even with the higher concentration of catalase (1,000 U/mL), no significant changes of TXA2 production were found in thrombin-stimulated platelets.
Catalase inhibits TXB2 production in collagen but not thrombin-stimulated platelets. Platelet TXB2production in collagen (10 μg/mL)-stimulated platelets added with and without 250 to 1,000 U/mL of catalase and in thrombin (0.1 U/mL)-stimulated platelets incubated with and without 1,000 U/mL of catalase. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01).
Catalase inhibits TXB2 production in collagen but not thrombin-stimulated platelets. Platelet TXB2production in collagen (10 μg/mL)-stimulated platelets added with and without 250 to 1,000 U/mL of catalase and in thrombin (0.1 U/mL)-stimulated platelets incubated with and without 1,000 U/mL of catalase. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01).
Changes in intracellular calcium concentration.
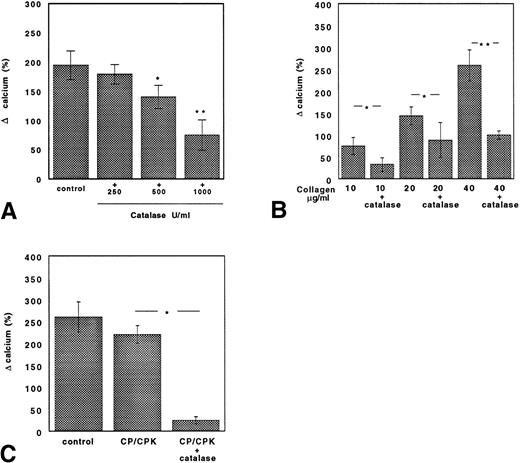
The changes in intracellular calcium concentration induced by collagen in control GFP or in GFP treated with ASA, catalase, or catalase plus ASA are reported in Fig 6. In samples stimulated with collagen (10 μg/mL), catalase dose-dependently inhibited intracellular calcium mobilization (Fig 6A). In ASA-treated platelets, the intracellular calcium mobilization induced by collagen (10 μg/mL) was reduced by about 70% but was restored by increasing collagen concentrations (20 and 40 μg/mL; Fig 6B). The addition of catalase to ASA-treated platelets again reduced intracellular calcium mobilization; this effect was evident even with very high concentration of collagen (40 μg/mL; Fig 6B).
Catalase inhibits collagen-induced calcium mobilization both in untreated and ASA-treated platelets. (A) Calcium mobilization in collagen-stimulated platelets added with or without 250, 500, or 1,000 U/mL catalase. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01). (B) Calcium mobilization in ASA (100 μmol/L)-treated platelets with or without catalase (250 U/mL) stimulated with scalar concentrations of collagen. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01). (C) Cytosolic calcium concentration induced by collagen (40 μg/mL) was reduced by catalase (250 U/mL) in platelets pretreated with aspirin (100 μmol/L) and the ADP scavenger system CP/CPK. Data are expressed as the mean ± SEM of five separate experiments (*P < .01).
Catalase inhibits collagen-induced calcium mobilization both in untreated and ASA-treated platelets. (A) Calcium mobilization in collagen-stimulated platelets added with or without 250, 500, or 1,000 U/mL catalase. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01). (B) Calcium mobilization in ASA (100 μmol/L)-treated platelets with or without catalase (250 U/mL) stimulated with scalar concentrations of collagen. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01). (C) Cytosolic calcium concentration induced by collagen (40 μg/mL) was reduced by catalase (250 U/mL) in platelets pretreated with aspirin (100 μmol/L) and the ADP scavenger system CP/CPK. Data are expressed as the mean ± SEM of five separate experiments (*P < .01).
The inhibition of calcium mobilization by catalase was also observed when aspirinated platelets were treated with the ADP scavenger system CP/CPK (Fig 6C).
PLC activation.
