Abstract
Simple collagen-related peptides (CRPs) containing a repeat Gly-Pro-Hyp sequence are highly potent platelet agonists. Like collagen, they must exhibit tertiary (triple-helical) and quaternary (polymeric) structure to activate platelets. Platelet signaling events induced by the peptides are the same as most of those induced by collagen. The peptides do not recognize the α2β1 integrin. To identify the signaling receptor involved, we have evaluated the response to the CRP, Gly-Lys-Hyp(Gly-Pro-Hyp)10-Gly-Lys-Hyp-Gly of platelets with defined functional deficiencies. These studies exclude a primary recognition role for CD36, von Willebrand factor (vWF), or glycoprotein (GP) IIb/IIIa. Thus, both CD36 and vWF-deficient platelets exhibited normal aggregation, normal fibrinogen binding, and normal expression of CD62 and CD63, measured by flow cytometry, in response to the peptide, and there was normal expression of CD62 and CD63 on thrombasthenic platelets. In contrast, GPVI-deficient platelets were totally unresponsive to the peptide, indicating that this receptor recognizes the Gly-Pro-Hyp sequence in collagen. GPVI-deficient platelets showed some fibrinogen binding in response to collagen but failed to aggregate and to express CD62 and CD63. Collagen, but not CRP-XL, contains binding sites for α2β1. Therefore, it is possible that collagen still induces some signaling via α2β1, leading to activation of GPIIb/IIIa. Our findings are consistent with a two-site, two-step model of collagen interaction with platelets involving recognition of specific sequences in collagen by an adhesive receptor such as α2β1 to arrest platelets under flow and subsequent recognition of another specific collagen sequence by an activatory receptor, namely GPVI.
COLLAGENS OF THE subendothelium are major determinants of the thrombogenicity of the blood vessel wall.1 In particular, the fibrous collagens I and III, exposed as a consequence of injury to the vessel wall, are strong platelet agonists that induce shape change, release reaction, and aggregation. The mechanism of collagen-platelet interaction involves both the direct recognition of collagen receptors and indirect binding of collagen to the platelet surface via intermediary proteins.2,3 As an example of the latter, von Willebrand factor (vWF) plays an essential role in hemostasis by complexing to collagen(s) in the vessel wall and concomitantly binding to specific receptors on the platelet surface,4,5 the glycoprotein (GP) Ib/V/IX complex, and the activated GPIIb/IIIa complex,6thus forming a bridge between collagen(s) and platelets.
A number of platelet membrane proteins have been proposed as collagen receptors, including integrin α2β1(GPIa/IIa; as reviewed by Santoro and Zutter7), CD36 (GPIIIb, GPIV), and GPVI (p62). Their precise role in the overall process of interaction and the relationship between them remains unclear.
Nieuwenhuis et al8 and Kehrel et al9 have described patients with mild bleeding disorders attributable to deficient expression of platelet α2β1. The platelets from these patients had impaired collagen-induced aggregation but responded normally to all other platelet agonists. Further evidence for the involvement of α2β1 is that monoclonal antibodies (MoAbs) directed against the receptor are able to inhibit adhesion of platelets to collagen under either static or flow conditions.7 10-12
CD36 binds to collagen type I fibers,13 and Fab fragments of polyclonal antibodies against CD36 have been reported to inhibit collagen-induced platelet aggregation.13,14 However, the role of CD36 as a platelet collagen receptor is not clear, because a significant proportion (1% to 4%) of Japanese and other East Asian populations lack this receptor on platelets but have no obvious bleeding disorder. Platelet adhesion to collagens I and III15,16 and platelet aggregation and secretion induced by these collagens is normal in CD36-deficient platelets.17 18
Other Japanese patients with mild bleeding disorders have been described whose platelets show reduced responses to collagen associated with functional deficiency in GPVI.19-21 One of these patients developed antibodies to GPVI, which, when added to normal platelets, caused their aggregation and whose Fab fragments were able to reduce platelet aggregation induced by collagen.21,22GPVI-deficient platelets showed defective second phase adhesion in flowing blood, a reaction that is attributable mainly to the platelet-platelet interaction.23
The interaction of platelets with collagen involves firstly adhesion and, subsequently, activation leading to second phase adhesion, secretion, and ultimately aggregation. Different mechanisms and receptor populations may be involved in these two processes.24,25 Collagen-induced platelet activation requires the expression by collagen of both tertiary (triple-helical) and quaternary (polymeric) structure.26-28 Recently, Morton et al29 have described simple collagen-related peptides (CRPs), comprising a repeating Gly-Pro-Hyp motif, that mimic the collagen tertiary (triple-helical) structure. They have shown that, when cross-linked via either lysyl or cysteine residues to impart quaternary (polymeric) structure, these peptides (CRP-XL) are extremely active platelet agonists, being more reactive than collagen fibers. In contrast, non–cross-linked (nonpolymeric) CRP antagonizes platelet activation stimulated by either native collagen or CRP-XL.29 CRPs possess the same tertiary structure as collagen and, like collagen, this conformation is essential for activity. Like collagen, CRP molecules spontaneously assemble into an organized quaternary structure and, again like collagen, the polymeric structure is essential for activity. It is therefore most likely that the potent platelet reactivity of CRP-XL reflects the platelet reactivity to collagen.
