Abstract
Hypersensitivity to cross-linking agents and predisposition to malignancy are characteristic of the genetically heterogeneous inherited bone marrow failure syndrome, Fanconi anemia (FA). The protein encoded by the recently cloned FA complementation group A gene, FAA, has been expected to localize in the nucleus as based on the presence of sequences homologous to a bipartite nuclear localization signal (NLS) and a leucine repeat motif. In contrast to this expectation, we show here that a functionally active FAA-green fluorescent protein (GFP) hybrid resides in the cytoplasmic compartment of human kidney 293 cells. In accordance with this finding, disruption of the putative NLS by site-directed mutagenesis failed to affect both subcellular localization and the capacity to complement hypersensitivity to the cross-linking agent mitomycin C in FA-A lymphoblasts. Furthermore, the N-terminal part of FAA with the putative NLS at amino acid position 18 to 35 showed no nuclear translocation activity when fused to GFP, while the first 115 N-terminal amino acids appeared to be indispensable for the complementing activity in FA-A cells. Similarly, mutagenesis studies of the putative leucine repeat showed that, even though this region of the protein is important for complementing activity, this activity does not depend on an intact leucine zipper motif. Finally, fusion of the NLS motif derived from the SV40 large T antigen to FAA could not direct the hybrid protein into the nucleus of 293 cells, suggesting that FAA is somehow maintained in the cytoplasm via currently unknown mechanisms. Thus, like the first identified FA protein, FAC, FAA seems to exert its function in the cytoplasmic compartment suggesting FA proteins to be active in a yet to be elucidated cytoplasmic pathway that governs hematopoiesis and protects against genomic instability.
FANCONI ANEMIA (FA) is an autosomal recessive disease characterized by bone marrow (BM) failure, variable congenital malformations, and cancer predisposition.1 Cells from FA patients have a characteristic hypersensitivity to cross-linking agents such as mitomycin C (MMC), cisplatin, and diepoxybutane2,3 due to their proneness to undergo apoptosis induced by these agents.4 The disorder is genetically heterogeneous, with at least five complementation groups having been identified in somatic cell fusion studies.5,6 These different groups, named A to E, suggest the existence of five distinct disease genes.7
The first FA gene to be cloned, the FA group C gene FAC encoding a 63-kD protein, has not revealed sequence motifs that could provide a clue to its function.8 So far, the molecular basis of the disease remains unclear, although the increased spontaneous and cross-linker–induced chromosomal instability found in FA has been interpreted as being indicative of a primary defect in DNA repair. However, conflicting evidence has been reported on the putative DNA repair defect in FA,3 while independent studies have shown that the FAC protein is localized and functionally active in the cytoplasmic compartment only, suggesting no direct role for FAC in DNA repair.9-11
Recently, a second FA gene FAA has been identified and found to encode a protein of 1,455 amino acids with a molecular mass of approximately 163 kD.12,13 Although no significant homology to other proteins has been found, a putative bipartite nuclear localization signal (NLS) as well as a leucine zipper motif could be distinguished. The presence of these domains lead to the expectation of the FAA protein being localized to the cell nucleus.12
Here we describe the use of chimeras of FAA and the reporter molecule green fluorescent protein (GFP) to examine the subcellular localization of the FAA polypeptide in human cells. GFP is a 238-amino acid protein from the jellyfish Aequorea victoria which retains its ability to fluoresce in vivo when expressed in heterologous cell types.14-16 In contrast to the expected nuclear localization of FAA, our results show that an FAA-GFP protein chimera, which retains full activity with respect to the correction of MMC hypersensitivity in FA-A lymphoblasts, is primarily localized to the cytoplasm. Consistent with this finding, disruption of the putative bipartite NLS and leucine repeat motifs by site-directed mutagenesis failed to impair FAA function or alter its subcellular localization. Furthermore, experiments designed to determine the requirement of a cytoplasmic localization for FAA to restore cross-linker resistance in FA-A lymphoblasts were hampered by the inability of a heterologous NLS motif to target FAA to the nucleus.
The cytoplasmic localization of both FAC and FAA suggests the existence of a novel and yet to be elucidated molecular mechanism involved in multiple cellular functions, such as maintenance of genomic stability and the control of programmed cell death.
MATERIALS AND METHODS
Plasmid construction, mutagenesis, and GFP fusion proteins. The cDNA encoding FAA was excised by Not I digestion from pREP-FAA, previously described as clone D,12 and subcloned in pBluescript SK− (Stratagene Cloning Systems, La Jolla, CA). Following determination of the orientation of the cDNA by restriction enzyme analysis, pSKFAA-5.5 was obtained with the 5′ end of the cDNA positioned at the T3 promoter site. Digestion of this plasmid with Kpn I, leading to deletion of the approximately 4-kb 3′ end of the cDNA, and subsequent religation yielded pSKFAA-BK1.5. pSKFAA-M116 was constructed by cutting pSKFAA-BK1.5 with Ava I, filling in recessive ends, religation, and subsequent completion of the open reading frame (ORF) by inserting the approximately 4-kb Kpn I-fragment derived from pSKFAA-5.5.