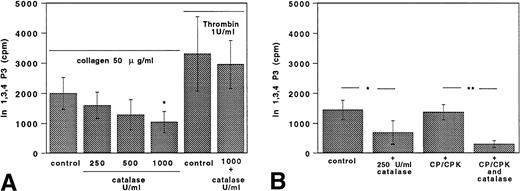
The 32P-labeled-Ins 1,3,4 P3 (IP3) production by collagen-stimulated platelets added with and without catalase, ASA, or ASA plus catalase is shown in Fig 7. As previously described,4,18 24 we had to use higher concentrations of collagen (50 μg/mL) and thrombin (1 U/mL) to measure IP3. Collagen-induced IP3 formation was inhibited by catalase in a dose-dependent fashion (Fig 7A). Thrombin-induced PLC activation was not affected by catalase (Fig 7A). In ASA-treated platelets stimulated with 50 μg/mL collagen, catalase elicited a further reduction of IP3 formation in comparison with platelets treated with aspirin alone. This effect was evident when aspirin-treated platelets were also incubated with CP/CPK (Fig 7B).
Catalase inhibits 32P-Ins 1,3,4 P3 (IP3) production. (A) Catalase (250 to 500 to 1,000 U/mL) inhibited32P-Ins 1,3,4 P3 (IP3) production by collagen (50 μg/mL) but was ineffective if thrombin (1 U/mL) was used as an agonist. Data are expressed as the mean ± SEM of five separate experiments (*P < .05). (B) In ASA (100 μmol/L)-treated platelets, 250 U/mL catalase inhibited 32P-Ins 1,3,4 P3 (IP3) production by collagen (50 μg/mL). Similar findings were obtained in samples added also with the ADP scavenger system CP/CPK. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01).
Catalase inhibits 32P-Ins 1,3,4 P3 (IP3) production. (A) Catalase (250 to 500 to 1,000 U/mL) inhibited32P-Ins 1,3,4 P3 (IP3) production by collagen (50 μg/mL) but was ineffective if thrombin (1 U/mL) was used as an agonist. Data are expressed as the mean ± SEM of five separate experiments (*P < .05). (B) In ASA (100 μmol/L)-treated platelets, 250 U/mL catalase inhibited 32P-Ins 1,3,4 P3 (IP3) production by collagen (50 μg/mL). Similar findings were obtained in samples added also with the ADP scavenger system CP/CPK. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01).
Tritiated AA release.
Platelets stimulated by collagen released 3H-AA, which was inhibited by catalase depending on the concentration used. Conversely, aspirin did not affect 3H-AA release (Table 2).
Superoxide production.
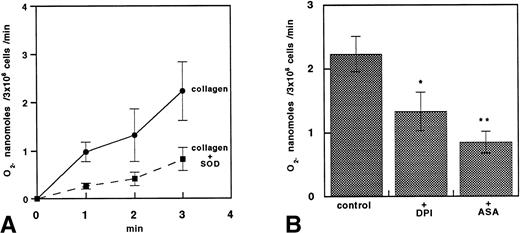
The O2− release measured by lucigenin CL increased time dependently upon platelet stimulation with collagen and was reduced 63% by SOD (n = 5; P < .01; Fig 8A), 40% by DPI (n = 5; P < .05), and 63% by ASA (n = 5; P < .01; Fig 8B).
O2− production in platelets stimulated with collagen. (A) Time course of O2− production estimated by lucigenin chemoluminescence in platelets stimulated with 1 μg/mL collagen. Data are expressed as the mean ± SEM of five separate experiments. (B) Bar groups showing O2−production estimated by lucigenin 3 minutes after stimulation with 1 μg/mL collagen in platelets incubated with or without 50 μmol/L DPI or 100 μmol/L ASA. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01).
O2− production in platelets stimulated with collagen. (A) Time course of O2− production estimated by lucigenin chemoluminescence in platelets stimulated with 1 μg/mL collagen. Data are expressed as the mean ± SEM of five separate experiments. (B) Bar groups showing O2−production estimated by lucigenin 3 minutes after stimulation with 1 μg/mL collagen in platelets incubated with or without 50 μmol/L DPI or 100 μmol/L ASA. Data are expressed as the mean ± SEM of five separate experiments (*P < .05; **P < .01).