The interaction of CRP-XL with platelets does not involve the integrin α2β1; thus, CRP supports static platelet adhesion that is not blocked by the α2β1-directed MoAbs, 6F1 and MoAb13.29 These same antibodies fully block α2β1-mediated adhesion to immobilized monomeric collagen. The MoAbs are also without effect on aggregation stimulated by CRP-XL.29 The recognition of α2β1 by collagen is essential for adhesion of platelets under flow. However, CRP-XL is unable to support adhesion under flow conditions.30 Furthermore, the purified I-domain of the α2 subunit, which mediates the adhesion of native collagens to α2β1, does not recognize CRP-XL,31 and affinity chromatography using CRP-XL does not extract α2β1 from platelet lysates (Barnes et al, unpublished data). All of this establishes CRP as an agonist that operates entirely independently from α2β1.
Despite the absence of recognition of the integrin α2β1, CRPs signal in platelets in precisely the same way as native collagen fibers.32,33 Specifically, they stimulate tyrosine phosphorylation of the Fc receptor γ chain,34 of p72syk, and of phospholipase Cγ2 (PLCγ2)35—all responses characteristic of platelet activation by collagen. CRPs are therefore valuable tools for the identification of the collagen receptor that recognizes the quaternary structure of collagen and is responsible for signaling leading to activation. To this end, we have evaluated the response to CRP-XL of platelets deficient in GPIIb/IIIa, CD36, vWF, and GPVI.
MATERIALS AND METHODS
Materials.
Ristocetin was obtained from Paesel (Frankfurt, Germany).
Human vWF was purified from Haemate HS (a kind gift of Centeon, Marburg, Germany), as previously described.36
The vWF-directed MoAb, 4F9 (Immunotech, Marseilles, France), was conjugated to fluorescein isothiocyanate (FITC) using the antibody conjugation kit from Sigma (Deisenhofen, Germany) according to the manufacturer's instructions. The F/P ratio, calculated from the FITC absorbance at 495 nm and the IgG content, was 1.4. FITC-coupled MoAbs recognizing CD62 (P-selectin, GMP140, and PADGEM) or CD63 (GP53 and granulophysin), clone CLB-thromb/6 and CLB-gran/12, respectively, were obtained from Immunotech (Marseilles, France).
Highly purified human fibrinogen (Enzyme Research Labs, South Bend, IN) was conjugated with FITC using the FITC-celite (Calbiochem, Bad Soden, Germany) method according to Xia et al37 but using pH 7.8 buffer for 48 hours at 4°C, resulting in an F/P ratio of 5.0 to 5.2.
Collagen type I for use as a reference reagent has been described previously.18
Patients.
All studies were performed with the patients or other blood donors giving informed consent. Samples from healthy controls were processed in parallel with all patient samples. None of the subjects had taken any medication affecting platelet functions for at least 2 weeks before the study.
The CD36-deficient platelets of a Japanese male blood donor Y.A. have been described before.18,38 Platelets from patient A.M. with Glanzmann's thrombasthenia type I were shown to contain less than 1% of normal amounts of GPIIb/IIIa.39 Patient P.R. had von Willebrand disease type 3 (according to the Sadler and Gralnick classification40). In addition, the patient suffered from an intestinal angiodysplasia, which is associated with life-threatening bleeding episodes, requiring frequent replacement therapy with vWF. The blood for the experiments was taken 2 weeks after the last treatment and directly before new medication. At that time the vWF:RCO was less than 1% of normal and vWF antigen was undetectable (Asserachrom vWF; Stago-Diagnostica, Asniers, France) in both the plasma and the platelets. The GPVI-deficient platelets of the female Japanese patient Y.A. have been described in detail.21,22 41
Platelet aggregation.
Platelet aggregation studies were performed in an aggregometer (Chrono-Log, Haverton, PA) within 3 hours of venipuncture using platelet-rich plasma (PRP) containing 2 × 108platelets/mL, as described by Born and Cross.42 Blood was anticoagulated with 0.1 vol of 3.8% Na-citrate and PRP was prepared by centrifugation at 200g for 10 minutes at room temperature.
Flow cytometric analysis of ristocetin-induced vWF binding to platelets.