For polymerase chain reaction (PCR)-based site-directed mutagenesis, the following oligonucleotide primers were used, in which restriction enzyme sites are underlined and nonmatching or mutated nucleotides are shown in lowercase letters: FAAmNLS (Ava I): (5′-taaGCCTCGGGCCAGGACCCAGGGGaCCtCCtGAG); FAA1474sp: (5′-gcgatttaggtgacactatagaAGGGGTGGGTGGAGAATGTG); FA3795S: (5′-aatacgactcactataggGATGGGCCTGCTGTCGTCACAT); FAAL3P (BamHI): (5′-AACGGATCCTCCcCCGCCTGCC); FAAL3,4V (BamHI): (5′-AACGGATCCTCgTCCGCCTGCCTTCGTCTGTCgTCTGC); FAA-X (HindIII): (5′-ggtcaagcttGAAGAGATGAGGCTCCTGGGACAGGT); FAAXH (Xba I/HindIII): (5′-GCAGAAGCTTCTAGAAGAGATGAGGCTCCTGGGACAGGT).
pSKFAA-5.5 was used as a template for PCR. The PCR product obtained with FAA-mNLS and FAA1474sp primers was digested with both Ava I and Kpn I, and exchanged with the corresponding fragment in pSKFAA-BK1.5. The PCR fragments generated with either FAAL3P or FAAL3,4V in combination with the FAAXH primer were digested with BamHI and HindIII, and subcloned in pSK−. Several subcloning steps were required to add the lacking ORF sequences in these FAA mutants. The PCR product obtained with the primers FA3795S and FAA-X, encompassing the 3′ portion of the FAA ORF in which the stop codon is absent, was digested with Sst I and HindIII and subcloned in pSK−. The 5′ end of the ORF was added by insertion of the approximately 4-kb Sst I-fragment obtained from pSKFAA-5.5. Subsequently, the 2.5-kb Kpn I-HindIII fragment was taken from this construct and fused in frame to the S65T-GFP mutant coding region,17 which was obtained as a BamHI-Xba I fragment derived from the peroxisome-targeted GFP-containing plasmids pcDNA3-PTS1- and PST2-GFP,18 in which the specific target had been deleted. After the exchange of DNA fragments derived from this construct and corresponding fragments with earlier generated plasmid constructs, ORFs encoding either wild-type or mutant FAA-GFP protein chimeras could be generated.
The SV40 Large T antigen NLS was fused to the N-terminus of FAA by using the oligonucleotides LTNLS-U (5′-GATCCATGCCGAAGAAAAAGAGAAAGGTGGAACTGTCCGACTCGTGGGTCCCGAACTCCGCC) and LTNLS-L (5′-CCGAGGCGGAGTTC-G G G A C C C A C G A G T C G G A C A G T T C C A C C T T T C T C T T T T-TCTTCGGCATG). After annealing, the resulting fragment was subcloned in BamHI/Ava I digested pSKFAA-BK1.5, and subsequently the FAA ORF was added with or without GFP. As control FAA was fused to mLTNLS-U and mLTNLS-L, which are identical to LTNLS-U and -L except for the substitution of the underlined nucleotides for C or G respectively, to harbor a hybrid FAA protein with an inactivated Large T NLS. In the same way GFP alone was fused to either a functional or mutated Large T NLS by using a similar stratagy to allow in frame fusion to the GFP ORF.
The Epstein-Barr virus (EBV)-based episomal expression vector pDR2 (Clontech Laboratories Inc, Palo Alto, CA), containing the Rous Sarcoma Virus LTR, was used for transient or stable expression of the FAA variants in different cell lines as indicated. To generate pDR-FAA, the 3.3-kb BamHI fragment was inserted in pDR2 yielding pDRFAA-3.3, and subsequently the ORF was completed with the approximately 4-kb Kpn I-Xba I fragment obtained from pSKFAA-5.5. The various FAA mutants in pDR2 with or without the GFP tag were generated by exchanging specific DNA fragments from plasmids described above and pDR-FAA. A detailed description of the generation of the constructs used in this study is available on request.
All the mutant FAA or FAA-GFP hybrid-containing constructs were tested for the presence of the introduced mutation or the desired in frame fusion by sequencing using the T7 Sequencing Kit (Pharmacia Biotech, Uppsala, Sweden). Restriction or modifying enzymes were purchased from GIBCO-BRL (Gaithersburg, MD) and oligonucleotides from Pharmacia.
Cell culture and transfection. HSC72 (FA-A) lymphoblastoid cells19 were cultured at 37°C in RPMI 1640 medium (GIBCO-BRL) containing 10% newborn calf serum (Hyclone, Logan, UT) under an atmosphere of 5% CO2 in air. Human fetal kidney 293 cells20 constitutively expressing the EBNA-1 protein from EBV (293-EBNA; Invitrogen Corp, San Diego, CA) were cultured in Dulbecco's Modified Eagle's Media containing glutamax-1 (GIBCO-BRL) supplemented with 10% fetal bovine serum and 250 μg/mL geneticin (G418 sulfate; GIBCO-BRL).
Determination of mitomycin C (MMC, Kyowa Hakko, Ltd)-induced growth inhibition was performed by the continuous exposure of cells in parallel cultures, starting with 5 × 104 cell/mL, to 0 to 100 nmol/L MMC as indicated. Growth inhibition was measured by cell counting using a Coulter counter (Coulter Immunology, Hialeah, FL) at the time that untreated cells had undergone three cell divisions (typically after 3 to 5 days). To assess growth inhibition the final cell count of untreated cultures was set at 100%. Electroporation was used to stably transfect HSC72 cells as described previously.4
293-EBNA cells were transfected using DOSPER liposomal transfection reagent (Boehringer Mannheim, Mannheim, Germany) basically according to the instructions of the manufacturer. Briefly, cells were dispensed on 6-well plates (3.5 × 105 cells/well) and 1 day later transfected by the addition of a 2-μg plasmid DNA/9-μg DOSPER mixture in 100 μL 20 mmol/L HEPES, 150 mmol/L NaCl. After incubation with the DNA/DOSPER mixture for 8 hours, the medium was discarded and replaced by fresh medium. Forty-eight hours later, cells were either isolated for the experiments as indicated or selected for stable expression by culturing in medium supplemented with 150 μg/mL hygromycin (Boehringer Mannheim).