DISCUSSION
This study shows that collagen-induced platelet activation is associated with a burst of H2O2 that was dependent on the concentration of collagen used. Platelet production of H2O2 was shown by DCFH oxidation and its disappearance on platelet preincubation with catalase. To assess whether this oxidant species has a role on platelet function, we investigated whether catalase, which destroys H2O2, affects platelet aggregation and specific pathways of platelet activation. First of all, we showed that catalase inhibited platelet aggregation. Such an effect was not aspecific, because catalase elicited platelet inhibition in a dose-dependent fashion and was ineffective when denatured. The role of H2O2 on platelet function was closely related to the agonist collagen, because other agonists such as ADP and thrombin did not provoke platelet H2O2formation; furthermore, catalase had no effect on platelet activation induced by ADP or thrombin. We then investigated whether H2O2 could behave as a second messenger by stimulating specific intracellular pathways such as arachidonic acid metabolism and PLC activation. Collagen-induced platelet TXA2 formation was significantly inhibited by catalase, suggesting that H2O2 interferes with arachidonic acid metabolism. Previous studies have shown that peroxides are an important stimulus for the activation of cyclooxygenase enzyme25; therefore, H2O2 could amplify platelet response to collagen by stimulating this enzyme. Alternatively, H2O2 could favor arachidonic acid release by platelet membrane, because oxidant species have been shown to stimulate phospholipase A2 enzyme.26Our findings are consistent with this suggestion, because catalase inhibited dose-dependently the arachidonic acid release by collagen.
The amplification of platelet response by H2O2was not restricted to the activation of arachidonic acid metabolism, because catalase was able to inhibit Ca2+ mobilization in both ASA-treated and non–ASA-treated platelets, suggesting that H2O2 could also stimulate PLC activation. Our findings are consistent with the hypothesis that H2O2 stimulates PLC, because catalase dose-dependently inhibited IP3 formation; however, our findings do not explain the molecular mechanism underlying this effect. Previous study showed that collagen-induced PLC activation is dependent on tyrosine kinase activation4 and that H2O2 plays a role in the activation of tyrosine kinase.27 Hence, it could be postulated that the activation of phospholipase Cγ2 by H2O2 could be mediated by the stimulation of tyrosine kinase, but we do not have conclusive data to support this hypothesis.
Several sources of oxidant species have been suggested to be activated upon platelet stimulation, but definitive evidence is still lacking. Activation of arachidonic acid metabolism could be theoretically an important source, because, in other cell lines, it has been shown that oxidant species are produced through the stimulation of this pathway.11 Preliminary data from our group showed that O2− results from the activation of arachidonic acid pathways because arachidonic acid-stimulated platelets produced this oxidant species and aspirin partly prevented it.19 Our findings are consistent with this suggestion, because the release of O2− by collagen-stimulated platelets was reduced 63% by aspirin. However, it is likely that other sources of O2− are present in platelets, because catalase interfered with platelet function also when cyclooxygenase pathway was inhibited, suggesting that H2O2 may be produced through alternative pathways. Even if it has been shown by other investigators28 and ourselves19 that DPI, an inhibitor of NADPH oxidase, inhibits platelet function and O2− platelet release, its lack of specificity does not allow to fully conclude that platelets produce O2− through the activation of NADPH oxidase. Whatever the source of O2−, the release of this oxidant species by collagen-stimulated platelets is an important step for the formation of H2O2, because O2− is enzymatically dismutated to H2O2 by SOD, an enzyme that is present in platelet cytosol.29
In conclusion, this study shows that H2O2 is produced by collagen-stimulated platelets and acts as second messenger by activating arachidonic acid metabolism and PLC pathway. Our findings provide a rationale to further analyze the relationship between antioxidants and platelet function and to investigate whether the antiatherosclerosis effect so far reported for this drug category is also related to some interference with platelet function.
Supported in part by Grant C.N.R.-FATMA, subproject 8-contract C.T.95.00834.PF41.
Address reprint requests to Pier Paolo Gazzaniga, MD, Dipartimento di Medicina Sperimentale e Patologia, Università degli Studi di Roma “La Sapienza,” Viale Regina Elena 324, 00161 Roma, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.