PRP was diluted to 5 × 107 platelets/mL with Tyrode's buffer, pH 7.4, to minimize the formation of platelet aggregates. Platelet suspension (200 μL) was added to 20 μL of ristocetin (at a range of concentrations in Tyrode's buffer, pH 7.4) without stirring to avoid aggregate formation. After 180 seconds, the platelets were fixed for 30 minutes with 1% formaldehyde (final concentration) in phosphate-buffered saline (PBS). Platelets were washed and resuspended in 200 μL of PBS, and FITC-conjugated anti-vWF MoAb was added at a saturating concentration (determined beforehand). After 1 hour of incubation at room temperature, 104 single platelets were analyzed in a flow cytometer (FACScan; Becton Dickinson, Heidelberg, Germany). Background binding obtained from parallel samples with FITC-conjugated isotype-specific mouse IgG was subtracted from each test sample. Excitation was at a wavelength of 488 nm. The FACScan was used in a standard configuration with a 530 nm bandpass filter. Data were obtained from fluorescence channels in a logarithmic mode.
Flow cytometric analysis of fibrinogen-FITC binding to CRP-activated platelets.
Diluted PRP (5 × 107 platelets/mL) was preincubated for 3 minutes at room temperature with 150 μg/mL fibrinogen-FITC (saturating concentration). Two hundred microliters of platelet suspension was added to 20 μL of a solution containing a range of concentrations of CRP-XL in 0.01 mol/L acetic acid at room temperature. The reaction was stopped after 120 seconds by fixation with 1% formaldehyde as described above. Platelets were washed, resuspended in 200 μL of PBS, and analyzed in a flow cytometer as described above. The nonspecific background labeling was determined using platelets from different patients with thrombasthenia type I and control platelets treated with 10 mmol/L of the inhibitory peptide GRGDSP (Novabiochem, Bad Soden, Germany) to prevent specific fibrinogen binding.
Flow cytometric analysis of CD62 and CD63 expression on CRP-activated platelets.
Two hundred microliters of diluted PRP (5 × 107platelets/mL) was added at room temperature to 20 μL 0.01 mol/L acetic acid containing CRP-XL at a range of concentrations. The reaction was stopped after 120 seconds with 1% formaldehyde, as described above. Platelets were washed and resuspended in PBS, and FITC-coupled MoAbs recognizing CD62 or CD63 were added and incubated for 1 hour at room temperature. The concentrations required for saturated binding of both antibodies were determined beforehand. After incubation, platelets were washed, resuspended in 200 μL of PBS, and analyzed as described above.
RESULTS
The interaction of CD36-deficient platelets with CRP.
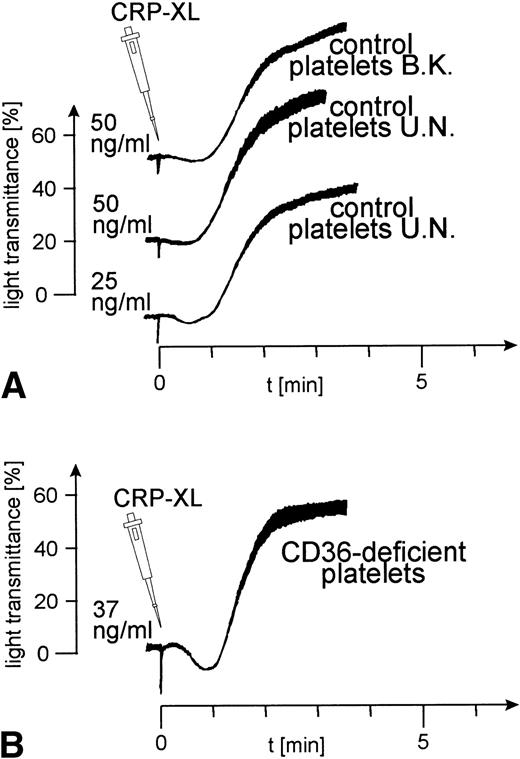
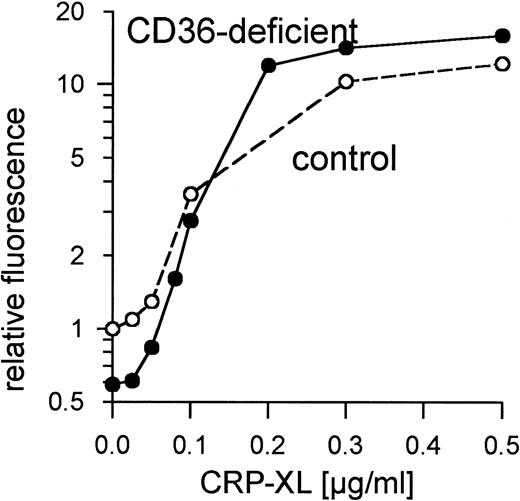
Platelets from 8 control donors were aggregated by CRP-XL at concentrations from 20 to 100 ng/mL. Aggregation curves from 2 such donors are shown in Fig 1A. The CD36-deficient platelets of donor Y.A. reacted as normal control platelets when treated with CRP-XL, which induced full aggregation at a concentration of 37 ng/mL (Fig 1B). Fibrinogen binding to CRP-XL-activated control and CD36-deficient platelets did not differ significantly (Fig 2). CD36-deficient platelets activated by CRP-XL expressed CD62 and CD63 to the same extent as control platelets (data not shown).