Microscopy and image capturing. Transiently or stably transfected 293-EBNA cells expressing GFP fusion proteins were grown on sterile glass coverslips placed in 6-well plates. After 24 hours living cells were either directly analyzed or treated with various concentrations MMC before mounting onto microscope slides. Stably GFP hybrid-expressing HSC72 cells resuspended in culture medium were spotted on slides and coverslips were sealed.
Cells illuminated by UV light were evaluated by microscopy using a Leica DM-RB microscope (Leica, Cambridge, UK), a fluorescein filter and a plan-apo oil-phase lens (Leica), magnification 63×. Images were captured using CytoVision software (Applied Imaging Corp, Santa Clara, CA) and processed using SCIL-Image software (SCIL-Image; Difa Vision, Breda, The Netherlands).
Immunoblotting and cell fractionation. Immunoblotting was performed as described earlier.4 Briefly, cell extracts, usually representing 105 cells per sample, were run on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to immobilon-P membrane (Millipore Corp, Bredford, MA). As molecular weight markers kaleidoscope prestained standards in the range of 200 to 6.5 kD were used (Bio-Rad Laboratories, Hercules, CA). After blocking with 5% dry milk in TBS (10 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl) for 1 hour at room temperature, the membrane was incubated overnight at 4°C in TBS containing 0.5% bovine serum albumin (BSA) with a 20,000-fold dilution of a GFP monoclonal antibody (Clontech Laboratories Inc). After several washing steps, incubation with biotinylated F(ab′)2 fragments of rabbit-antimouse IGgs (DAKO, Glostrups, Denmark), additional washing, and subsequent incubation with peroxidase-conjugated streptavidin (DAKO), the blots were developed by enhanced chemiluminescence according to the instructions of the manufacturer (Amersham, Arlington Heights, IL).
For subcellular fractions, transfected 293 cells were washed with ice-cold phosphate-buffered saline (PBS), resuspended in PBS, and transferred to a tube. Pelleted cells were carefully resuspended in 5-pellet volumes hypotonic buffer (10 mmol/L Tris-Hcl pH 7.5, 10 mmol/L KCl, 1.5 mmol/L MgCl2 ), immediately centrifuged for 5 minutes at 1,200 rpm and resuspended in 3-pellet volumes hypotonic buffer. Cells were allowed to swell for 10 minutes on ice and were homogenized by pushing the cell suspension five times through a 25-gauge needle. Accurate lysis was confirmed by microscopy and nuclei were pelleted by centrifugation for 15 minutes at 4,000 rpm. The supernatant was collected as the cytoplasmic fraction and nuclei were gently washed in ice-cold PBS and subsequently resuspended in 0.5 vol low salt buffer containing 20 mmol/L Tris-HCl pH 7.5, 25% glycerol, 20 mmol/L KCl, 1.5 mmol/L MgCl2 , 0.2 mmol/L EDTA. After the addition of 0.5 vol high-salt buffer (20 mmol/L Tris-HCl pH 7.5, 25% glycerol, 1.2 mol/L KCl, 1.5 mmol/L MgCl2 , 0.2 mmol/L EDTA) nuclei were extracted by rotation for 30 minutes at 4°C, followed by centrifugation for 30 minutes at 14,000 rpm. The supernatant was dialyzed against a buffer containing 20 mmol/L Tris-HCl pH 7.5, 20% glycerol, 100 mmol/L KCl, 0.2 mmol/L EDTA to obtain the nuclear extract. All buffers used were suplemented with 0.5 mmol/L dithiothreitol (DTT) and 0.1 μg/mL Pefabloc (protease inhibitor; Boehringer Mannheim). Supernatants were taken and protein content was determined using the Bio-Rad protein assay (Bio-Rad) according to the manufacturer's protocol.
RESULTS
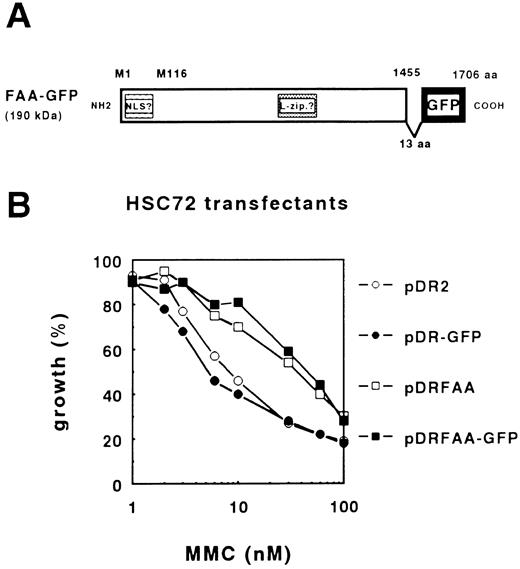
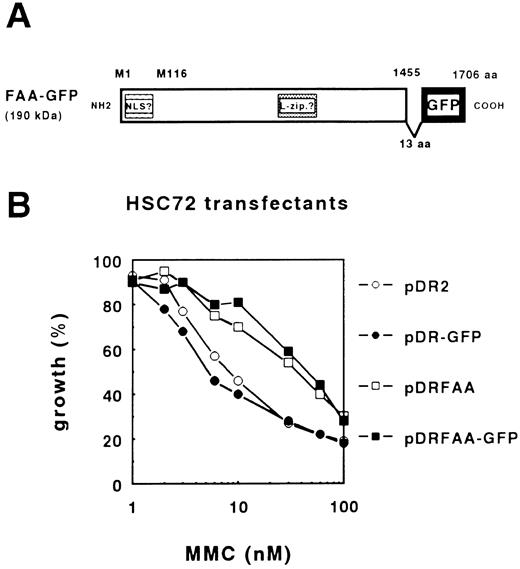
Functional FAA-GFP localizes to the cytoplasmic compartment. To determine the subcellular localization of FAA, a protein chimera was generated in which a modified GFP gene, GFPS65T,17 was fused to the C-terminus of full-length FAA. As shown in a schematic representation of FAA-GFP (Fig 1A), the cloning strategy lead to the insertion of an additional stretch of 13 amino acids (aa), KLDIEFLQPGGST, between the last residue in the FAA ORF and the start of the GFP coding region.
The FAA-GFP chimera complements MMC-hypersensitivity in FA-A lymphoblasts. (A) Schematic representation of FAA-GFP. Indicated are the relative positions of both the putative bipartite nuclear localization domain (NLS) and leucine repeat motif (L-zip.). The cloning strategy used resulted in the insertion of 13 amino acids (aa), KLDIEFLQPGGST, leading to a hybrid protein encompassing 1,706 aa with a molecular weight of approximately 190 kD. (B) MMC-dependent growth inhibition in stably transfected HSC72 (FA-A) cells. The expression vector pDR2 alone or containing the GFP, FAA, or FAA-GFP coding regions were stably transfected in HSC72 cells and MMC-complementing activity of the constructs was determined.
The FAA-GFP chimera complements MMC-hypersensitivity in FA-A lymphoblasts. (A) Schematic representation of FAA-GFP. Indicated are the relative positions of both the putative bipartite nuclear localization domain (NLS) and leucine repeat motif (L-zip.). The cloning strategy used resulted in the insertion of 13 amino acids (aa), KLDIEFLQPGGST, leading to a hybrid protein encompassing 1,706 aa with a molecular weight of approximately 190 kD. (B) MMC-dependent growth inhibition in stably transfected HSC72 (FA-A) cells. The expression vector pDR2 alone or containing the GFP, FAA, or FAA-GFP coding regions were stably transfected in HSC72 cells and MMC-complementing activity of the constructs was determined.
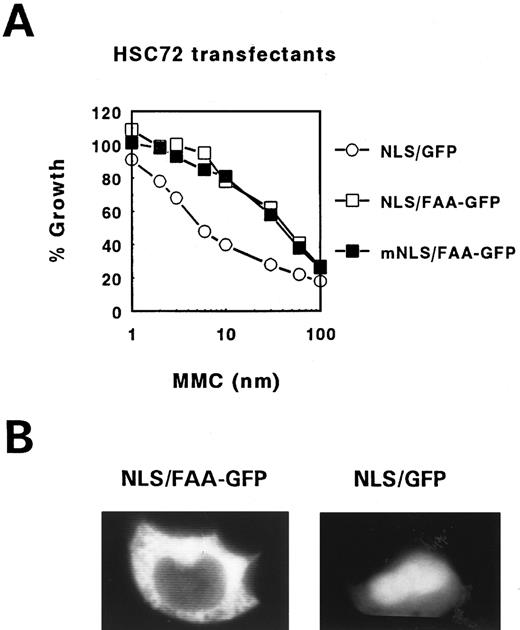
First, we tested the efficacy of FAA-GFP to complement cross-linker sensitivity in the FA-A lymphoblastoid cell line, HSC72. The hybrid ORF encoding an approximately 190-kD fusion protein was subcloned in the episomal EBV-based expression vector pDR2 and transfected into HSC72 cells followed by the determination of MMC-dependent growth inhibition. Figure 1B shows that FAA-GFP is as efficient as FAA in complementing MMC hypersensitivity, indicating that this hybrid protein is functional. Based on this finding the possibility that the GFP tag caused mislocalization of the hybrid protein was considered unlikely.
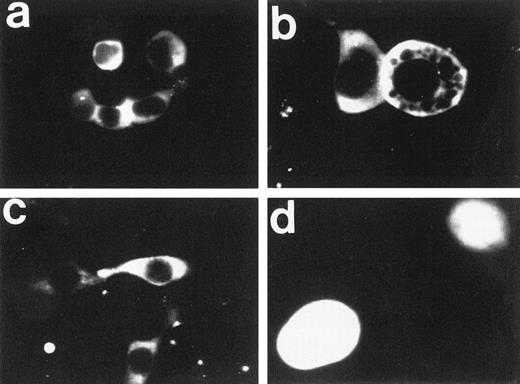
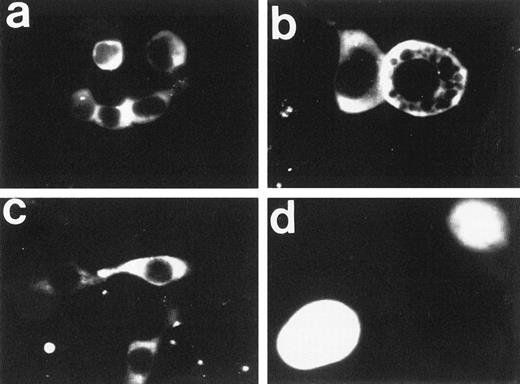
Next, the subcellular localization of FAA-GFP was examined by fluorescence microscopy in human kidney 293 cells, which were selected for their favorable transfection properties and use in previous studies of the FA phenotype.21 To facilitate efficient expression from EBV-based expression vectors in 293 cells, a variant cell line was used that constitutively expresses the EBV-derived protein, EBNA1 (see Materials and Methods). Both transiently and stably transfected 293-EBNA cells were evaluated for GFP-based fluorescence. Interestingly, FAA-GFP appeared to be primarily localized to the cytoplasmic compartment (Fig 2a through c). In the large majority of cells FAA-GFP appeared to be evenly distributed throughout the cytoplasm. However, in some cases, a more granular or vesicular distribution pattern was observed (not shown), a phenomenon that is currently being investigated. In addition, treatment with various concentrations of MMC failed to affect the subcellular distribution pattern (not shown). Transfection with the parental vector expressing GFP alone resulted in a diffuse fluorescence throughout the cytosol and nuclear compartments (Fig 2d), which is in agreement with earlier reports on the intracellular distribution of GFP.22 This latter finding was as expected, because relatively small proteins with a molecular weight below 40 to 45 kD are known to diffuse freely between cytoplasm and nucleus, whereas larger proteins require an active transport mechanism to be translocated into the nucleus.23 24
Fluorescence microscopy of 293-EBNA cells stably expressing FAA-GFP or GFP. (a through c) The images obtained with FAA-GFP. The distribution of GFP is depicted in (d). (b and d) A twofold magnification of the originally recorded image.
Fluorescence microscopy of 293-EBNA cells stably expressing FAA-GFP or GFP. (a through c) The images obtained with FAA-GFP. The distribution of GFP is depicted in (d). (b and d) A twofold magnification of the originally recorded image.
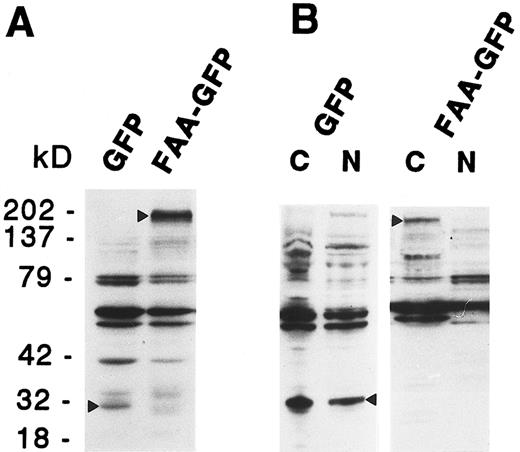
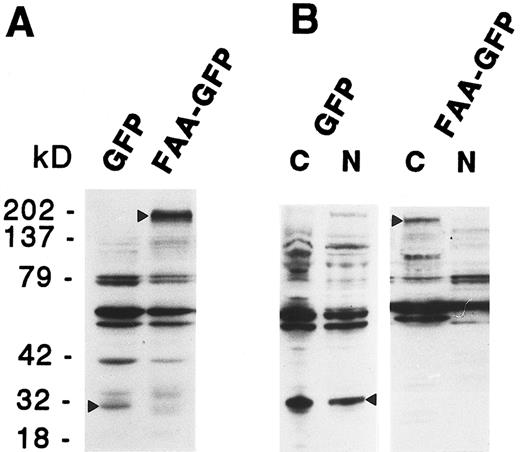
To examine the possibility that shorter protein variants derived from the hybrid coding region caused the observed cytoplasmic staining, due to the lack of potential nuclear targeting domains, expression of FAA-GFP was monitored by immunoblotting analysis using a mouse monoclonal antibody directed against GFP. As shown in Fig 3A, in total cell extracts derived from 293-EBNA cells transfected with pDRFAA-GFP predominantly a GFP-specific band of approximately 190 kD was detected, representing the expected molecular weight of the complete FAA-GFP fusion protein. In pDR-GFP transfected cells a band of approximately 30 kD was detected, in agreement with the size of GFP (Fig 3A). The subcellular distribution of FAA was also assessed by cell fractionation studies in which nuclear and cytoplasmic cell fractions of 293-EBNA cells expressing FAA-GFP were analyzed by immunoblotting. As shown in Fig 3B, FAA-GFP was detected in the cytoplasmic fraction only, whereas GFP alone was found in both subcellular fractions.
Immunoblotting analysis on total cell extracts (A) or subcellular fractions (B) derived from 293-EBNA cells stably transfected with FAA-GFP or GFP. The GFP specific bands are indicated by arrowheads; cytoplasmic and nuclear cell fractions are indicated with C and N, respectively.
Immunoblotting analysis on total cell extracts (A) or subcellular fractions (B) derived from 293-EBNA cells stably transfected with FAA-GFP or GFP. The GFP specific bands are indicated by arrowheads; cytoplasmic and nuclear cell fractions are indicated with C and N, respectively.
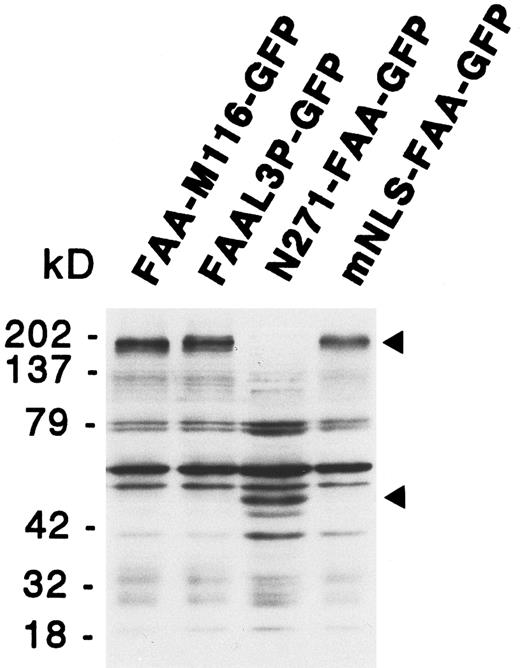
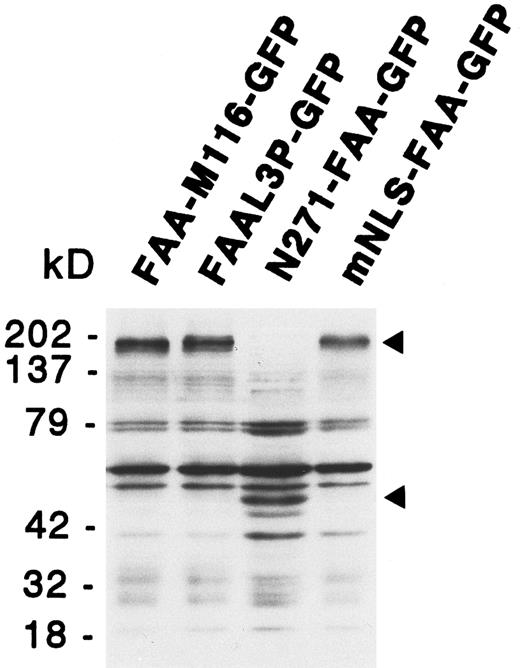
Functional analysis of the putative NLS and leucine repeat. The unexpected finding of FAA-GFP being localized to the cytoplasm suggested no function for the putative bipartite NLS and, in addition, made a role for the potential leucine repeat questionable. To examine this notion in more detail several FAA mutants were generated in which these putative domains were either deleted or modified by site-directed mutagenesis. These mutants, which are described below, were generated both with and without the GFP tag and tested for their ability to complement the MMC hypersensitivity of HSC72 cells. In addition, the subcellular localization of the GFP tag-containing mutants was examined in 293-EBNA cells. Predicted protein sizes of the various GFP chimeras were confirmed by immunoblotting, as shown for several FAA mutants in Fig 4. The results obtained with these mutants are summarized in Table 1.
Immunoblotting analysis of 293-EBNA cells transfected with deletion or site-directed mutants of FAA-GFP. The positions of the GFP fusion protein specific bands are indicated by arrowheads. See text for a description of the various mutants.
Immunoblotting analysis of 293-EBNA cells transfected with deletion or site-directed mutants of FAA-GFP. The positions of the GFP fusion protein specific bands are indicated by arrowheads. See text for a description of the various mutants.
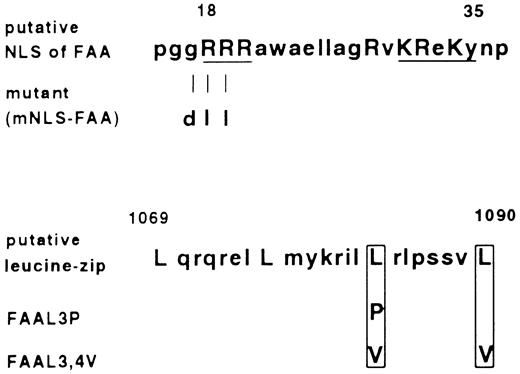
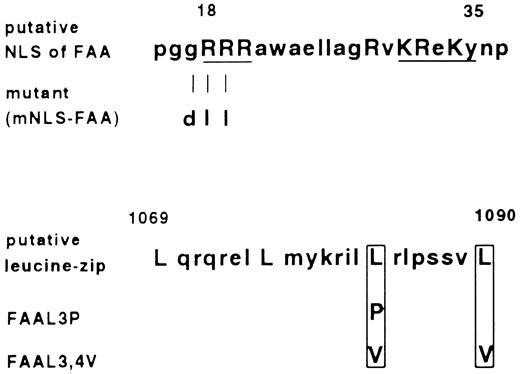
First, the relevance of the putative bipartite NLS domain for FAA function was explored. Active transport of proteins through nuclear pores has been shown to depend on the presence of short stretches of basic, positively charged residues in the target protein.23-25 Aside from the well-characterized SV40 Large T nuclear targeting sequence consisting of seven amino acids, a more complex NLS such as that present in nucleoplasmin can consist of two clusters of basic amino acids which are necessary for nuclear import.26 From the latter one, as well as other bipartite nuclear targeting signals, a consensus sequence has been derived consisting of two basic amino acids, a spacer region of any 10 amino acids and a cluster in which three out of five amino acids must be basic.27 As shown in Fig 5, in FAA the amino acids present between 18-35 are in agreement with this consensus. We deleted this putative domain by making use of the Ava I site present in the FAA ORF at base pair position 30, relative to the adenine in the first ATG referred to as base pair position 1. After the digestion with Ava I, filling in the recessive ends and religation, a frame shift was introduced leading to the occurrence of a stop codon, TAA at position 105. In this way an ORF was created which encodes a N-terminal truncated FAA protein starting at methionine 116, named FAA-M116. In vitro translation of this modified FAA ORF and subsequent electrophoresis under denaturating conditions confirmed the translation of a truncated protein of the expected size (not shown). When stably expressed in HSC72 cells, FAA-M116 with or without the GFP tag failed to complement hypersensitivity to MMC, indicating that the 115 N-terminal amino acids of FAA are essential for this function of FAA (see Table 1). Nevertheless, FAA-M116-GFP was found to have a similar subcellular distribution pattern as FAA-GFP. To further scrutinize the relevance of the putative bipartite NLS, an FAA mutant was generated in which the arginines at position 18 and 19 were substituted for neutral leucines in combination with the substitution of glycine 17 for the acidic residue aspartic acid (see Fig 5). Disruption of this first basic cluster in the bipartite NLS has been previously shown to impair nuclear translocation.25,28 When transfected into HSC72 cells, this FAA variant with a mutated putative NLS, designated mNLS-FAA, was functional in restoring MMC resistance (Table 1). Furthermore, fluorescence microscopy of this mutant fused to GFP showed a similar cytoplasmic distribution as found for FAA-GFP (not shown). Finally, the potential of the bipartite NLS to translocate a heterologous protein to the nucleus was tested, a feature that is characteristic for domains that function as NLS.23 Therefore, the first 271 amino acids of FAA were fused to GFP, yielding a hybrid protein with a predicted mass of approximately 60 kD (N271-FAA-GFP), and the subcellular distribution of the chimera was examined. No nuclear GFP-based fluorescence could be detected in 293-EBNA cells transfected with this construct, thus confirming that the sequence in FAA resembling a bipartite NLS has no nuclear targeting activity.
Amino acid sequence of the putative bipartite nuclear localization signal (NLS) and leucine repeat found in FAA, and their mutated derivatives. Amino acids that are in agreement with the consensus sequences are indicated with capital letters. In case of the bipartite NLS, basic residues that fit the consensus best are underlined. Depicted are the amino acid substitutions introduced by site-specific mutagenesis.
Amino acid sequence of the putative bipartite nuclear localization signal (NLS) and leucine repeat found in FAA, and their mutated derivatives. Amino acids that are in agreement with the consensus sequences are indicated with capital letters. In case of the bipartite NLS, basic residues that fit the consensus best are underlined. Depicted are the amino acid substitutions introduced by site-specific mutagenesis.
Second, the relevance of the putative leucine repeat motif was investigated. As depicted in Fig 5, this motif encompasses amino acids 1069-1090 and exists of four leucine residues spaced by six amino acids, which matches the well-characterized leucine repeat consensus present in many transcription factors and known to function as a dimerization interface.29,30 However, in FAA the presence of a proline at position 1086, between the third and fourth leucine in the repeat, casts doubt on its potential function because this residue is known to distort the putative α helical structure in the leucine repeat.31,32 To obtain experimental proof for a putative function of this repeat in FAA, the motif was mutated by substituting the third leucine for a proline and by replacing the third and fourth for valine residues (Fig 5). Both mutations have previously been shown to disrupt the structure and/or function of the repeat.32 33 After transfection of these mutants into HSC72 cells, named FAA-L3P and FAA-L3,4V, their ability to complement MMC sensitivity was determined. As summarized in Table 1, FAA-L3,4V had wild-type FAA activity while FAA-L3P was not able to complement HSC72 cells. The results obtained with FAA-L3,4V show that the motif present in FAA is apparently not functioning as a leucine repeat. However, the finding that substitution of leucine 1083 for proline impairs the cross-linker complementation function indicates that this domain somehow contributes to the proper functioning of FAA.
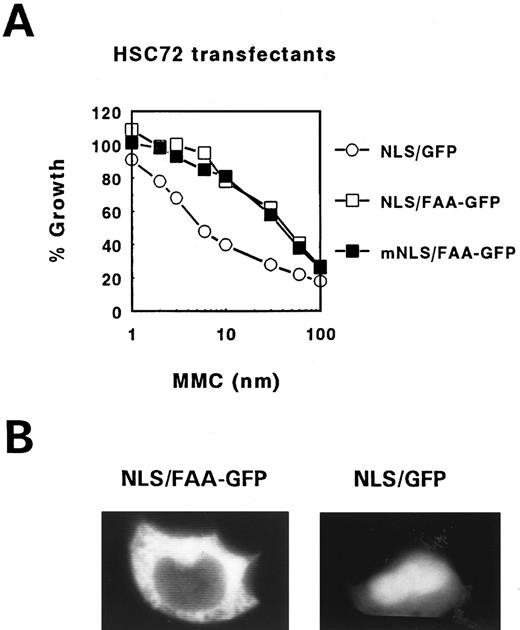
FAA tagging with SV40 Large T NLS fails to induce translocation into the nucleus. To address the question whether the cytoplasmic localization of FAA is essential for its cross-linker complementation function a similar approach was used as previously employed to show that FAC exerts this function in the cytoplasmic compartment only.11 The NLS motif derived from the SV40 Large T antigen (NLS, MP-LLLRLV-EL) was fused to the N-terminus of FAA and the hybrid ORF either with or without GFP tag was subcloned in pDR2 . As a control the same construct was made with an inactivated Large T NLS by substituting lysine at position 128 in the originally described motif for threonine (mNLS, MP-LTLRLV-EL), known to completely disrupt its nuclear targetting function.34 Interestingly, stable transfection of these constructs in HSC72 cells and subsequent testing of their MMC resistance-conferring potential showed that the foreign NLS-containing FAA hybrids remained fully functional as shown for the GFP tagged constructs in Fig 6A. However, examination of their subcellular localization in 293 cells showed a similar cytoplasmic distribution pattern as found with FAA-GFP (Fig 2). In addition, inspection of the transfected HSC72 cells also failed to show nuclear accumulation of heterologous NLS-containing FAA-GFP (not shown). This in contrast to Large T NLS-tagged GFP alone, which showed the expected enhanced nuclear staining in 293 cells (compare Fig 2d and Fig 6B), despite its constant leaking into the cytoplasm because of its relatively small size. These results indicate that Large T NLS mediated nuclear translocation of FAA in these cells is somehow prevented by yet unknown means. For this reason these experiments provide no further insight on whether the cross-linker complementation function of FAA is predominantly dependent on its location in the cytoplasmic compartment.
MMC complementation and subcellular localization of FAA-GFP tagged with the SV40 Large T NLS. (A) MMC survival curve of FA-A lymphoblasts stably transfected with FAA-GFP tagged with either a functional (NLS/FAA-GFP) or inactive (mNLS/FAA-GFP) Large T NLS. As control, FA-A cells transfected with the Large T NLS fused to GFP (NLS/GFP) were used. (B) Fluorescence microscopy of 293-EBNA cells transfected with NLS/FAA-GFP or NLS/GFP.
MMC complementation and subcellular localization of FAA-GFP tagged with the SV40 Large T NLS. (A) MMC survival curve of FA-A lymphoblasts stably transfected with FAA-GFP tagged with either a functional (NLS/FAA-GFP) or inactive (mNLS/FAA-GFP) Large T NLS. As control, FA-A cells transfected with the Large T NLS fused to GFP (NLS/GFP) were used. (B) Fluorescence microscopy of 293-EBNA cells transfected with NLS/FAA-GFP or NLS/GFP.
DISCUSSION
In this study using GFP-tagged FAA protein we have shown FAA to be primary localized to the cytoplasm of human 293 cells. Because FAA-GFP was fully functional in complementing MMC-hypersensitivity in FA-A lymphoblasts, it is unlikely that the GFP tag has caused mislocalization of the hybrid protein. The possibility that the level of FAA-GFP expression may have affected the subcellular localization was not substantiated by the finding of cytoplasmic staining in both intensly and weakly fluorescent 293-EBNA cells (see also Fig 2). In addition, HSC72 cells that have less intense GFP-based fluorescence as compared with 293 cells due to a relatively weak Rous Sarcoma Virus (RSV) promoter activity in lymphoblastoid cells (F.A.E.K., unpublished results, 1996) also showed FAA-GFP to reside in the cytoplasmic compartment. Furthermore, by using a functional FAC-GFP chimera we have confirmed the cytoplasmic localisation of FAC in living cells (Q.W., F.A.E.K., unpublished results, 1996), which was previously reported in immunofluoresence microscopy studies with rabbit polyclonal antisera against FAC.9 10 This result further indicates that the GFP tag seems not to affect both the function and subcellular routing of the known FA proteins.
It should be stressed that the subcellular localization experiments described were performed in the absence of exogenously added cross-linking agents. However, experiments in which various concentrations of MMC were applied for different periods of time to FAA-GFP expressing cells failed to alter the subcellular distribution pattern (data not shown). Although more detailed studies are in progress to examine possible changes in FAA distribution upon treatment with various DNA damaging agents and agents that primarily affect cell-cycle progression, to date we have no evidence for possible nuclear translocation of FAA upon exposure to cross-linkers in 293 cells.
The immunoblotting experiments performed in this study indicate that apparently FAA is not extensively modified or processed via posttranslational mechanisms. The predicted molecular weight of the FAA-GFP hybrid in 293 cells matches well the estimated size of approximately 190 kD found on immunoblots (Figs 3 and 4). More evidence for this notion comes from immunoprecipitation experiments on metabolically labeled COS cells, using a polyclonal antibody raised against the N-terminal part of bacterially expressed FAA, showing predominantly one specific band representing nontagged FAA with an estimated size of 165 kD (H.Y., manuscript in preparation).
The mutational analysis of FAA performed in this study showed no function for the putative bipartite NLS and leucine repeat motif, although the portions of FAA that comprise these sequences appeared to contribute to its proper function. The precise role of these domains remains elusive until more is known about the molecular function of FAA. However, the finding that substitition of the third leucine in the zipper motif for proline inactivated FAA, whereas substitution of the last two leucines for valine residues had no effect, may indicate that α helical structures in this part of FAA are indispensible for its function. In fact, prediction of the secondary structure confirmed the presence of an α helical structure between residues 1064 to 1083 that was unaffected in the L3,4V mutant (not shown). In contrast, substituting leucine-1083 for proline reduced the length of the predicted α helix to end at amino acid position 1079 and enforced the formation of a β sheet, thus pointing to the importance of protein secondary structure in this region.
For FAC it has been shown that forced translocation into the nucleus by fusion to the Large T SV40 NLS abolishes its complementing activity, thus indicating that cytoplasmic localization is essential for the activity of FAC.11 In this study a similar approach failed to provide insight on the subcellular positional requirement for FAA's complementation function since FAA-GFP could not be forced into the nucleus by the Large T NLS in the cell lines used (Fig 6). The mechanism underlying the inability of FAA to be targeted to the nucleus of these cells remains elusive. Several explanations could account for this phenomenon such as a possible masking of the NLS due to the structural conformation of FAA, binding of other factors to FAA that might prevent entrance into the nucleus, the presence of yet unknown more powerfull other subcellular routing signals in FAA (eg, nuclear export sequences), or a combination of these possibilities.
The cytoplasmic localization of FAA indicates that both FA proteins identified so far are localized in the same subcellular compartment. Although direct evidence is currently lacking, a potential physical interaction between FAC and FAA seems to be unlikely because FAC has been shown to interact in vitro predominantly with three cytosolic proteins with molecular masses of 65, 50, and 35 kD,35 neither of which equals the molecular weight of the relatively large FAA protein. Furthermore, the cytoplasmic localization of the two FA proteins adds up to the notion that FA genes may not be directly involved in the repair of cross-linked DNA and suggests the existence of a novel pathway that confers resistance to cross-linking agents. The recently found predisposition to apoptosis in FA-C cells induced by cross-linker treatment,4,36 the FAC-mediated suppression of apoptosis induced by interleukin-3 withdrawal in hematopoietic factor–dependent cell lines,37 as well as the observed hypersensitivity to γ-interferon in murine progenitor cells obtained from ‘fac knock-out’ mice,38 indicate that the function of FA proteins may relate to apoptosis regulatory mechanisms involving cytokine-dependent signal transduction pathways. In addition, and based on recent findings,39 these signaling pathways may be linked to mechanisms that regulate the activity of the cyclinB/cdc2 kinase complex that controls G2/M cell cycle progression, thereby providing an explanation for the spontaneous and low-dose cross-linker-induced G2 delay/arrest observed in FA cells. In these contexts, the cytoplasmic localization of FA proteins identified so far appears to be consistent with their anti-apoptotic activities. More systematic studies designed to elucidate the molecular function of FA proteins are now required to obtain more insight on the process(es) affected by mutated FA genes.
ACKNOWLEDGMENT
We thank Dr Stephen J. Gould for kindly providing the construct pPTS1- and PST2-GFP, Mark Strunk for performing in vitro translation assays, and Jeroen Beliën for helping with the image processing.
Supported by the Dutch Cancer Society. H.Y. is supported by grants from the National Institutes of Health HL52138 and DK49216.
F.A.E.K. and Q.W. equally contributed to this work.
Address reprint requests Frank A.E. Kruyt, PhD, Department of Human Genetics, Free University, Van der Boechorststraat 7, NL-1081 BT Amsterdam, The Netherlands.