Normal platelet aggregation induced by CRP-XL in platelet-rich plasma (PRP). (A) Control platelets; (B) CD36-deficient platelets from donor Y.A. The platelet counts were adjusted to 2 × 108/mL. The experiment shown is representative of three similar experiments.
Normal platelet aggregation induced by CRP-XL in platelet-rich plasma (PRP). (A) Control platelets; (B) CD36-deficient platelets from donor Y.A. The platelet counts were adjusted to 2 × 108/mL. The experiment shown is representative of three similar experiments.
Normal binding of fibrinogen to CD36-deficient platelets and control platelets activated with CRP-XL measured by flow cytometry using FITC-labeled fibrinogen. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Normal binding of fibrinogen to CD36-deficient platelets and control platelets activated with CRP-XL measured by flow cytometry using FITC-labeled fibrinogen. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
The reaction of CRP with thrombasthenic platelets.
Because, as expected, specific fibrinogen binding after exposure to agonist was not observed in these GPIIb/IIIa-deficient platelets, activation was determined on the basis of the surface expression of CD62 (Fig 3A) and of CD63 (Fig 3B). Expression of both in GPIIb/IIIa-deficient platelets treated with CRP-XL was not lower than in controls.
Normal expression of CD62 (A) and of CD63 (B) on GPIIb/IIIa-deficient platelets and control platelets induced by CRP-XL, measured by flow cytometry after labeling with anti-CD62-FITC or anti-CD63-FITC, respectively. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Normal expression of CD62 (A) and of CD63 (B) on GPIIb/IIIa-deficient platelets and control platelets induced by CRP-XL, measured by flow cytometry after labeling with anti-CD62-FITC or anti-CD63-FITC, respectively. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
The reaction of CRP with platelets in von Willebrand disease.
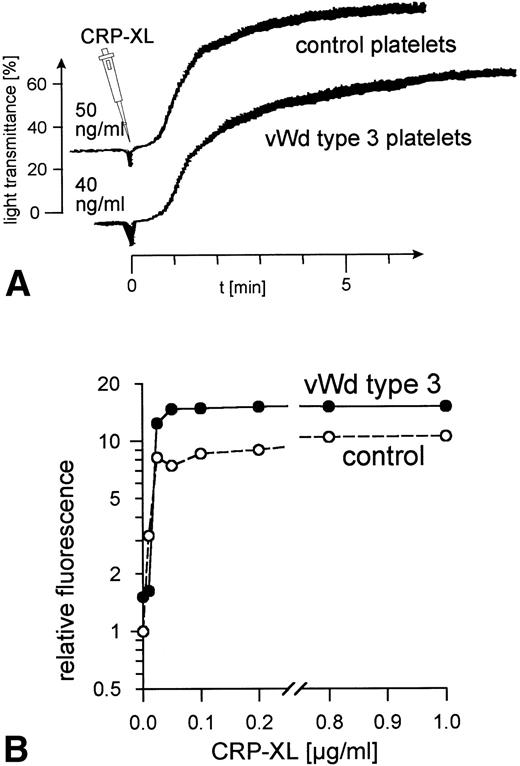
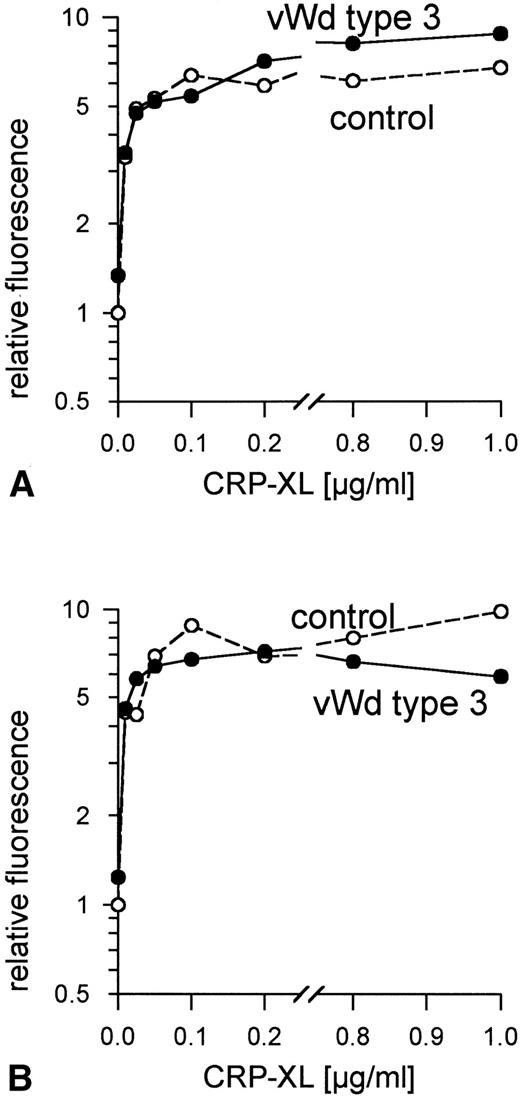
Ristocetin, at concentrations up to 4 mg/mL, did not induce vWF-mediated platelet agglutination in PRP from patient P.R. (data not shown). The addition of human vWF (10 μg/mL) corrected the defect. No vWF binding to the surface of the patient's platelets was observed when these platelets were treated with ristocetin at concentrations ranging from 0.2 to 2 mg/mL, whereas 1.0 mg/mL induced maximum vWF binding to control platelets (data not shown). No vWF was detectable in samples of the patient's platelets and plasma taken at the time these platelets were activated with CRP-XL. CRP-XL induced normal aggregation of vWF-deficient platelets in vWF-deficient plasma (Fig 4A) and induced fibrinogen binding to a somewhat greater extent than in controls (Fig 4B). The surface expression of CD62 (Fig 5A) and of CD63 (Fig 5B) induced by CRP-XL in PRP of patient P.R. were identical in terms of both extent and concentration dependence to that occurring in control platelets.
Normal platelet aggregation induced by CRP-XL in PRP from a von Willebrand disease type 3 patient and from a control (A). Normal binding of fibrinogen to vWF-deficient platelets and to control platelets in vWF-deficient plasma activated with CRP-XL, measured by flow cytometry using FITC-labeled fibrinogen (B). Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Normal platelet aggregation induced by CRP-XL in PRP from a von Willebrand disease type 3 patient and from a control (A). Normal binding of fibrinogen to vWF-deficient platelets and to control platelets in vWF-deficient plasma activated with CRP-XL, measured by flow cytometry using FITC-labeled fibrinogen (B). Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Normal expression of (A) CD62 and (B) CD63 on vWF-deficient platelets in vWF-deficient plasma and control platelets in control plasma, induced by CRP-XL, measured by flow cytometry using FITC-labeled anti-CD62 antibody or anti-CD63 antibody, respectively. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Normal expression of (A) CD62 and (B) CD63 on vWF-deficient platelets in vWF-deficient plasma and control platelets in control plasma, induced by CRP-XL, measured by flow cytometry using FITC-labeled anti-CD62 antibody or anti-CD63 antibody, respectively. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
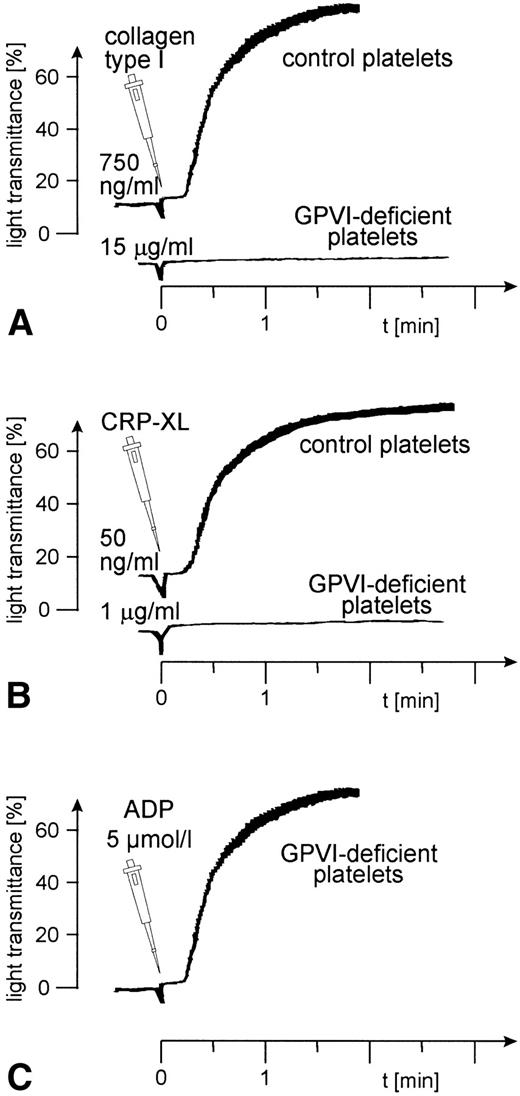
The reaction of CRP with GPVI-deficient platelets.
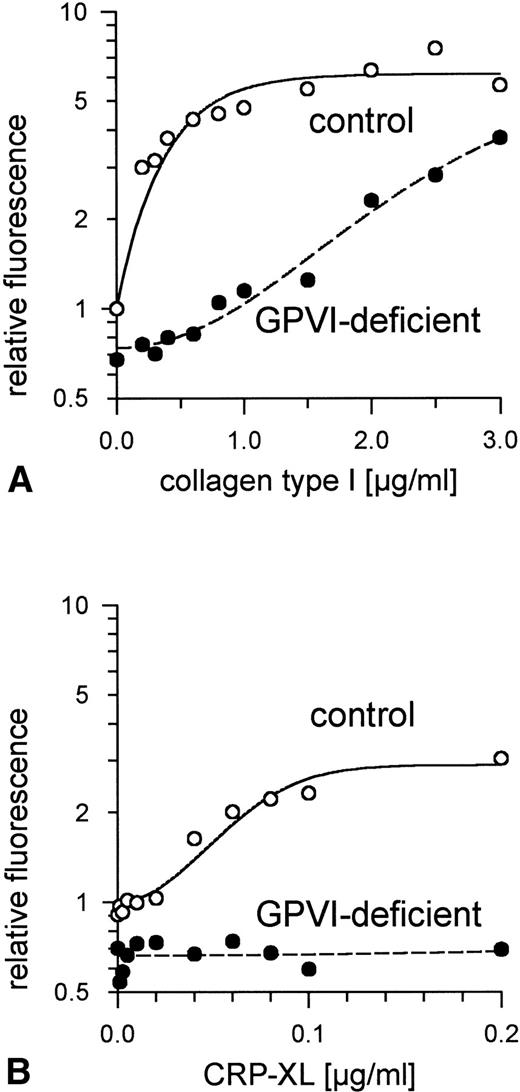
Aggregation of GPVI-deficient platelets was totally absent even in the presence of a concentration of either collagen or CRP-XL 20-fold higher than was needed to aggregate normal platelets (Fig 6A and B). In contrast, aggregation of these platelets by ADP was normal (Fig 6C). Neither collagen nor CRP-XL induced any surface expression of CD62 (Fig7A and B) or CD63 (Fig 8A and B). There was no expression of fibrinogen binding in response to CRP-XL (Fig 9B), although collagen-induced fibrinogen binding was not absent but expressed to a decreased extent compared with normal platelets, especially at low concentrations of collagen (Fig 9A).
Aggregation of GPVI-deficient and control platelets induced by (A) collagen type I, (B) CRP-XL, and (C) ADP. The platelet count had to be adjusted to 1.8 × 108/mL because the patient was slightly thrombocytopenic. Collagen and CRP-XL did not induce platelet aggregation in GPVI-deficient platelets, although these platelets were able to aggregate in response to ADP. Data shown are representative of three similar experiments.
Aggregation of GPVI-deficient and control platelets induced by (A) collagen type I, (B) CRP-XL, and (C) ADP. The platelet count had to be adjusted to 1.8 × 108/mL because the patient was slightly thrombocytopenic. Collagen and CRP-XL did not induce platelet aggregation in GPVI-deficient platelets, although these platelets were able to aggregate in response to ADP. Data shown are representative of three similar experiments.
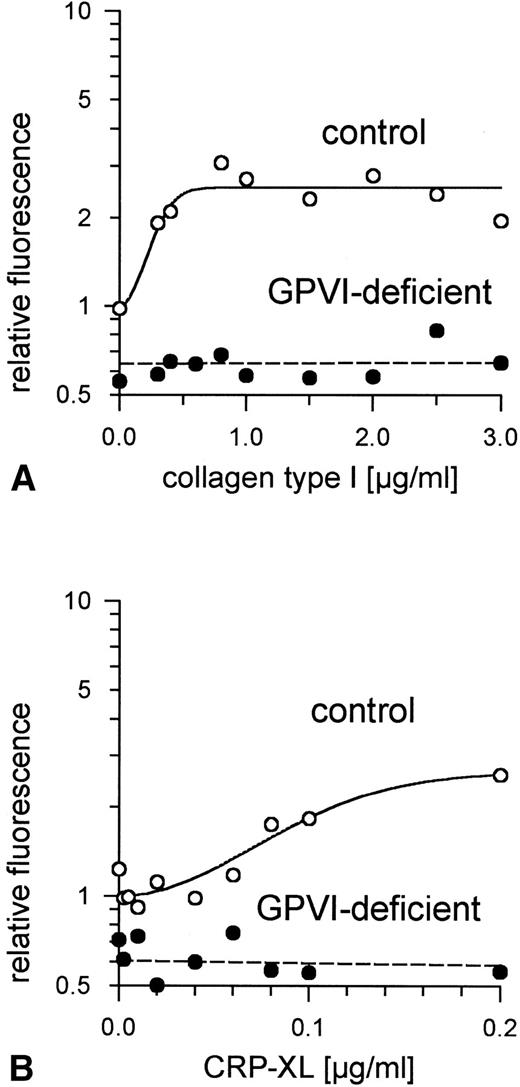
Expression of P-selectin (CD62) on GPVI-deficient platelets and control platelets induced by (A) collagen type I and (B) CRP-XL, measured by flow cytometry using FITC-labeled anti-CD62 antibody. Neither collagen nor CRP-XL induced CD62 expression on GPVI-deficient platelets. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Expression of P-selectin (CD62) on GPVI-deficient platelets and control platelets induced by (A) collagen type I and (B) CRP-XL, measured by flow cytometry using FITC-labeled anti-CD62 antibody. Neither collagen nor CRP-XL induced CD62 expression on GPVI-deficient platelets. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Expression of CD63 on GPVI-deficient platelets and control platelets induced by (A) collagen type I and (B) CRP-XL, measured by flow cytometry using FITC-labeled anti-CD63 antibody. Neither collagen nor CRP-XL induced CD63 expression on GPVI-deficient platelets. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Expression of CD63 on GPVI-deficient platelets and control platelets induced by (A) collagen type I and (B) CRP-XL, measured by flow cytometry using FITC-labeled anti-CD63 antibody. Neither collagen nor CRP-XL induced CD63 expression on GPVI-deficient platelets. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Binding of fibrinogen to GPVI-deficient platelets and control platelets activated by (A) collagen type I and (B) CRP-XL, measured by flow cytometry using FITC-labeled fibrinogen. In GPVI-deficient platelets, there was no expression of fibrinogen binding in response to CRP-XL; collagen-induced fibrinogen binding was expressed to a decreased extent, especially at low concentrations of collagen. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
Binding of fibrinogen to GPVI-deficient platelets and control platelets activated by (A) collagen type I and (B) CRP-XL, measured by flow cytometry using FITC-labeled fibrinogen. In GPVI-deficient platelets, there was no expression of fibrinogen binding in response to CRP-XL; collagen-induced fibrinogen binding was expressed to a decreased extent, especially at low concentrations of collagen. Fluorescence of unstimulated control platelets is defined as 1. Data shown are representative of two similar experiments.
DISCUSSION
All collagens share a common tertiary structure, the collagen triple helix. The collagen molecule contains three α chains, each with a repeating Gly-X-Y sequence in which X and Y are frequently the amino acids, Pro and Hyp, respectively. The Gly-Pro-Hyp triplet represents approximately 12% of the primary sequence of type I collagen. Within the molecule each of these chains forms a left-handed polyproline II helix and the three helices are intertwined to form a right-handed superhelix, the collagen triple helix. Molecules then associate very specifically to yield the highly ordered quaternary structure of the collagen fiber.43
The native triple helical structure of collagen is required for platelet secretion and aggregation. Furthermore, collagen molecules must also assemble into fibrous form for collagen to be an effective agonist.26-28 In contrast, monomeric collagen immobilized on plastic can serve as a very effective substrate for adhesion without any activation.44 This adhesion is mediated by the integrin α2β1 and can be blocked by antibodies to α2β1.7 10-12
Although evidence has been presented suggesting that signaling in platelets by collagen is subsequent to recognition of α2β1,35,45-47 it is not clear how far α2β1 is directly responsible for signaling leading to platelet activation. Although collagen-induced phosphorylation events may under some circumstances be attenuated by blockade of α2β1 by antibodies, persistence of signaling has been reported in response to collagen or CRP-XL.29,34,35,47 Indeed, in platelets lacking GPVI, but in which α2β1 is expressed normally, collagen is able to activate the tyrosine kinase c-src, but not p72syk, PLCγ2, or p125fak.41 All of these are regarded as important signaling molecules in the activation of normal platelets by collagen. This indicates the possibility that GPVI (rather than α2β1) may be crucial as an activatory receptor for collagen in platelets.
A triple-helical synthetic analogue of collagen, composed simply of a repeating Gly-Pro-Hyp sequence, has been shown in polymeric form (CRP-XL) to elicit a full aggregatory response from human platelets without the involvement of the integrin α2β1.29 The triple-helical non–cross-linked peptide (CRP) is not activatory but inhibits platelet activation by collagen and by CRP-XL.29 This corroborates the existence of a signaling receptor on platelets other than, or in addition to, α2β1, which requires the tertiary and quaternary structures of collagen for platelet activation.
As shown here, CD36-deficient platelets bind fibrinogen and exhibit normal secretion and aggregation in response to CRP-XL (as is true for collagen15-18). CD36 cannot, therefore, be this collagen receptor.
Platelets bind indirectly to collagen, via vWF, through GPIb and GPIIb/IIIa complexes.48 This binding confers resistance to the shear forces generated by blood flowing along the vessel wall.49 50 However, platelets from a Glanzmann's thrombasthenia type I patient, totally deficient in GPIIb/IIIa, responded normally to CRP-XL, which induced normal secretion, measured as expression on the plasma membrane of CD62 (from the α-granules) and CD63 (from the dense bodies). These data exclude GPIIb/IIIa as the primary signaling receptor that recognizes the quaternary structure of collagen.
The same can be concluded for vWF. Despite the absence of vWF, both in the platelets and plasma of patient P.R., CRP-XL induced normal fibrinogen binding, normal secretion (CD62 and CD63 expression), and normal aggregation.
These results confirm the view that CD36, GPIIb/IIIa, and vWF are not essential for platelet activation by collagen.
In contrast, GPVI-deficient platelets were unresponsive to CRP-XL, showing that GPVI is a signaling receptor for the polymeric cross-linked triple-helical Gly-Pro-Hyp motif. Our findings are in line with the observation that antibody-induced cross-linking of GPVI in normal platelets results in activation of src and p72syk in parallel with activation of PLCγ222 and that CRP-XL stimulates tyrosine phosphorylation of PLCγ2 and p72sykin normal platelets independent of the integrin α2β1.35
In GPVI-deficient platelets CRP-XL did not induce the activation-dependent conformational changes in GPIIb/IIIa that are necessary for fibrinogen binding. However, it is interesting that fibrinogen binding was induced in these platelets by increasing collagen levels, although to a lower degree than in equivalent controls (Fig 9A). Collagen (but not CRP-XL) contains binding sites for α2β1, and GPVI-deficient platelets express α2β1, which may possibly be involved in signal transduction by collagen, sufficient for partial activation of GPIIb/IIIa. Nevertheless, collagen fibers provided insufficient signals to cause platelet aggregation in GPVI-deficient platelets, even at collagen levels 20-fold higher than are needed to aggregate normal platelets. Granule release was completely absent, as with CRP-XL (Figs7 and 8). Recently, the activation of c-src, but not of p72syk or PLCγ2, has been shown in GPVI-deficient platelets stimulated with collagen,41 providing firm evidence for signaling, but not sufficient to cause aggregation, arising from a second population of collagen receptors as well as GPVI. It is plausible that GPVI may be linked more to positive feedback events, such as thromboxane production, a process essential for the aggregation of platelets by collagen, or granule release, providing further activation mediated by secreted ADP, so leading to full activation of GPIIb/IIIa. Results from these and other studies suggest that, in platelets, α2β1 has a major role in adhesion to collagen and a less-well defined role in platelet activation in support of GPVI. These ideas are consistent with the two-site two-step model of platelet activation by collagen.24,25 51 The major role for α2β1 may be to provide a stable interaction, especially under nonstatic conditions, to allow subsequent interaction of the Gly-Pro-Hyp motif with other, activatory receptors. This study establishes GPVI as an activatory receptor crucial for the full activation of platelets by collagen.
Un–cross-linked CRP, although not causing platelet activation, does inhibit aggregation by both CRP-XL and collagen fibers, which may suggest that GPVI can recognize the monomeric molecule. Lack of activation by monomeric CRP suggests, in turn, that clustering of GPVI molecules may be necessary for activation.
In view of the potency of the repeating Gly-Pro-Hyp sequence in triple-helical, polymeric form, we believe that Gly-Pro-Hyp in collagen may be a highly specific recognition sequence for GPVI. However, we cannot rule out the possibility, as we have recently conjectured,29 that GPVI recognizes the triple helix per se and that any assembly of Gly-X-Y amino acid triplets generating a triple-helical structure may be active. Further studies are in progress to affirm the crucial importance of the Gly-Pro-Hyp motif in the recognition of collagen by GPVI.
ACKNOWLEDGMENT
The authors thank all blood donors and patients involved in this study for their kind cooperation and Joachim Kardoeus for preparation of the figures and editorial assistance.
Supported in part by the Deutsche Forschungsgemeinschaft (Grant No. Ke397/1-3) and the Gesellschaft für Thrombose- und Hämostaseforschung (travel support). K.J.C. was supported by Grant No. 31-42336.94 from the Swiss National Science Foundation. R.W.F., C.G.K., and M.J.B. were supported by the Medical Research Council, UK, to whose External Scientific Staff M.J.B. belongs.
Address reprint requests to Beate Kehrel, PhD, Experimentelle Haemostaseforschung, Medizinische Klinik und Poliklinik, Innere Medizin A, Universität Münster, Domagkstrasse 3, D-48129 Münster, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal