The description of skin dendritic cells (DC) by Langerhans in 1868 was followed by prolonged speculation as to their function. Steinman and Cohn identified mouse spleen DC in 19731 and initiated a series of experiments that established lymphoid tissue–derived DC as potent stimulators of primary immune responses.2-5 The observation that similar cells were present in the nonlymphoid tissues of both rodents6-8 and humans,9,10 combined with early evidence that they played an important role in heart and kidney transplant rejection,11-14 generated further interest in DC. However, a paucity of markers for DC, the difficulty distinguishing DC from monocytes/macrophages (Mo/MØ), and the problems involved in purifying DC made for slow progress. Nonetheless, several laboratories persisted with their investigations, leading to the current acceptance that DC represent discrete leukocyte population(s) of specialist or “professional” antigen presenting cells (APC), with an extraordinary capacity for initiating primary (and secondary) T-lymphocyte responses.15 16
It is now possible to grow DC-like cells in culture17-21 and the first monoclonal antibodies (MoAbs)22,23 that react with human DC are available. DC migration24 is probably directed by a range of mediators and recent data suggest that reciprocal T-lymphocyte feedback provides antigen (Ag)-specific control of DC Ag presentation.16,25 26 This new understanding of DC function may provide opportunities for therapeutic intervention in bone marrow transplantation (BMT), solid organ transplantation, and autoimmune disease. Protocols for clinical immunotherapy programs, targeted on malignant cell Ag or infectious agents, are being designed to exploit DC as “nature's adjuvant” for optimal therapeutic vaccination.
A practical issue facing hematologists is the lack of a clear definition of a DC. This, combined with uncertainty as to the ontogeny of DC, limited data as to the interrelationship of different DC populations, and the fact that activation/differentiation events may dramatically change the morphology, molecular expression (phenotype), and function of DC, has created difficulties. This review will concentrate mainly on the myeloid lineage–derived DC, which associate with T lymphocytes within lymphoid tissue, to provide critical APC activity for initiating specific T-lymphocyte activation and proliferation.15,27,28 Follicular dendritic cells (FDC), which are present within the B-lymphoid follicle, do not originate from the BM, have a different phenotype, and retain Ag for prolonged periods, thereby restimulating B lymphocytes and perhaps T lymphocytes, will not be reviewed. Nor will dendritic epithelial cells, a specialized subset of T lymphocytes with extensive membrane processes, which are found in epithelial surfaces in mice but are rare in humans. Myeloid DC should be further distinguished from thymic DC, which appear to derive from a lymphoid stem cell.29 Thymic DC (and perhaps other lymphoid lineage–derived DC) have an entirely different role30,31; they delete maturing T lymphocytes and the prediction that they may have different properties from the myeloid-derived DC, although controversial, has received experimental support.32
The cardinal properties of the myeloid lineage–derived DC (hereafter DC) include (1) the ability to take up, process, and present Ag; (2) the ability to migrate selectively through tissues; and (3) the ability to interact with, stimulate, and direct T-lymphocyte responses. DC may be the only cell capable of stimulating a naive T lymphocyte but other “nonprofessional” APC (and DC) can stimulate experienced (activated or memory) T lymphocytes. As such, DC have unique cell interaction capabilities, some of which relate to their extensive cell membrane processes that are acquired late during DC differentiation/activation (Fig 1). DC have been defined, as of necessity, by these specialized functional characteristics15,27 33 to distinguish them from Mo and MØ.
The morphology of human (see text for description) DC. (A) Fresh “lineage negative” blood DC, isolated without a period of tissue culture, using immunoselection.57 (May-Grünwald-Giemsa [MGG], original magnification [OM] × 1,433). (B) CMRF-44 sorted, Nycodenz gradient purified cultured blood DC77 MGG, OM × 1,433). (C) Tonsil low-density cultured DC stained with anti–HLA-DR. The veils and dendritic processes are more obvious in these preparations (OM × 1,433). (D) Mo-derived DC preparation stained with CMRF-44 using an immunoenzyme (brown, Peroxidase-DAB) technique (in preparation) (OM × 1,433). (E and F ) Fresh “lineage negative” blood DC clustered with CD4+ purified autologous T lymphocytes in the presence of staphylococcal entertoxin A (SEA) and MGG stained. The DC is stained in another cluster (F ) for the costimulator molecule CD86 using an immunoenzyme (Alkaline Phosphatase-Fast Blue) technique (OM × 1,197). (G) EM appearances of a CMRF-44–positive cultured blood DC. Note the mitochondria endosomes and lysosomal vacuoles (OM × 17,000). (H) DC in the interstitial tissues of rat heart identified by anti-MHC class II staining (immunofluorescence) (OM × 479). (I) Dermal CMRF-44+ DC (red, Peroxidase-AEC) and T lymphocytes (blue, Alkaline Phosphatase-Fast Blue) within a section of normal skin adjacent to a hair follicle (OM × 479). (J) Lymph node interfollicular (T lymphocyte) area containing CMRF-44+ IDC (brown, Peroxidase-DAB) compared with CD14 Mo and CD20 B lymphocytes (blue, Alkaline phosphatase-Fast Blue) (OM × 143). (K) Lymph node interfollicular region with CMRF-44+ IDC (blue, Alkaline phosphatase-Fast Blue) showing nuclear labeling for the transcription factor Rel B (brown, Peroxidase-DAB) (OM × 1,197). Bar = 10 μm.
The morphology of human (see text for description) DC. (A) Fresh “lineage negative” blood DC, isolated without a period of tissue culture, using immunoselection.57 (May-Grünwald-Giemsa [MGG], original magnification [OM] × 1,433). (B) CMRF-44 sorted, Nycodenz gradient purified cultured blood DC77 MGG, OM × 1,433). (C) Tonsil low-density cultured DC stained with anti–HLA-DR. The veils and dendritic processes are more obvious in these preparations (OM × 1,433). (D) Mo-derived DC preparation stained with CMRF-44 using an immunoenzyme (brown, Peroxidase-DAB) technique (in preparation) (OM × 1,433). (E and F ) Fresh “lineage negative” blood DC clustered with CD4+ purified autologous T lymphocytes in the presence of staphylococcal entertoxin A (SEA) and MGG stained. The DC is stained in another cluster (F ) for the costimulator molecule CD86 using an immunoenzyme (Alkaline Phosphatase-Fast Blue) technique (OM × 1,197). (G) EM appearances of a CMRF-44–positive cultured blood DC. Note the mitochondria endosomes and lysosomal vacuoles (OM × 17,000). (H) DC in the interstitial tissues of rat heart identified by anti-MHC class II staining (immunofluorescence) (OM × 479). (I) Dermal CMRF-44+ DC (red, Peroxidase-AEC) and T lymphocytes (blue, Alkaline Phosphatase-Fast Blue) within a section of normal skin adjacent to a hair follicle (OM × 479). (J) Lymph node interfollicular (T lymphocyte) area containing CMRF-44+ IDC (brown, Peroxidase-DAB) compared with CD14 Mo and CD20 B lymphocytes (blue, Alkaline phosphatase-Fast Blue) (OM × 143). (K) Lymph node interfollicular region with CMRF-44+ IDC (blue, Alkaline phosphatase-Fast Blue) showing nuclear labeling for the transcription factor Rel B (brown, Peroxidase-DAB) (OM × 1,197). Bar = 10 μm.
TOWARD A DEFINITION OF DC
The Existence of a Lineage?
The original description of mouse spleen DC drew attention to their irregular shape with numerous cell membrane processes, including spiny dendrites, bulbous pseudopods, and lamellipodiae or veils.1 Distinctive electron microscopic features with a paucity of intracellular organelles and prominent mitochondria were also described. The presence of endosomes and lysosomes essential for Ag processing have been emphasized more recently.34,35 Mouse spleen DC adhere to plastic initially but become nonadherent after overnight tissue culture1 and this critical property, plus their low density, allowed them to be separated from MØ. Spleen DC were further distinguished from MØ on the basis of cytochemical reactions, a lack of phagocytic activity, and an apparent lack of Fc and complement (C) receptors, features which may be present in less mature DC. Similar cells were identified in lymph node (LN) preparations.2 Subsequent studies (reviewed by Steinman15 ) showed that DC express major histocompatibility complex (MHC) molecules, particularly class II loci products, in high density (mouse MØ have low-density MHC class II) and are exceptionally effective stimulators of primary T-lymphocyte responses, both in allogeneic mixed leukocyte reactions (allo-MLR)3 and Ag-specific systems.36
Leukocytes with a high density of MHC class II molecules and irregular cell membrane processes were noted in the interstitium of most rat tissues, except the immuno-privileged sites of brain and testes.6-8,37 These interstitial DC were shown to be BM-derived, relatively poorly phagocytic, and to have similar characteristics to the lymphoid tissue–derived DC.7 Interstitial DC stimulate strong allograft responses13,14 and isolated interstitial DC stimulate T lymphocytes.24 Human interstitial DC were described in kidney initially9 and subsequently in other tissues.10
Initial emphasis on their Fc and C receptors as MØ-like features delayed appreciation that Langerhans cells (LC) represent a stage of DC differentiation.33 Electron microscopy distinguished the Birbeck granule as a distinctive membrane inclusion within LC33 but this was difficult to follow technically and is rarely present in other DC types. When techniques were evolved to isolate LC from both mouse38 and human39 skin, cultured LC were shown to develop the morphological and phenotypic features of lymphoid tissue–derived DC. Functional studies confirmed that LC could stimulate a strong primary allo-MLR, but in some studies only after a significant period of culture.40-42 Similar allostimulatory properties were attributed to veiled cells and soon it was appreciated that these migrating DC gave rise to the interdigitating dendritic cell (IDC) within draining nodes.
Thus, the concept grew that DC have a growth and differentiation pathway (see A DC Hematopoietic Lineage and Migration Pathway), which interrelates with their migration24 and functional activation.15,16 BM precursors2,7 give rise to maturing DC, which can be distinguished from other myeloid cell types within human BM.44 Small numbers of DC precursors (and mature or recirculating forms) circulate in the blood and exit from the bloodstream into the tissues. These interstitial DC (LC form a subset in the skin33 and other epithelia) have extended cell processes and act as sentinel cells. After exposure to Ag or inflammatory stimuli, interstitial DC migrate out of the tissues into afferent lymph, where they are identified as veiled cells.45 Upon entering draining LN the DC are found as IDC in the T-lymphoid areas of LN. IDC are also found in tonsil and the white pulp of spleen.15,16,46 The spleen contains a second population of (red pulp, ie, blood-derived) marginal-zone DC.47
Functional Activity of DC
Although DC lack substantial phagocytic activity, this relative deficiency has been over-emphasized.16 The lack of phagocytic activity is a feature of more differentiated or mature DC and less differentiated or immature DC have selective phagocytic activity48-50 (see Antigenic Material). In vitro studies show DC to have marked cell motility and the ability to extend/retract their cell-membrane processes.51
The allo-MLR has been used widely to test DC Ag presenting activity.3,52 Although certain features (notably the high-responder T-lymphocyte precursor frequency) are not typical of a true naive primary immune response,53,54 the ability of DC to stimulate a potent allo-MLR distinguished them from other leukocytes. Activated B lymphocytes also have allostimulatory activity in vitro55,56 but are poor allostimulators compared with DC.57 58
DC stimulate an autologous MLR,59-61 which may equate to a primary response to exogenous Ags (see Autologous Ag).
The extraordinary ability of DC to take up and present Ag for primary specific T-lymphocyte responses, in vitro62 and in vivo,63-65 is their main distinguishing feature.
The unique properties of DC become most evident when pure populations of DC, Mo, and B lymphocytes are compared.15,27 B lymphocytes probably contribute little to primary immune responses in vivo.66 67 DC are also active APC for secondary responses as are MØ, endothelial cells, and activated B lymphocytes.
Isolation of DC
A comprehensive review of methodology27 is available. DC purifications generally use one of two main approaches.
The first exploits the fact that DC lack certain lineage-specific markers expressed on other defined leukocyte populations (Fig 1A), eg, T-lymphocyte (CD2 [at least in high density], CD3), B lymphocyte (CD19, CD20, CD24), natural killer (NK) cell (CD16, CD56, CD57), Mo (high-density CD14), and granulocyte (CD15) markers. Various MoAb cocktails have been used with a variety of immunoselection systems to isolate “lineage negative” populations of DC from cell suspensions of hematopoietic tissue or after enzymatic digestion of other tissues such as skin.
The second approach has been to capitalize on the low density of DC, which results after a period of in vitro culture.68 Culture of peripheral blood mononuclear cells (PBMC) (often sheep red blood cell [SRBC] rosette negative), particularly in fetal calf serum at 37°C, activates DC and changes their size as well as their density, aiding purification over density gradients.17,69,70 Various gradient media have been used and the original bovine serum albumin (BSA) gradient71 has been replaced by metrizamide,51,72,73 Nycodenz (Nycomed Pharma, Oslo, Norway),68 or Percoll (Amrad, Pharmacia Biotech, Aukland, New Zealand).17,69,70 The Nycodenz gradient has proved very effective (Fig 1B).68 The gradient media all perform a similar function but these compounds and their osmotic effects may induce phenotypic or functional changes.25,74 For example, Kabel et al75 have suggested that metrizamide may alter Mo cells so that they resemble DC.
The major selective procedure in mouse DC preparation, transient adherence of DC to plastic,1,15 is ineffective in humans.16 Human DC, Mo, and other cell types appear to segregate variably after adherence to both plastic and teflon surfaces. Adherence enriches human Mo but this does not lead to reliable increases in stimulatory cells.16 In addition, adherence to different surfaces may influence the phenotype of Mo76 and perhaps DC.
Elutriation has been used27,75 but its application is limited by the availability of equipment. Methods that used other physical characteristics which may discriminate DC from other cells, eg, phagocytosis and clustering,27 have fallen into abeyance.
Sophisticated flow cytometric and immunomagnetic bead selection have now become the norm and a new era of positive selection with the CD83 and CMRF-44 reagents has made high-purity preparations of low-density DC feasible.77,78 An effective method to prepare DC for immunostimulation involves gradient separation over a standard density gradient to obtain PBMC, T-lymphocyte depletion (optional), overnight incubation for 16 hours in autologous serum/medium (±cytokines), and Nycodenz gradient separation. A significant lineage-negative, CMRF-44+ cell population (Figs 1B and 2) can then be isolated by positive selection.77 A similar approach using CD83 to select DC from a metrizamide gradient is also effective.78
The costimulatory capacity of blood derived CMRF-44 purified DC compared with CD14+ Mo and CD19+ B lymphocytes. (a) The flow cytometry labeling of Nycodenz low-density cells is shown before sorting CMRF-44+ CD14− DC and CMRF-44− CD14+ Mo. (b) Allogeneic T-lymphocyte proliferative responses measured with 3H thymidine, are shown using each FACS purified cell populations as stimulators.77 The allostimulatory capacity of sorted DC, Mo, and B lymphocytes (CD19+ sorted) are compared at a ratio of 1 APC to 50 allogeneic T lymphocytes. (c) DC and Mo are compared in their ability to process and present the soluble protein antigens to KLH and tetanus toxoid to autologous T lymphocytes at an APC:T lymphocyte ratio of 1:20.
The costimulatory capacity of blood derived CMRF-44 purified DC compared with CD14+ Mo and CD19+ B lymphocytes. (a) The flow cytometry labeling of Nycodenz low-density cells is shown before sorting CMRF-44+ CD14− DC and CMRF-44− CD14+ Mo. (b) Allogeneic T-lymphocyte proliferative responses measured with 3H thymidine, are shown using each FACS purified cell populations as stimulators.77 The allostimulatory capacity of sorted DC, Mo, and B lymphocytes (CD19+ sorted) are compared at a ratio of 1 APC to 50 allogeneic T lymphocytes. (c) DC and Mo are compared in their ability to process and present the soluble protein antigens to KLH and tetanus toxoid to autologous T lymphocytes at an APC:T lymphocyte ratio of 1:20.
Cytochemical Characteristics
DC lack many of the enzymes associated with classic Mo/MØ. Cytochemical reactions show that DC lack myeloperoxidase1,7,44,57,79 and have low levels of 5′ nucleotidase, dipeptidyl peptidase (DPP1), and cathepsin B activity.33,73,80 Other intracellular enzymes (eg, nonspecific esterase [NSE], acid phosphatase) or lysosomal Ags (eg, CD68)46 may be present in DC but their intensity is less or their intracellular distribution differs from Mo and MØ. NSE staining decreases with DC activation.81
Problems Defining the DC Lineage
There are notable species differences. In mice and rats, BM and blood DC precursors express few MHC Ags and are poor stimulators in the allo-MLR.20,82,83 However, in humans, BM is allostimulatory44,84 and at least one or more fresh DC populations in blood are potent allostimulatory cells.52,57 85 Other surface molecules on DC have different patterns of expression between the species, eg, CD11c86 and CD187 (see Cell Surface and Other DC-Associated Molecules).
Inbred mouse and rat strains7,14 have different DC counts and their number may decrease with age.88 89 Informal reports (routine blood DC counts are just available) suggest considerable individual variations in DC numbers circulating in humans.
The different methods of purifying DC have considerable and variable effects on DC activation, which may modify DC phenotype and function. It remains a problem as to how to select for “resting” DC in human blood and thereby remove the contaminating small lymphoid population, stem cells, basophils, endothelial precursors, etc. Other cell populations, notably adherent cells, may be contaminated by numbers of highly activated DC.16 Thus, the original adherent preparations of Mo and MØ for APC studies were performed, not surprisingly, without regard for the existence of DC. Only recently, when CD14+ Mo were selected to exclude DC, did it become clear that Mo were poor APC compared with DC.57 80
A Working Definition of DC
The putative DC lineage encompasses DC populations with very different properties, characteristic of their differentiation/activation state. Although DC were named because of their distinctive morphology,1 7 this feature is insufficient to define a DC. A definition of a DC must emphasize its ability to migrate and to stimulate a primary T-lymphocyte response. A consensus working definition might be:
The ability to stimulate a primary T-lymphocyte response (this may require differentiation/activation of the DC population).
Marked cell motility and the ability to extend and retract cell membrane processes freely at 37°C in vitro. The ability to migrate through tissues and track to the T-lymphocyte–dependent areas of lymph node.
Relatively specialized phagocytic activity (in vitro) — DC uptake of extracellular material is probably greater than hitherto realized in vivo (see Antigen Uptake, Processing, and Presentation by DC). Active fluid-phase endocytosis is a feature of DC.
Spontaneous initial and rapid clustering with T lymphocytes at 37°C in vitro.
A cell-surface Ag phenotype distinguishing it from other leukocytes, notably Mo/MØ and B lymphocytes (see Cell Surface and Other DC-Associated Molecules).
Expression of certain DC-associated Ags according to their differentiation/activation state (see Cell Surface and Other DC-Associated Molecules).
Cytochemical reactions, which differ from Mo/MØ (see earlier).
Two additional features, “dendritic” morphology and high-density membrane MHC class II Ag, are confirmatory only (particularly in humans), as other cells, notably B lymphocytes46,90 and fibroblasts, can adopt a very similar appearance. DC morphology is also influenced by temperature.21 Note that FDC, dendritic epithelial cells, and lymphoid DC (for the moment) are excluded by these criteria.
CELL SURFACE AND OTHER DC-ASSOCIATED MOLECULES
DC express a repertoire of molecules common to other leukocytes, eg, MHC molecules, CD45 (leukocyte common) isoforms, adhesion molecules etc. These have dominated attempts to produce DC lineage specific MoAbs but persistence has resulted in some useful new reagents (Table 1).
Some Discriminating Leukocyte Differentiation Antigens Expressed by Human DC
| Antigen/Molecule . | Blood DC (uncultured) . | DC activated/Differentiated* . | Blood Mo . | MØ . | Activated B Lymphocyte . |
|---|---|---|---|---|---|
| DC lineage | |||||
| CMRF-44 | (+) | +++ | ± | (+) | ++ |
| CMRF-56 | − | ++ | − | (+) | (+) |
| CD83 | − | + | − | − | (+) |
| DEC-205‡ | NAρ | ++ | NA | ± | ± |
| S100 | NA | ++ | ± | NA | − |
| Mo/MØ lineage | |||||
| CD14 | − | − | ++ | + | ± |
| CD68 | + | + | +++ | ++ | − |
| CD115 (M-CSFR) | − | − | ++ | + | − |
| Myeloid/lymphoid | ++ | ||||
| CD1 | − | (++)1-155 | − | (+) | − |
| CD4 | + | ± | + | + | − |
| CD5 | + | ++ | + | + | (+) |
| CD13 | + | + | + | + | − |
| CD33 | + | + | + | ± | − |
| Fc receptors | |||||
| CD64 | ± | − | ++ | ++ | − |
| CD32 | + | ± | ++ | ++ | + |
| CD16 | − | − | (+) | + | − |
| Adhesion molecules | |||||
| CD11a (LFA-1) | ++ | ++ | ++ | + | ++ |
| CD11b | − | (+) | ++ | + | − |
| CD11c | ± | ++ | + | + | (+) |
| ICAM-1 (CD54) | + | +++ | + | + | +++ |
| ICAM-2 (CD50) | + | + | |||
| ICAM-3 (CD102) | +++ | +++ | + | + | − |
| LFA-3 (CD58) | ++ | ++ | ++ | ++ | ++ |
| Costimulator molecules | |||||
| CD40 | ± | ++ | + | + | ++ |
| CD80 | − | ++ | − | + | ++ |
| CD86 | − | ++ | + | + | ++ |
| Leukocyte common | |||||
| CD45RA | + | ± | + | − | − |
| CD45RO | − | ++ | − | + | ++ |
| MHC | |||||
| HLA-ABC | ++ | +++ | + | ++ | ++ |
| HLA-DP | + | +++ | + | ++ | ++ |
| HLA-DQ | ++ | +++ | + | ++ | ++ |
| HLA-DR | ++ | +++ | + | ++ | ++ |
| Antigen/Molecule . | Blood DC (uncultured) . | DC activated/Differentiated* . | Blood Mo . | MØ . | Activated B Lymphocyte . |
|---|---|---|---|---|---|
| DC lineage | |||||
| CMRF-44 | (+) | +++ | ± | (+) | ++ |
| CMRF-56 | − | ++ | − | (+) | (+) |
| CD83 | − | + | − | − | (+) |
| DEC-205‡ | NAρ | ++ | NA | ± | ± |
| S100 | NA | ++ | ± | NA | − |
| Mo/MØ lineage | |||||
| CD14 | − | − | ++ | + | ± |
| CD68 | + | + | +++ | ++ | − |
| CD115 (M-CSFR) | − | − | ++ | + | − |
| Myeloid/lymphoid | ++ | ||||
| CD1 | − | (++)1-155 | − | (+) | − |
| CD4 | + | ± | + | + | − |
| CD5 | + | ++ | + | + | (+) |
| CD13 | + | + | + | + | − |
| CD33 | + | + | + | ± | − |
| Fc receptors | |||||
| CD64 | ± | − | ++ | ++ | − |
| CD32 | + | ± | ++ | ++ | + |
| CD16 | − | − | (+) | + | − |
| Adhesion molecules | |||||
| CD11a (LFA-1) | ++ | ++ | ++ | + | ++ |
| CD11b | − | (+) | ++ | + | − |
| CD11c | ± | ++ | + | + | (+) |
| ICAM-1 (CD54) | + | +++ | + | + | +++ |
| ICAM-2 (CD50) | + | + | |||
| ICAM-3 (CD102) | +++ | +++ | + | + | − |
| LFA-3 (CD58) | ++ | ++ | ++ | ++ | ++ |
| Costimulator molecules | |||||
| CD40 | ± | ++ | + | + | ++ |
| CD80 | − | ++ | − | + | ++ |
| CD86 | − | ++ | + | + | ++ |
| Leukocyte common | |||||
| CD45RA | + | ± | + | − | − |
| CD45RO | − | ++ | − | + | ++ |
| MHC | |||||
| HLA-ABC | ++ | +++ | + | ++ | ++ |
| HLA-DP | + | +++ | + | ++ | ++ |
| HLA-DQ | ++ | +++ | + | ++ | ++ |
| HLA-DR | ++ | +++ | + | ++ | ++ |
Based on summarized data referenced in text.
Cultured overnight or longer in the presence of serum. IDC downregulate CD11c, CD13, and CD33.
Key: −, negative; ±, variable; +, positive; ++, strong positive; +++, extremely strong; ( ), subset only positive.
Mouse data only at present
ρ NA, not available.
Langerhans cells.
Relatively DC-Specific Ags
The difficulties in isolating pure DC for immunization and screening of MoAb undoubtedly limited endeavors and hence the reagents available. It seems unlikely that DC lack unique membrane features and cell-surface molecules. Indeed, when sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) protein analysis of pure human tonsil DC proteins was compared with other leukocytes,91 DC appeared to have a very different protein composition, even if this level of resolution did not identify DC-specific molecules.
Mouse DC
The 33D1 rat MoAb92 identifies a low-density Ag on mouse (marginal zone) spleen DC. The antibody does not stain DC in cryostat sections and does not react with LC. No biochemical data on the Ag are available. Nonetheless, this antibody has proved extremely useful for C lysis of mouse spleen DC.
The hamster MoAb N41886 reacts with an epitope of the β2 integrin family heterodimer p150/95 (CD11c), which is expressed in high density on mouse DC and other leukocytes including MØ.
The rat NLDC-145 MoAb was raised by immunizing with mouse lymphoid tissue stroma and screening for reactivity on DC in tissue sections.93 It stains DC in T lymphocyte areas, including spleen white pulp but not spleen marginal-zone DC or LC. The Ag is also expressed on activated MØ and thymic cortical epithelium.93 Subsequent isolation of the Ag recognized by NLDC-145 and protein sequencing enabled a full-length mouse cDNA to be isolated.94 Now renamed DEC-205 (as a result of a revised molecular weight), the molecule, which has some similarities with mannose receptors,95 is postulated to act as a receptor for Ag uptake.94
Other Animal Species
Another potential member of the integrin family that is preferentially expressed on rat DC is defined by the MoAb MRC OX62.96 The Ag is found on other cells but has considerable potential for defining DC.49
Although DC have been studied in pigs, sheep, and monkeys, no reagents specific to DC in these species have been produced. Sheep and rabbit, but not mouse, DC express CD1.
Humans
The CD1 gene family includes at least three relatively well-characterized gene products: CD1a, CD1b, and CD1c, which have similarity with MHC class I molecules and are expressed by cortical thymocytes. LC express CD1a and variable amounts of CD1c87,97 while CD1b has been reported on dermal and migrating LC.98
IDC in LN draining the skin may express CD1a, CD1b, and CD1c.99 Blood57,70 and tonsil46 DC do not express CD1a, although the former have been reported to both express CD1c78 and to lack CD1c.51 CD1 expression may be influenced by exogenous β2 -microglobulin levels in media.87 The CD1 Ags are inducible on Mo100 and probably on other DC populations in certain circumstances.18,101 102
CD11c (p150/95) reagents have been investigated in humans as potential DC markers. This integrin has been described on a wide range of human leukocytes including Mo and MØ.103 CD11c reagents probably differ in the epitopes recognized,104 and have not proved particularly useful for identifying human DC. Nonetheless, CD11c expression on a subset of activated freshly isolated blood DC has been reported,78,85,105 although it is absent from tonsil DC.46
Other reagents, including an MoAb, X-11, which detects a neo-epitope of C9106 and the “HLA-DQ–associated Ag” defined by RFD-1,107 also found on MØ, have received less attention. These Ags and the molecule present within LC identified by the Lag MoAb108 have yet to be characterized biochemically. An antibody, IRAC, which stains IDC in LN sections,109 has a wider tissue distribution by flow cytometry. Factor XIIIa, which identifies a dermal cell with dendritic morphology,110 has been suggested to be a marker for a tissue MØ population.104
Two new reagents, HB15 (CD83) and CMRF-44, which react with DC membrane Ags are contributing toward better identification of DC in humans.22 23 These MoAbs and a third reagent, CMRF-56 (submitted), react with separate differentiation/activation Ags, which appear in higher density on cultured blood DC, as follows:
The CD83 antibody HB15 has limited reactivity with activated B lymphocytes but stains cultured human blood DC,78 LC74, and some interdigitating cells in LN.23,74 The Ag is encoded by a cDNA that defines a member of the Ig gene superfamily.23 The mouse homologue maps to the MHC region.111 As yet it has no assigned function, although preliminary data suggest soluble CD83 may influence T-lymphocyte proliferation.
The MoAb CMRF-44 reacts strongly with cultured human blood DC22 and identifies a small population of freshly isolated DC in human blood.77 The fact that these DC subsequently express CD83 upon activation77 suggests that these are partially differentiated/activated cells rather than recirculating cells. The CMRF-44 Ag is expressed in moderate density on isolated LC, consistent with the postulated DC differentiation/activation pathway.74 Although inducible on Mo/MØ by high doses of interferon-γ (IFN-γ),22 the CMRF-44 Ag expression on Mo/MØ is more limited in physiologic circumstances. Thus, the substantially higher density of CMRF-44 Ag expressed on DC allows positive selection of DC.77 The Ag is also weakly expressed on B lymphocytes. Biochemical studies have yet to fully characterize the Ag.
The third reagent CMRF-56 identifies a DC differentiation/activation Ag of unknown function, which is not present on Mo/MØ (submitted).
Finally, the sequence for human DEC-205 has been obtained (submitted) and reagents to this molecule are eagerly anticipated.
Non–Lineage-Restricted Membrane Ags on DC
Broadly Expressed Molecules
DC express the CD45 Ag60 including the CD45RA, CD45RO, and CD45RC isoforms.51,78,112,113 The CD45RO is probably induced by activation.105 Signaling via the phosphatase active CD45 cytoplasmic portion may contribute to DC function.60
Myeloid Ags
In humans, most blood DC express CD33, an early marker of myeloid differentiation.44,80 LC and dermal DC express some CD33.116,117 Expression of CD33 appears to be reduced on tonsil DC,46,118 suggesting that it downregulates as DC differentiate. CD33 has been postulated to be a sialoadhesin119 but its function on DC is unknown.
CD13 is expressed on blood DC precursors but not on more differentiated DC.46,85,120 In contrast, tissue MØ are CD13+.103
The CD14 molecule is expressed in high density on Mo and acts as a lipopolysaccharide (LPS) receptor.121,122 A soluble form is released by direct secretion and by cleavage of the phosphatidylinositol (PI)-linked membrane form which may bind to other cells. Low and variable levels of CD14 have been reported on blood DC80,85 while other flow cytometry studies suggest DC are CD14−.57,77,78,123 The CD14 gene structure makes reverse transcriptase polymerase chain reaction (RT-PCR) analysis of CD14 mRNA difficult124 but the presence or absence of CD14 transcripts in DC needs clarification. Given that CD14 binds LPS,121 which triggers cellular responses, including DC migration,24 this is a pertinent question. The expression of CD14 can be modified by cytokines, notably IL-4125 and IFN-γ.126 CD14 bright cells have little allostimulatory activity57,58 but there may be a CD14+ stage of DC differentiation.84 127
Lymphoid Ags
Freshly isolated human blood DC probably express low levels of CD278,130 as do rat DC. However, cultured human blood DC,70,130 LC,117 and tonsil DC131 lack CD2 Ag. The CD5 Ag is found on blood DC.78,85,120 The CD4 Ag was identified on tonsil DC,46 LC,97 and recently on blood DC.105,120,132 Lymphoid-derived mouse DC express CD8.133
Adhesion Molecules
Clearly, DC are involved in a number of adhesive interactions during their migration and subsequent interaction with T lymphocytes. The initial tethering and rolling of DC precursors on endothelium may prove to be mediated by selectins. Firmer DC adhesion and migration through vessel walls is likely to involve integrins and intercellular adhesion molecules (ICAMs) or other Ig superfamily members. Integrins, the CD44 Ag or the syndecans may mediate interstitial tissue interactions. The more stable DC interactions with epithelia probably involve the cadherins.
The Ig superfamily members ICAM-1(CD54), ICAM-2 (CD50), and ICAM-3 (CD102), which are all ligands for LFA-1(CD11a), are expressed on DC.134-136ICAM-1 is present in low density initially on blood DC but its expression is readily upregulated.135 ICAM-2 is present and its density seemingly changes little with activation.136 In contrast, ICAM-3 is present in highest apparent density as judged by flow cytometry staining and appears to be the most important LFA-1 ligand for initial DC-T lymphocyte adhesion.135 ICAM-1, which upregulates,137 and ICAM-3, which is not upregulated by culture in vitro, are also expressed by LC.138-140
LFA-3 is a PI-linked protein that is expressed on DC and LC.134 This molecule probably has an adhesive function as well as signaling via T lymphocyte CD2.
Another family member, V-CAM (CD106), although not present on resting DC, is inducible at least on gut DC,141 possibly by IL-4 exposure.
Several integrin family members are expressed on DC. Certain β1 integrins (VLA subfamily α1-α9, αV ) are expressed selectively on LC.142 VLA-5 (fibronectin receptor) and VLA-6 (laminin receptor) may be involved in DC interstitial reactions. The β2 integrin, LFA-1 (CD11a), is expressed on mouse and human DC in high density.134,143 The expression of CD11b is limited to, at most, a subset of DC with low-density expression.57,85 CD11c appears to be present in higher density (N418 staining) on mouse lymphoid tissue–derived DC but it is also on a subset of human blood DC.85,105 CD11b and CD11c are also reported on human LC.104 144 The β3 subfamily (α11b) and the vitronectin receptor (αv) are probably absent from DC. Data on the β4-β8 integrins are awaited.
The cadherins are transmembrane glycoproteins that localize to adhering cell junctions. E-cadherin is present on both mouse145 and human146 LC. This molecule is expressed on blood DC and downregulates as LC migrate, suggesting an isologous adhesion interaction with skin epithelial cells.
The selectins are likely to be expressed on DC and may account for some of their trafficking. It is reported that human blood DC lack L selectin (CD62L),78 which is widely expressed on other leukocytes.147 However, it is shed after cell activation and thus perhaps during DC preparation. LC express the E-selectin ligand, cutaneous lymphocyte–associated Ag (CLA).148 The carbohydrate (CHO) moiety, sialyl Lewis X (CD15s), on DC is related to the ligands for E and P selectins.
The CD44 molecule is present in very high density on DC134 and the V3, V6, V9 isoforms are preferentially expressed on Mo-derived DC (Mo-DC).149A comprehensive analysis of CD44 isoforms in DC is justified, as CD44 acts as a ligand for hyaluronate, a connective tissue component likely to stabilize DC interactions with the tissue interstitium. CD44 may also have costimulatory functions.
The syndecans (CD138) bind growth factors and matrix molecules (fibronectin, collagen, laminin, and vitronectin) extracellularly and associate with the intracellular cytoskeleton. Thus, their presence on DC is of great interest.
Low levels of surface CD68 on DC may act as a ligand for E selectin.150 DC stain strongly for endoglin (CD105),78,113 a molecule found on endothelium, which may also have adhesion properties and act as part of the TGFβ receptor complex.151 They also express neurothelin (CD147), another endothelial expressed molecule with a suggested adhesion function.113 The fact that DC express the urokinase plasminogen activation receptor (CD87) implies this may be involved in tissue extravasation.113 Finally, CD36 may encourage DC binding to wound surfaces.152
Potential Ag Uptake (C, Fc, pattern recognition) Receptors
Intact pathogens or antigenic components must bind to DC via opsonization (C and antibody) or other nonspecific recognition receptors.
Trace amounts of C receptors are detected on DC, particularly mouse24,40 and human153 LC. The CR1 (CD35) receptor is absent, as is CR2 (CD21),46 but trace levels of CD11b (CR3, see above) are documented. CD11c on DC may have potential as another C receptor. C5a receptors (CD88) are present on dermal DC and a subpopulation of LC.154 Blood DC are CD55+ and CD59+, providing some protection against C-mediated damage.
Early in their differentiation, DC probably express some CD32 (FcγRII),80,97,155 and CD64 (FcγRI)155 but no CD16 (FcγRIII).80 These may be downregulated with activation. A recent publication emphasized that the preparative method may affect FcR expression by DC and suggested CD64 and CD32 were both present in high density and functional (to a lesser degree than Mo).155 Human LC express CD32 and the high-affinity receptor for IgE (FcεRI)156 as well as the low-affinity FcεRII (CD23).157 The FcεRI on DC is a multimeric structure containing FcεRI α and γ chains but lacks the β chain present in basophils.158 A molecule with considerable homology to the poly Ig receptor, the CMRF-35 antigen, has been described159 and similar molecules are expressed on human blood DC, LC, and tonsil DC (submitted).
Nonspecific “pattern recognition” receptors exhibit binding specificities for structural patterns typically displayed by cell surface molecules of many microorganisms (eg, LPS and glycans) but not normally found on the surface of host cells.150Membrane-bound proteins, including the mannose receptor,95,160 DEC-205,94 or other lectin-like molecules,161 may act as non–self-recognition receptors on DC. Functional assays have recently identified the MØ mannose receptor on Mo-DC,162,163 although previous studies on rat DC were negative.164 The DEC-205 molecule on DC is predicted to have glycan binding activity associated with its CHO recognition domains.94 CD11c165 may be more relevant than CD14 as a DC LPS receptor. The scavenger receptors include the two class A MØ receptors SR-AI and SR-AII,150 belonging to a cysteine-rich (SRCR) family of proteins, which may be expressed on some DC.166 A third class A scavenger receptor described only on MØ to date, MARCO,167 remains to be studied on DC. The class B scavenger receptors CD36 and SR-BI are primarily lipid binding proteins150; the former is present on DC.78 The collectins are soluble serum proteins with lectin like specificity168 that may also interact with DC.
Costimulatory/Signaling Molecules (see DC Functional Properties section for functional data)
The CD40 molecule was first identified on human tonsil DC46 and then LC39 after activation in vitro. Blood DC express low levels of CD40 and upregulate its expression in vitro and after cytokine exposure.26
CD80/86 Ags are not found on resting blood DC,25,169 may be on LC,170 and are upregulated rapidly upon DC activation (see DC Functional Properties section).
The heat stable antigen (HSA) identified in mice as a heavily glycosylated molecule with a small PI-linked protein core has been described on a subset of mouse spleen DC, LC,171,172 liver173 and thymic DC.174 The human homologue, CD24,175 was described initially as an Ag recognized by several MoAbs, many reacting with CHO epitopes. CD24a (IgM) antibodies react with a CD24 CHO epitope (contained within mucin) and CD24b antibodies (predominantly IgG) react with the CD24 protein core.176 Blood DC were found to be negative for CD24 using both CD24b MoAb and RT-PCR but specific staining with CD24a MoAb recognized CHO epitopes on a potentially novel molecule(s) (DC24) on DC.177 This reactivity was associated with a more immature DC phenotype.
Activation Markers
Several inducible (activation) surface Ags are found on DC. CD25 (IL-2R39,81 ) is induced on DC (cytokine receptors are discussed in Molecular Events Involved in DC Clustering and Signaling to T Lymphocytes section). Other markers, eg, CD98 (4F2)113 and CMRF-37178 are found on DC. The Reed Sternberg/Hodgkin cell (RSC/HC) associated CD30 Ag179 has not proved inducible on DC in limited studies to date. CD38 is expressed on tonsil DC (in preparation).
Inhibitory Molecules
Migrating LC undergo apoptosis in vitro.180 DC have also been shown to undergo steroid and UV light–induced apoptosis.181 They are predicted to express the Fas Ag (CD95)16,182 but confirmation of a functional effect on DC is awaited. Human DC express Fas-ligand. A subset of mouse CD8+ spleen DC express Fas-ligand.183
Cytoplasmic Molecules
The hamster MoAb M342 stains an intracellular granule-associated Ag within mouse IDC and some B lymphocytes.47 Absent from freshly isolated spleen DC, the Ag is induced by culturing. Another MoAb, MIDC-8, stains similarly, but this Ag is not present in B lymphocytes.47
The lysosomal-associated CD68 Ag (FAII in mice,184 ED1 in rats49 ) has been a moderately useful marker.185 CD68 staining has a limited (dot) perinuclear distribution in DC compared with a widespread cytoplasmic distribution in MØ.185-188 The S100 MoAb detects a series of intracellular isomers that were first considered specific to nerve tissues. Technical factors limit its use as an intracellular marker of activated DC for histological studies on formalin fixed section.189 190
A 55-kD actin bundling protein that is induced in B lymphocytes by Epstein-Barr virus (EBV) infection has been shown to be expressed in the cytoplasm of the majority of human blood DC but not other leukocytes.191 Restin is a novel 160-kD cytoskeletal protein found in the RSC/HC and is associated with the intermediate filament cytoskeletal network.192 RT-PCR detected its expression by human DC (unpublished).
Novel DC-associated proteases that might be involved in processing Ag or other DC-secreted products (apart from chemokines or cytokines), which might contribute to DC migration via the extracellular matrix, may be anticipated.193
Transcription Factors
It is logical that certain transcription factors (or combinations) will be associated selectively with the DC lineage. The early data reinforce the concept that DC form a unique lineage, with distinct transcriptional control of several genes.
Rel-B is expressed in murine thymus, spleen, and LN DC.194 Immunoblotting techniques have shown expression of c-rel, rel-B, NF-κBp65, and NF-κBp50 in DC grown from peripheral blood with granulocyte-macrophage colony-stimulating factor (GM-CSF ).195 Interestingly, these DC lacked the widely expressed transcription factor SP-1. The lack of CD14 on DC may relate to the fact that SP-1 is critical for the expression of CD14 on monocytes (Mo).196 Rel-B has also been identified in the Mo-DC197 and human tonsil DC (Fig 1K).198
Fascinating studies with v-rel transformed chicken BM precursors suggested that when v-rel was induced, B-lymphoid cells resulted: suppression of v-rel caused the transformed cells to generate DC-like characteristics.199 The absence of rel B in gene-targeted mice is associated with a lack of functional mature DC (discussed later).200 201
Novel DC Molecules
The search for novel molecules that play a role in the special functions of DC as an APC continues apace using both MoAbs and the new differential display technology. Several novel molecules expressed relatively selectively in DC have been identified, and further information on these will soon be available.
A DC HEMATOPOIETIC LINEAGE AND MIGRATION PATHWAY
Immunophenotypic and functional analyses have defined the different DC populations (Fig 3). The interrelationship of nonlymphoid and lymphoid tissue DC has been inferred from the behavior of murine LC both in vitro and in vivo, the homing of DC injected into recipients and Ag tracking studies. The ability of DC to migrate into the tissues and from there to the LN is critical to their overall function as APC.
A putative hematopoietic differentiation pathway for myeloid and lymphoid DC. Lymphoid DC have different properties (?tolerizing) from the myeloid DC, which is immunostimulatory in most circumstances. The surveillance tissue-based DC:LC in the skin, DC in the respiratory tract, gut, or other nonlymphoid tissues, migrate after exposure to infection, tissue damage/inflammation, or antigen (ie, danger signals) via the afferent lymphatics to the T-lymphocyte–dependent areas of the draining LN. It is possible that epithelial based CD1+ DC have an independent derivation from the stem cell. The ability of Mo and Mø to convert to DC in vivo has yet to be established.
A putative hematopoietic differentiation pathway for myeloid and lymphoid DC. Lymphoid DC have different properties (?tolerizing) from the myeloid DC, which is immunostimulatory in most circumstances. The surveillance tissue-based DC:LC in the skin, DC in the respiratory tract, gut, or other nonlymphoid tissues, migrate after exposure to infection, tissue damage/inflammation, or antigen (ie, danger signals) via the afferent lymphatics to the T-lymphocyte–dependent areas of the draining LN. It is possible that epithelial based CD1+ DC have an independent derivation from the stem cell. The ability of Mo and Mø to convert to DC in vivo has yet to be established.
Bone Marrow
BM isolated from mice20 and rats83 does not have constitutive allostimulatory activity. Mouse BM cultured in low21 or high concentrations82 of GM-CSF, with further mechanical (decanting) selection against granulocytic development, enables a DC-like population to emerge. Curiously, these cell preparations lacked significant expression of the mouse DC markers, NLDC-145 and N418,20 but were allostimulatory and homed to LN. Subsequent reports by others describe the generation of DC from mouse BM using GM-CSF and interleukin-4 (IL-4).202,203 OP/OP M-CSF–deficient mice204 have deficiencies in MØ populations but have been reported to have normal LC and DC networks,205 although LC were said to be reduced by 40% in number and abnormal in morphology.206
In rats, conditioned medium, GM-CSF,83 and a novel cytokine combination including M-CSF with IL-3, linoleic acid, α tocopherol, and cholecalciferol,207 generated DC-like cells from BM.
Human BM contains allostimulatory DC-like cells.44 Further fractionation of the morphologically heterogenous allostimulatory “lineage negative” population of BM mononuclear cells established that some of the CD34+ progenitor cells were also allostimulatory.84 Putative precursors of LC-type DC have been identified in human BM18,102,208,209 using CD1a labeling. CD1a is inducible on Mo by GM-CSF100 and is not present on the uncultured BM allostimulatory population44 or fresh-blood DC.57 More recently the CLA Ag was shown to define a subpopulation of blood CD34+ cells, which appear to give rise to a CD1a+ Lag+ LC.210
Analysis of human BM precursor growth in semisolid media has enabled DC-like cells to be defined morphologically,18,127,211 which when isolated had functional properties very akin to DC. The cells isolated by Young et al212 have virtually all the characteristics of DC and it is noteworthy that a significant proportion are CD1a−.
Human BM cultured in liquid suspension84,213 with a range of cytokines has produced DC-like populations (see In Vitro Cultivation of DC and DC Lines). A CD14 stage of DC differentiation may emerge in vitro,84,127,213 although no CD14+ precursor was evident in directly sorted BM.44,84 The purity of the resulting DC-like populations has been hard to assess but these cell populations certainly include potent allostimulatory cells. Curiously, few CMRF-44+ or CD83+ cells have been identified in these first culture studies and prolonged culture in specific conditions may be required.84 127
It is possible that a second DC differentiation pathway commits independently from a CD34+ precursor to provide Lag+ CD1+ epithelial-associated LC.210
Galy et al214 suggested a human CD10+ lymphoid precursor gave rise to T, B, NK cells, and DC. In addition, a CD34+ CD38 (dim) human thymic progenitor has been shown to give rise to T lymphocytes, NK cells, and DC.215 These intriguing results will encourage more functional studies on putative lymphoid lineage DC.
Not surprisingly, SCF (c-kit ligand) improves yields of DC generated in vitro from human BM.211,212,216 It also increases DC (and MØ) colony yields in semisolid media.211 The alternative growth factor flt-3 ligand has been suggested to drive both myeloid and lymphoid DC growth in mice217 and facilitates human DC growth twofold.218 GM-CSF, either exogenous or endogenous, seems important in vitro for DC growth but lower doses than generally used may be sufficient.211 Curiously, both types of DC are found in mice in the absence of GM-CSF,219 suggesting other cytokines control DC production in vivo. IL-6216 and IL-4 almost certainly contribute to DC hematopoiesis. The latter downregulates CD14125 and suppresses MØ generation.220 Interestingly, IL-13 may reduplicate this IL-4 effect,221 as may IL-7. Tumor necrosis factor (TNF ) may contribute more to late DC differentiation/activation.
The high cytokine concentrations in vitro may drive aberrant costimulatory function and skew differentiation of committed Mo/MØ lineage cells. It will be reassuring to see culture conditions evolved that allow significant upregulation of “DC lineage” markers. Studies using CD34+ cord blood generated DC are discussed in the In Vitro Cultivation of DC and DC Lines section.
Blood DC
Mouse peripheral blood mononuclear cells generate DC-like cells when cultured in GM-CSF alone,19 suggesting a circulating DC precursor. These are destined to provide interstitial DC in nonlymphoid tissues, including the skin and liver.49
Human peripheral blood DC were first isolated using methods similar to those used for the isolation of murine splenic DC.71 DC circulate in low numbers in blood and were difficult to isolate in quantity or high purity at this time.61 71 Most workers estimate DC (and their precursors) to represent only 0.1% of PBMC (a routine 300 to 400 mL blood donation provides 105 to 106 DC).
Highly purified human blood Mo, including a CD16 subpopulation,222 are poor stimulators in the allo-MLR compared with “lineage-negative” DC-enriched fractions.57,58 These “freshly isolated” blood DC57,58,105,155 or “immature” DC are smaller and lack dendritic morphology but have the nuclear features of DC (Fig 1A). A small population of larger cells are CMRF-44+ may be activated. These immature DC develop classic DC-like features after culture. It is noteworthy that these “lineage-negative” fresh DC preparations are not homogeneous and may include five or more cellular subsets.77
Gradient separation of cultured T-lymphocyte–depleted PBMC removes contaminating small “lymphoid cells” (perhaps even some DC precursors) and with further cell sorting, relatively homogeneous DC preparations result, perhaps by coordinating the differentiation/activation of the DC precursor and CMRF-44+ DC populations. These have the following (MGG stain) cytological features: an indented nucleus with open chromatin pattern and rare nucleoli, substantial by a large pale blue cytoplasm with a prominent light Golgi (perinuclear) zone, minimal granules but some minor vacuolation, and more extensive membrane protrusions, veils, or dendrites (Fig 1B).
In the presence of human serum (ie, perhaps normal physiological conditions) human Mo differentiate into MØ,223,224 apparently distinct from DC. These results and the separation of circulating DC precursors (±an activated circulating DC population) from Mo suggest two separate lineages evolve from a common precursor (Fig 3). Recent data127 again support this concept but an independent CD1a+ intermediate precursor of LC remains a definite but elusive possibility.
As illustrated in Fig 3, a third differentiation pathway may also occur at least in vitro whereby, in certain conditions,223,225 nonproliferating Mo differentiate into Mo-DC (Fig 1D). This involves high concentrations (? nonphysiological) of cytokines in combinations, which may not occur in vivo. The critical differentiation event in this process appears to be cytokine downregulation of the M-CSF receptor, which allows a DC phenotype to emerge197 (see In Vitro Cultivation of DC and DC Lines).
Nonlymphoid Tissue-Derived Interstitial DC
There is an extensive network of interstitial DC encompassing virtually all organs except the brain, parts of the eye, and the testes.7,10,37,226 These cells develop from precursors in the blood7 and provide a sentinel system of APC. LPS,115 GM-CSF,227 IL-6, and probably other stimuli recruit precursors to the tissues. Mo-DC migrate in response to classical chemoattractants (formyl peptides and C5a) and some chemokines (MCP-3, MIP-1α, and RANTES) but not IL-8.228 A basal rate of tissue entry is boosted (5- to 10-fold) by tissue damage/inflammation, ie, “danger” signals.229 The kinetics of the DC response in the lung are rapid, similar to the neutrophil response. Freshly recruited mouse LC and other interstitial DC are N418−, NLDC-145− but become NLDC-145 + in time and may express low levels of the macrophage-associated F4/80 Ag.
“Danger” signals, ie, tissue damage/inflammation, also mobilize DC from the tissues. After sensing inflammatory change230 or antigenic exposure,229 tissue DC migrate into afferent lymph.231 The properties of fresh LC and cultured LC28 (and fresh and cultured interstitial DC from other sites24 173 ) define two separate DC immune functions: (1) Ag acquisition and processing, and (2) Ag presentation to naive T lymphocytes in association with costimulatory signals.
LC
Epidermal LC form an extensive suprabasal network with branched cytoplasmic processes extended in physical contact with multiple adjacent LC.33 CD1a+ LC containing Birbeck granules are found in the dermis97 and may represent cells moving into the afferent lymphatic system. The number of LC (≈1,000/mm2 ) vary according to site.232 LC in the skin are subject to neuroendocrine control and are intimately associated with nerve endings. A low level of in vivo LC proliferation (? recently entered blood precursors) has been described.233 A population of CD1a− dermal DC (immunohistology97 ) or CD1adim (immunofluorescence/isolated cells) is also present.74 Factor XIIIa positive dermal DC may be another potential subset110 among a substantial dermal MØ population.104
The isolation of LC from skin is achieved by separating epidermal sheets from the dermis by short (<1 hour) incubations in trypsin39,42,138,234,235 or dispase.74 Epidermal cell suspensions are then produced by mechanical disruption and keratinocytes depleted. LC may be isolated at this stage, or after a period of culture, by density gradient centrifugation. Positive selection using CD1 or MHC class II MoAbs allows high purity LC preparations.235,236 Dermal DC are isolated by collecting the cells that migrate from dermal fragments.98 137
As with blood DC, there are clear phenotypic and functional differences between fresh236,237 and cultured LC.39,42,234 Periods of culture, particularly in the presence of keratinocytes which produce copious cytokines, notably GM-CSF and IL-1, stimulate upregulation of MHC class II Ags, CD40, and allostimulatory activity. Low-level expression of functional Fc and C receptors on LC40 are lost on activation.231 Culture causes LC veiled processes to increase in number and length, whereas acidic organelles and Birbeck granules decrease or disappear. The characteristic CD1a marker also disappears. Normal skin contains only a small proportion of activated CD1a+ CMRF-44+ CD83+ DC (Fig 1I) mainly in association with T lymphocytes.74 CD1a+ DC have recently been identified in other epithelial surfaces such as the urothelium238 and gut epithelium.232
Interstitial DC
First noted as strongly MHC class II positive cells with irregular membrane processes in rat tissue sections,6-8,37 these DC are distinguished from MØ by phenotype and radiosensitivity.239 Similar DC have been observed in mouse heart and kidney,43 although the level of MHC class II expression in the mouse appears reduced, hindering detection. In humans, DC have been documented in most organs, including liver,185 kidney,9 heart, and other connective tissue and tend to be associated with vascular structures.10
Liver DC are preferentially associated with portal triads whereas Kupffer cells are located in the sinusoids. Their histological location suggests that DC enter lymphatics in portal triads or capsular lymphatics.185 DC in liver were poorly phagocytic cells compared with Kupffer cells,7 but recently divided BM-derived DC precursors enter the liver with phagocytic capacity for colloidal carbon.49 Rat liver DC have now been isolated and shown to have a relatively immature DC phenotype and functional capability,173 differentiating when exposed to type I collagen.
Interstitial DC in the heart (Fig 1H) show close association with small capillaries.7,239 They are not found in heart valves. Isolated mouse heart DC (N418− and NLDC-145−) have limited phagocytic capacity and upregulate allostimulatory activity upon in vitro culture — characteristics reminiscent of the LC.43
Interstitial DC in kidney are prominent in the cortex and are noted between renal tubules close to renal capillaries.6,9 They also occur in the medulla. Again they show limited phagocytic capability and upregulate allostimulatory activity in vitro.43 DC are found throughout the rest of the urogenital system, particularly associated with vascular structures below the urothelium. The presence of DC in the sclera and limbus of the eye, with a decreasing number of DC more centrally in the cornea, has been documented.226,240 Recently, DC have been isolated from the rat iris — a technical tour de force, and these too have similar properties to LC.241
Interstitial DC are found in endocrine organs, notably thyroid, adrenal, and islet cells.8 These cells have no unique characteristics as yet but it is curious to note that thyroxine, vitamin D, and other medications influence DC function. DC were not obvious in nervous tissue7 but DC-like cells are reported in rat pineal.242
Skeletal muscle and other supporting tissues all contain interstitial DC.10 Indeed, DC are found in major blood vessels, below the endothelium, and have been suggested to contribute to the atheromatous reaction.150
DC are associated with the supporting structures of normal and abnormal joints. Arthritic synovial fluid is a source of human tissue-derived DC (see Autoimmune Disease).
Interstitial DC, like the epithelial associated LC, are believed to form a sentinel network of Ag-receptive cells, which subsequently move Ag centrally to provide activated APC and generate T-lymphocyte responses. Although both subpopulations are considered to parallel each other in terms of putative (? independent) DC differentiation pathways (Fig 3), the differential CD1a and CLA expression is notable and probably functionally important.
Mucosal Surface-Associated DC
An interdigitating sentinel epithelial network of DC has been described in the mucosa of the oral cavity, intestinal tract, and the respiratory tract, of mice, rat, and humans.243-246 These mucosal DC mature after weaning and their phenotype is responsive to external environmental influences.247 Inflammatory stimuli also recruit DC to the mucous membranes.248 Mucosal DC are also likely to have properties peculiar to the epithelial (external environment) surface that they serve.
Isolated oral mucosal DC express CD1a and contain Birbeck granules.243 CD1a+ cells are found in the oral and rectal mucosa.232 MHC class II positive DC have been identified in the epithelium of stomach, small and large bowel, lamina propria,244,249 and in the vagina and cervix.232 The draining mesenteric lymphatic DC population contains strongly MHC class II positive allostimulatory cells, with inclusion bodies indicative of a postphagocytic history.164
The network of rat respiratory tract epithelial DC extends from the trachea, into the bronchi, to the terminal bronchioles,32,245,246 with their density decreasing toward the peripheries. It has been possible to prepare respiratory epithelium and isolate the DC that have similar characteristics to LC. DC have also been purified from human lung parenchyma on the basis of weak adherence, lack of FcR, and strong MHC class II staining.250,251 These cells are distinguished from alveolar MØ by surface staining and intracellular structures. Lung DC cannot be obtained by bronchiolar lavage, unlike MØ: their isolation requires tissue digestion,250 consistent with their interstitial location. Pulmonary epithelial-derived DC present Ag to T-cell hybridomas in both rats251 and humans.250 Lung interstitial DC stimulate strong allo-MLR responses whereas alveolar MØ do not; indeed, MØ may inhibit the MLR.251
Afferent Lymphatic DC (veiled cells)
Various stimuli, including contact sensitizing agents, micro-organisms, and inflammatory mediators stimulate migration of DC from the skin, other nonlymphoid interstitial sites, and the mucosal surfaces into afferent lymph, where they are recognized as veiled cells.64,252-254 The agents, which mediate these effects, have all proved to be relatively nonspecific, eg, LPS, IL-1, and TNF-α.230,255 Thus, the activation of DC and the subsequent trafficking appears to be initiated by the Ag independent “danger signals.”229 Active changes in DC, dependent on tyrosine kinase activity, are involved in DC emigrating from tissues.171 Large mononuclear cells with veiled morphology were first isolated from pig45 and human256 lymph and constituted up to 20% of lymph cells. Peripheral DC migrate toward afferent lymphatics and traffic in the draining lymph to the LN where, after entering via the cortical sinus, the DC migrate to the T-lymphocyte–rich paracortical areas.257 This changes the DC homing characteristcs such that mouse spleen DC are unable to enter skin or heart transplants.258
Skin-Derived Lymphatic DC
Microscopic studies on skin have identified LC migrating from the epidermis into dermal lymphatic channels.231 Veiled cells have been identified in skin lymphatics.256,259 In addition to their dramatic morphological appearances some cells remain CD1a+259 or, in the mouse, retain the NLDC-145 marker. Some appear to retain Birbeck granules.260 LC induced to migrate on contact with Ag present the Ag as the draining lymphatic DC.65,261 LC migrate out of mouse skin grafts forming cords of exiting NLDC-145+ cells in the lymphatics231 and migrate from human skin fragments placed in vitro.98 137 LC when reinfused home to the skin.
Nonlymphoid Organ DC
Visceral organs are presumed to respond to similar stimuli with an efflux of interstitial DC into draining lymphatics. The close association of DC with vascular structures make this likely and migration from rat liver to coeliac nodes has now been observed. Tissue DC migrate out of transplanted heart and kidneys into the circulation and the spleen.262 In rats peritoneal inflammation induces DC traffic into lymphatics.
Mucosal Surface-Derived Lymphatic DC
Similar cells are found in the afferent lymph from rat gut.164,263 Increased numbers of these cells are obtained by removing mesenteric LN and direct thoracic duct cannulation. They are also mobilized by LPS.264 The ultimate fate of these cells in the intact host is not yet clear.
Purification of lymphatic DC is readily achieved.27 Veiled cells show high-density MHC class II expression and can also take up Ag,265 form spontaneous clusters with T lymphocytes in vitro252 and stimulate resting T-lymphocyte proliferation after in vivo priming.265 They increase their allostimulatory activity and change surface phenotype upon culture.81
Lymph Node and Tonsil DC
Practical issues have dictated that, apart from immunohistological studies in humans, functional data on LN-derived DC have been confined virtually entirely to other animals. Once again, it is prudent to consider LN draining skin, visceral organs, and mucosal (gut and respiratory) tissues as somewhat different. It has been assumed (there have been few direct comparisons) that the DC isolated from intact lymphoid tissue2 represent the IDC observed by light and electron microscopy266 and immunophenotyping.267
Subsequent studies have isolated fluorescein- or Ag-tagged DC which have migrated from the site of Ag exposure.63 65
The possibility of an alternative CD8+ DC subset in mouse LN DC was mentioned earlier.
Tonsils from routine surgery provide the most readily available source of human lymphoid tissue–derived DC from otherwise healthy donors. Cultured low-density tonsil DC were obtained with high purity (Fig 1C) and used for initial functional studies relating to the mechanisms of T-lymphocyte activation.268 Mo/MØ and “null” lymphoid population contamination is low27 and the omission of enzymatic tissue digestion precludes contamination with FDC. Cultured tonsil DC express high-density MHC products, low-level CD4, and certain CD14 epitopes detected by some MoAbs.46,269 They are potent APC in allo-MLR46,60,134 and oxidative mitogenesis assays.269
Recent work has identified two DC populations within uncultured tonsil DC isolated using an immunoselection technique (in preparation). The majority of these CD13−CD33− DC were not activated, ie, CMRF-44− and CD83− and the small proportion of CD80 and CD86 activated DC probably reflect the CMRF-44+ and CD83+ IDC noted in LN by direct in situ staining (Fig 1J). These CMRF-44+ cells express CD80 and CD86 in situ.169,270 The major population of “unactivated” DC could be identified within the T-lymphocyte areas as DC-24+, 2.7+ and CD38+ DC, but were CMRF-44−, CD83−, CD80−, and CD86−. A similar population of CD4+ CD11c−, CD13−, CD33− DC have also been described as corresponding to the “plasmacytoid T lymphocyte.”118 These populations may represent newly arrived IDC which have yet to upregulate these DC activation/differentiation Ags and costimulator molecules. Alternatively, they may represent IDC that have downregulated these molecules after interacting with T lymphocytes. This and other data74 localize the main events of DC costimulation to the lymphoid anatomical compartment. Some of these IDC may be relatively long-lived APC for stimulating T-lymphocyte memory, but the majority probably undergo apoptosis.118 A third population of CD4+ CD11c+, CD13+, CD33+ DC has also been identified within tonsil germinal centers.271 They had little capacity to phagocytose or take up soluble Ag but were allostimulatory. These DC are postulated to promote the T-B lymphocyte interaction within germinal centers and may regulate B lymphocytes.
Splenic DC
Given the spleen's different functions and dependence on a blood and not a lymphatic supply for cellular entry and exit, it seemed likely that splenic DC populations might have different characteristics.16 At least two subpopulations of DC have been defined in mouse spleen.47 The first, situated in the T-lymphocyte area in a periarteriolar distribution (ie white pulp), does not express the DC marker 33D1 or HSA but are NLDC-145+/DEC-205+ and can be induced to express M342 by culture.47 This population equates to the IDC described above. Direct visualization identifies a second population of DC in mouse spleen at the peripheries of the white pulp — the marginal zone. These cells have the inverse phenotype, ie, they are 33D1+ HSA+ NLDC-145− M342−.47 LPS appears to induce the marginal zone DC to mature and migrate into and then out of the T-lymphocyte area.272 These marginal zone, 33D1, and HSA-positive DC may represent a circulating blood-derived DC more equivalent to human blood DC and comparative studies with other forms of lymphoid tissue–derived DC are merited. Indeed, GM-CSF generates DC from mouse splenocytes that resemble LC in phenotype and function.273 In contrast, different techniques isolate a (? lymphoid-derived) CD8+ DC subset from mouse spleen with different properties183 — their anatomical location is yet to be established.
Human splenic DC have been phenotyped in situ79 and significant regional variation occurs as in the mouse. Human periarteriolar (white pulp) DC lack lysozyme, NSE, FcR, and CD11b but are CD4+ and CD14±. The marginal zone cells appear to have a more MØ-like phenotype. Isolating DC from human spleen is difficult but possible,274 and the resulting cells are indistinguishable from cultured tonsil DC.
DC FUNCTIONAL PROPERTIES
Gross phagocytic and proteolytic activity are more features of professional scavengers such as MØ.15 In contrast, relatively limited phagocytic/pinocytic capabilities provide DC with enough Ag to be processed into peptide for T-lymphocyte activation. After Ag uptake DC migrate, meet, and initiate membrane interactions with T lymphocytes and costimulate specific T-lymphocyte responses.
DC form distinct clusters275 with T lymphocytes at 37°C (Mo do not form stable clusters at this temperature) and these (Fig 1E and F ) are further stabilized as the interaction proceeds.36,59 Activated B lymphocytes have been reported to have different clustering characteristics with T lymphocytes.143 Analysis of DC-T lymphocyte clusters shows a high frequency of specific responding CD4+ T lymphocytes.
DC prime CD8+ T lymphocytes within clusters for cytotoxic responses and may do this via CD4+ help or independently of helper T-lymphocyte function.276-279 B lymphocytes are also incorporated into DC-T lymphocyte clusters and antibody secretion occurs in vitro.5
NK cells were reported to regulate the mouse MLR280 but inhibition of allogeneic DC generated MLRs in humans by NK cells was inconsistent (unpublished results).
Stimulation of T-Lymphocyte Responses
Alloantigen
DC are considerably more effective than purified blood B lymphocytes and Mo, as stimulators in an allo-MLR.46,57,58 Tonsil B lymphocytes are often poor stimulators of an MLR.46 On the other hand, activated B (memory) lymphocytes, either LPS stimulated or EBV-derived human B-lymphoid lines, have significant allo-MLR activity55,56,143,281 and likewise, cytokine-activated and differentiated Mo and endothelial cells may acquire greater MLR activity. Indirect responder APC contributions are minimal.57 The allogeneic MHC- peptide complex may involve self-antigens, including processed self MHC molecules or acquired exogenous or endogenous56 viral Ags. Extensive DC differentiation and activation may occur during the time course of an MLR and it does not measure Ag uptake and processing.
In vivo data are discussed later (see DC and Transplantation).
Autologous Ag
Purified mature DC stimulate significant autologous T-lymphocyte proliferative responses — the autologous MLR.46,52,60 61 These “background” results have been downplayed but may be significant, eg, in peptide-based cancer immunotherapy (see later). It is assumed that the preparation of DC leads to activation and expression of endogenous autoantigens or exogenous Ags. Some reduction in autologous MLR occurs when foreign Ags are removed from preparative media. In mice, increased specific responses upon rechallenge with BSA support the notion that fetal calf serum is a significant sensitizing Ag.
Exogenous Ags
Purified Ags have been used to prime mouse DC to generate primary immune responses. In vivo administration of splenic DC after in vitro incubation with ovalbumin,62 sperm whale myoglobin,63 or hen egg lysozyme63,67 have been shown to stimulate strong Ag-specific responses when T lymphocytes from these mice are rechallenged with Ag in vitro. Similar results with fluoroscein isothiocynate65 and other Ags282,283 have been obtained using mouse LC. Pulsing DC with PPD or an immunodominant 19-kD protein derived from M tuberculosis primes for strong specific proliferative responses in vivo.284,285 A variety of tumor-derived peptide Ags have been used to prime DC in vivo (see DC Vaccination and Immunotherapy) for T-lymphocyte helper and cytotoxic responses. In vitro priming has also been studied, mainly in mouse models, and primary in vitro responses using peptide pulsed DC have been reported.286
Strong cytotoxic responses to viral Ags have been generated in DC-immunized mice.287-289 Viral-specific cytotoxic T lymphocytes have also been demonstrated after in vitro priming with DC. In addition to influenza virus,290 mouse DC are effective APC for Herpes simplex virus,288 Moloney leukemia virus,289 and Sendai virus.289
Strong humoral responses to keyhole-limpet hemocyanin (KLH) and SRBC have been shown in mice after priming with Ag and DC.291
A variety of Ags have been tested in humans for their ability to generate DC-primed T-lymphocyte responses in vitro. Human DC exposed to KLH77,292 generate primary T-lymphocyte proliferative responses in vitro (Fig 2). Other studies have mainly used potential recall Ags: PPD,77,292-295 tetanus toxoid,52,77,292 Herpes simplex virus, C trachomatis,296 and C albicans,52 amongst others, to produce strong proliferative responses. Presentation of Leishmania48 and mycobacterial Ags295 by DC obtained from nonexposed individuals generates strong, almost certainly primary, responses.
DC elicit vigorous human CTL responses to influenza A virus from primed donors276 but their ability to generate primary CTL responses in vitro may be Ag dependent. Thus, primary CD8+ CTL responses to HIV in autologous DC-pulsed cultures297 may be variable but, in vitro, DC-generated cytotoxic responses to HIV proteins are possible.123,189 298
Other Systems
DC are accessory cells for CD3 mitogenesis — a paradoxical and variable result, which presumably reflects either contaminating FcR-positive cells or validates reports of the expression of CD64 and CD32 on less differentiated DC. DC also stimulate in oxidative mitogenesis assays for APC function.71,73 269
Finally, DC act as potent APC for bacterial superantigens that crosslink surface MHC molecules with responding T-lymphocyte T-cell receptor (TCR) complexes.299
Inhibition of T-Lymphocyte Responses
There are at least three different scenarios in which DC may mediate alternative APC functions by deleting inhibiting or anergizing T lymphocytes.
Thymic DC
The majority of thymic DC are located at the cortico-medullary junction.30,300,301 Extensive mouse studies using congenic strains, transgenic animals, and artificial thymus cultures have established that a high proportion of early thymocytes receive a negative, apoptotic signal if the evolving T-lymphocyte TCR binds with high affinity to “self antigenic peptide plus MHC” on APC within the thymus.302,303 Thymic DC, which were until recently presumed to be of myeloid origin, are thought to be the APC responsible for the induction of tolerance. It is suggested that low concentrations of Ags from nonthymic sources are available to thymic DC to ensure systemic tolerance but there are problems with this concept.229 The outcome of thymic DC Ag presentation was thought to depend on the stage of T-lymphocyte differentiation (mature or immature) and not any intrinsic property of thymic DC. Both isolated splenic and thymic DC can initiate primary in vitro responses when they are cocultured with mature T lymphocytes. Conversely, splenic DC can induce T-lymphocyte tolerance after introduction into thymic lobes.31
Lymphoid Precursor-Derived DC
Shortman et al have promoted the alternative concept that thymic DC are derived from a common lymphoid committed progenitor. The progenitors seed the thymus coincidentally in proportion with thymocyte progenitors29 and the resulting CD8+ thymic DC deliver tolerogenic signals.32,133,304 Mouse thymic DC appear to deliver a FasL dependent negative signal to CD4+ T lymphocytes183 and an alternative inhibitory signal to CD8+ T lymphocytes.32 Similar CD8+ DC have been isolated from peripheral lymphoid tissues, including mouse spleen.133 These DC, described as a subpopulation in lymphoid tissue preparations, may play a role in maintaining peripheral tolerance.
Human thymic DC have been isolated,305,306 but curiously they are CD4+ and CD8−. An early thymic, apparently lymphoid DC progenitor has been described in humans.215 The CD10+ human lymphoid progenitor-derived DC observed in vitro214 may predict for a peripheral equivalent, possibly with immunosuppressive capabilities.
Peripheral DC Lacking a Costimulator Phenotype
The postulated differentiation and activation of myeloid DC into fully competent potent APC is a regulated event16 and there is considerable control of the expression of several crucial DC costimulator molecules.25,26 In a normal inflammatory/immune response, DC are thought to upregulate these molecules (Fig 4, top). However, DC costimulator activity is likely to remain low in the absence of inflammation229 and these cells may migrate into LN. As a result DC could tolerize or anergize T lymphocytes they encounter in the peripheries.16 Furthermore, DC may downregulate costimulator activity in certain circumstances, thereby rendering them ineffective as APC. Neonatal respiratory tract DC are also hyporesponsive.247 Further evidence that myeloid DC may be tolerogenic comes from recent data suggesting DC targeted with the 33D1 antibody induced specific T- and B-lymphocyte tolerance to rat IgG2b antibody.307 Immature DC, which lack costimulator molecules, may tolerize allograft recipients.308 309
Molecular interactions between DC and T lymphocytes. DC undergo three or more stages to become fully active APC. Firstly, DC are activated by environmental danger signals. Secondly, initial adhesion and antigen-specific recognition takes place. Thirdly, full activation of DC costimulator activity follows, including cytokine production. This may be followed by programmed DC death.
Molecular interactions between DC and T lymphocytes. DC undergo three or more stages to become fully active APC. Firstly, DC are activated by environmental danger signals. Secondly, initial adhesion and antigen-specific recognition takes place. Thirdly, full activation of DC costimulator activity follows, including cytokine production. This may be followed by programmed DC death.
Terminally Differentiated DC
Few DC are found in the efferent lymph. It is probable that central migration of Ag loaded DC and subsequent T-lymphocyte activation triggers DC terminal differentiation resulting in DC death.16 No direct observational data of DC death within LN has been reported but in vitro analysis suggests that DC are susceptible to apoptotic signals,181 after induction of Fas or alternative death signaling molecules. It is feasible that DC, which have interacted productively with T lymphocytes, receive late negative signals which render them ineffective APC.16 This would reduce multiple Ag processing and the possibility of inappropriate autoreactive APC function.
DC Depletion Studies
No natural model of DC deficiency exists. Immunodeficient nude mice15 and rats7 have normal or increased numbers of DC. Likewise, RAG1/RAG2 deficient mice, which lack T- and B-lymphoid cells, have normal DC numbers, including thymic DC.
Two transgenic mouse phenotypes, which include at least a partial deficiency of DC, have been described. The first310 takes advantage of the fact that the LTR promoter of HIV appears to be relatively selectively activated in DC. Mice transgenic for an LTR- thymidine kinase (Herpes-derived) construct delete 90% of their DC in the presence of the drug ganciclovir.310 These animals have reduced APC function and other immune deficiencies. A second model resulted from targeted200 and accidental201 gene insertion mediated disruptions of the rel-B gene in mice. This disrupts normal hematopoiesis and also the production of mature DC in the homozygous knockout animals. They have impaired cellular immunity in contact sensitivity experiments. Curiously, LC were not affected in either mouse model providing further evidence to distinguish this “immature” tissue subset from the “mature” lymphoid tissue–derived DC. Lack of functional rel-B affects T-lymphocyte production of IL-3 and GM-CSF311 and it is possible the absence of DC in rel-B–deficient mice is an indirect rather than a direct effect. Thus, LC might persist in these mice as a result of epithelial driven cytokine production.
These models, if they are sufficiently robust — the potency of the residual DC may cause difficulties — will provide important experimental opportunities.
ANTIGEN UPTAKE, PROCESSING, AND PRESENTATION BY DC
The ability of DC to take up Ag and process it is highly dependent on the stage of DC differentiation. In vivo this relates to DC location. In vitro it relates to the time in culture, degree of activation, and antigen/T-lymphocyte exposure.
Antigenic Material
MØ may be more effective than DC as scavengers as a result of their portfolio of different nonspecific Ag (scavenger) receptors.150 In early studies, intravenous (IV) administered material such as colloidal carbon, peroxidase, and other Ags were found predominantly in MØ, although lesser quantities were noted in nonlymphoid tissue DC.2,7 Immunogenic fragments of foreign proteins are found in mouse spleen DC after IV administration.312 Phagocytosis of whole cells by DC in vivo has been reported,313 and DC draining the gut appear to contain phagocytic remnants.81,164 Liver DC precursors have phagocytic capability and migrate quickly (retaining immature cytological appearances) into the afferent lymph where they are no longer phagocytic.49 Several different investigators have now documented that administration of sensitizing Ag to the skin surface can be traced to LC and then to afferent lymph and IDC.65,312 Intradermal or mucosal administration of Ag primes afferent lymph DC in mice,67,314 rats,245,263 sheep,252 and humans50 to stimulate T-lymphocyte responses.
Several investigators have noted limited phagocytosis of large particles, ie, intact cells, bacteria, and synthetic beads of various types opsonized with a variety of compounds in vitro.155,315,316 BM-derived DC in mice are capable of active phagocytosis,284,315 but this has yet to be studied in humans. LC have been reported to take up bacteria including staphylococci and Leishmania,48,317 although this may vary between mouse strains. Moll et al318 described LC transporting Leishmania from infected skin to the draining LN. Viral particles can be endocytosed by sheep lymph-derived DC,50 a result which contradicts the findings reported with rat liver lymphatic DC.49
MØ may contribute by lysing large particles, eg, bacteria, viruses or tumor cells before transferring the degraded products to DC.63,268 MØ-DC cooperation in presenting mycobacterial Ags has been reported in some295 but not other situations.319
The size cut off for fluid phase Ag uptake (endocytosis, pinocytosis) is conventionally taken to be less than 0.5μm.49 Freshly isolated DC, ie, blood DC or LC, are highly active in this regard. Mouse and human LC bind surface Ag and internalize at least small portions of their plasma membrane.320 Direct visualization of fluoresceinated Ag has been observed within mouse LC and DC.62,65 Tonsil DC show considerable BSA uptake, whereas B-cell lines take up little by comparison.91 This active fluid phase endocytosis almost certainly explains why DC acquire a low density in vitro. The Mo-DC generated by GM-CSF/IL-4 treatment of PBMC take up extracellular fluid actively processing approximately 100× their volume per hour.162
Nonspecific inflammatory triggers may be required to initiate DC phagocytosis, pinocytosis and even expression of “antigen receptors,” eg, DEC-205. Equally, the downregulation of DC phagocytosis/pinocytosis upon exiting the tissues46 makes good sense as this reduces the risk of extraneous Ag presentation by DC. It may also switch the cellular machinery of the DC into Ag processing and presentation phases in preparation for T-lymphocyte costimulation phases.
Ag Uptake
Preliminary data suggest DC use a variety of membrane receptors for taking up Ag. The resulting receptor-antigen complexes may traffic between cell types321 and perhaps even from MØ to DC. “Cross-priming,” ie, Ag transfer from one cell to APC, may complicate interpretation of in vivo experiments.
FcR
The FcR repertoire on DC was described in the Potential Ag Uptake (C, Fc, Pattern Recognition) Receptors section. Specific antibody presumably acting via these receptors facilitates APC function of sheep lymphatic DC.252,322 Antibodies that bind to isolated LC are internalized.323 Fanger et al155 have used bispecific antibodies to confirm that CD64 and CD32 stimulate human blood DC to phagocytose ox red blood cells but at less than half the rate of Mo. A secondary tetanus toxoid T-cell line response was increased when specific antibody was added to Mo-DC and Ag.149 Culture of blood DC and LC downregulates their FcR and this presumably prevents further Fc Ag loading.
C Receptors
The trace levels of receptors were described in the Potential Ag Uptake (C, Fc, pattern Recognition) Receptors section. C components on the surface of organisms act as opsonins for the uptake of material in LC.33 C5a receptor (CD88) on DC have not been tested for Ag uptake.154 No data on the function of CR3 or indeed CR1 and CR2 on fresh blood DC are available but these receptors downregulate upon culture and activation of LC.324
Pattern Recognition Receptors
Bacteria express a large number of complex CHO molecules on their surface which may bind to pattern recognition receptors,150 many of which have a lectin-like specificity. Mannose containing residues bind to a mannose receptor protein present in high density on MØ,95,160 but there are subtleties as to the exact sugar specificities of binding, indicating several receptors may be involved.325 Related mannose binding proteins circulate in plasma.168 The mannose receptor binds bacteria and stimulates phagocytosis by MØ.160 Studies on mouse DC suggest mannose-like receptors are present317 and recent experiments in humans show that rapid uptake of mannose-conjugated BSA by GM-CSF/IL-4–differentiated Mo-DC is inhibited by mannose.162
A rabbit antiserum to mouse DEC-205 stimulated an antibody response to rabbit Ig94 and was interpreted to suggest active receptor uptake by DEC-205. The relative efficacy of this DEC-205 and CHO specificity for loading into DC compared with other surface receptors remains to be determined. The function of the other scavenger receptors,95 326 described earlier, on DC has yet to be established.
MHC and Other Membrane Cycled Molecules
Cycling of surface MHC class I molecules (the class I groove is closed) as an Ag uptake mechanism is thought to be very limited, although some peptide exchange may happen at the surface. The CD1 molecule may be associated with Ag uptake, particularly nonprotein Ag.320,323,327 328
A proportion of DC surface MHC class II molecules may be incorporated into a second and probably minor endocytotic class II compartment.35,329,330 Some Ags enter and bind in the open-ended surface MHC class II groove as peptides or even intact molecules. Thus, certain rare Ag, eg, fibrinogen, may be presented without processing by fixed DC.331 Bacterial superantigens binding alternative sites on MHC class II molecules may also be incorporated into the DC.
Ag Processing
Endogenous Ag is degraded by intracellular proteases and peptidases to provide peptides in the cytosol. These peptides, bind to transporter (TAP) gene products and are delivered to the MHC class I compartment to be incorporated in the nascent peptide binding groove generated by folding of the class I molecule before moving to the cell surface. This does not occur in the presence of proteasome or Golgi transport inhibitors and defines a conventional pathway. In DC, several additional mechanisms also operate allowing exogenous Ags to be presented on MHC class I molecules.332 Firstly, it is now clear that DC take up Ags by macropinocytosis and release Ag into the cytosol for classical TAP-dependent MHC class I presentation.333 Secondly, peptides produced in high concentration externally may exchange with peptides on mature MHC class I molecules. A third mechanism may involve carrier molecules or chaperones such as hsp96, capturing peptides and delivering then via the DC surface to the endoplasmic reticulum, where they are released, processed, and incorporated into MHC class I molecules.
For influenza Ags at least, material synthesized endogenously is presented more effectively via DC MHC class I molecules, although exogenous material can also be presented.276,290 Soluble protein, eg, albumin, is presented by DC to class I–restricted T lymphocytes.333 Peptides are readily presented by DC, providing good evidence that these alternative pathways operate in DC at least to some extent.123
Exogenous Ag, eg, intact organisms, particulate Ag or soluble Ag entering DC via a phagocytic or endocytic pathway, must undergo degradation and proteolysis before interacting with the first and major, MHC class II compartment, where peptide loading of class II molecules occurs. Class II α and β chains are synthesized and combine to be transported in association with HLA-DM to a class II compartment where DM dissociates. The class II molecules are membrane-bound with their peptide binding site protected by class II invariant protein (CLIP). Further maturation of these vesicles occurs after fusion with the endocytic lysosomal pathway (CD68 and acid phosphatase staining), prominent in DC.50 This lowers the pH and invariant chain (peptide?) dissociates, allowing appropriate peptides to bind with the MHC. This pathway has been studied recently in both mouse and human DC in a series of elegant immuno-electron microscopic studies.34 35
Levine and Chain334 have used a flow cytometric assay to show that fluid-phase endocytosis, especially traffic through late endosomes, is as active in DC as in other APC. Previous studies of endocytosis on DC used long pulses of Ag and therefore emphasized the accumulation of Ag in a terminal degradative organelle rather than traffic through late endosomes and the related processing organelle.334 Enzymatic degradation of proteins into peptides and their association with class II molecules probably takes place in acidic lysosomes as chloroquine blocks DC pulsing with certain protein Ags.234 Fresh LC contain many lysosomal and Ag containing membrane bound vesicles, which disappear with culture,329 coincidental with loss of Ag processing capacity. In contrast, differentiated murine spleen DC have a limited lysosomal system.1 The association of novel Ags with mature DC intracellular vesicles (and B cells)47 indicates specialized function for these APC organelles.
Tonsil DC degrade ingested BSA.91 Chain et al335 have likewise demonstrated proteolysis of exogenous protein by DC. Several soluble Ags have been processed by purified DC to be presented effectively to peptide specific T-lymphocyte clones336-339 or to generate primary or secondary in vitro responses.77,123,292,340,341 Preparations of mouse DC have been shown to degrade antigenic material from the surface of beads without ingestion and process it effectively (possibly externally) for a T-lymphocyte response.337 DC may express cell-surface peptidases, eg, CD13, but these have yet to be studied. Serum proteases may contribute by digesting proteins to peptides342 for DC binding.
Finally, other leukocytes may contribute to the proteolytic degradation of Ag prior to recovery by DC.268
MHC-Peptide Presentation
DC actively synthesize MHC class I molecules and mature DC show high-density surface expression.15 Again, MHC class II molecule synthesis is active in fresh blood DC35 and LC62 and surface MHC Ag expression increases markedly on culture, eg, LC in GM-CSF.38,343 It is noteworthy that low-dose GM-CSF decreases synthesis of class I in mouse MØ.21 IL-4 also upregulates MHC class II production125 as of course does IFN-γ. Isolated mouse liver DC are MHC class II− and require interaction with collagen to induce its expression.173 After in vitro culture DC synthesis of MHC Ags is reduced,344 however, cultured LC can passively acquire Ag fragments that do not need processing.234 Indicative of their specialized function, DC retain class II antigenic peptide complexes for prolonged periods (1 to 2 days) in culture,62 whereas MØ have a turnover measured in hours.345 The biochemical explanation for a lesser glycosylation of DC MHC molecules346 and any functional consequence remain elusive.
An early report suggested CD1b presented glycolipid to the T-lymphocyte TCRαβ328; other data suggested a special role for peptide presentation by CD1b to T lymphocytes with TCRαβ.347 CD1a and CD1c has also been postulated as a target recognition structure for TCRαβ348 and TCRγδ T lymphocytes.348 349
Once DC have phagocytosed/pinocytosed Ag, processed it, and delivered the antigenic peptide to surface MHC molecules, they are unable to process and present new peptides.324,343 Only a few hundred MHC-antigenic peptide complexes amongst the several million molecules on the DC surface may be required for a specific response324 and occupancy of only 0.1% of DC surface class II molecules with superantigen is sufficient to induce T-cell proliferation.299 Having achieved this, DC mobilized by “danger” signals (from Ag receptors) prepare for the next phase of molecular events: migration into LN and clustering with T lymphocytes.
MOLECULAR EVENTS INVOLVED IN DC CLUSTERING AND SIGNALING TO T LYMPHOCYTES
The formation of DC-T lymphocyte clusters in vitro only occurs after multiple transient DC-T lymphocyte interactions.60,275,350 It is an active process and may start in the afferent lymph.187 Cluster formation requires an intact DC cytoskeleton and protein kinase C activation350 to initiate adhesion interactions.60 This is initially an Ag-independent process275 but, once cognate-specific T-lymphocyte recognition occurs, additional events stabilize the cluster (Fig 4).
The molecular interactions from this point are complex and their kinetics may also contribute to the outcome. DC appear to upregulate costimulator activity as a result of Ag independent stimuli in vitro, but Ag-dependent control of DC antigen-presenting function may be more relevant in vivo.
DC must be viable to initiate T-lymphocyte activation. Thus, DC “fixed” with paraformaldehyde early in differentiation lack the ability to change their cell membrane molecular constitution and cannot costimulate T lymphocytes.331 However, activated or cultured DC expressing a full repertoire of costimulator molecules (and surface peptide) can stimulate after fixation.331 Soluble factors derived from DC alone are clearly insufficient to drive T-lymphocyte responses.59
The term “accessory” molecules describes the additional signal(s) (signal 2) apart from Ag (signal 1) required to activate a T lymphocyte. This includes essential adherence interactions but also other critical costimulator molecules. Several other signals may contribute to regulating this process (see Fig 4). Specific TCR binding to MHC-antigenic peptide might provide a permissive signal to DC. The DC also receives reciprocal signals from other T-lymphocyte membrane ligands. Cytokine signals either from the T lymphocyte or from elsewhere in the environment will also influence DC function. Data26,74 135 now suggest that T-lymphocyte recognition of specific Ag effectively converts DC into relatively Ag-specific APC (Fig 4) — somewhat of a paradigm shift for immunologists.
The character of the T-lymphocyte response may be influenced directly by the DC or by the local environment (eg, other cells and/or cytokines).
Adherence
The DC–T-lymphocyte interaction is stabilized by adhesion molecules.59,70,134,135,269 ICAM-3, which is constitutively expressed on DC in high concentrations, provides the predominant initial adhesion interaction with LFA-1 on T lymphocytes.135 ICAM-2 on DC does not appear to contribute much to this particular adherence interaction.136 ICAM-1 is expressed in relatively lesser amounts on resting DC but is induced rapidly on DC as a result of retrograde signaling from the T lymphocyte,135 thereby stabilizing the cluster.
LFA-3 on DC may also increase cluster stability by binding the CD2 ligand on T lymphocytes.134,269 LFA-1 on DC participates in reciprocal interactions. The relative paucity of CD43,114 a major contributor to cell-surface change, may facilitate DC membrane contact with T lymphocytes. Other adhesion molecules present or induced (eg, VCAM) on DC may play a part.
Costimulator Molecules
CD80 (B7.1) and CD86 (B7.2)
An excellent review in Blood describes the properties of these two members of the Ig superfamily.354 They bind different sites on the CD28 molecule (present on 80% of human T lymphocytes), thereby delivering a significant cyclosporin A–independent costimulatory signal. A second CD80/86 ligand, CTLA-4, is expressed on activated T lymphocytes and provides an alternative negative signal.355 Differential T-lymphocyte responses are attributed to CD80 stimulation (TH1 ) or to CD86 stimulation (TH 2 ) in vitro and in vivo.356
Resting human blood DC lack CD80 mRNA and surface Ag.169 They also lack CD86 surface Ag but have preformed CD86 mRNA present.25 This probably accounts for the rapid surface upregulation of CD86, followed by CD80 mRNA upregulation and subsequent surface expression of both Ags after culture.25 Isolated human LC express slightly increased amounts of CD80/CD86 expression compared to blood DC. This may relate to increased differentiation/activation,74,170,357 or simply be a consequence of the isolation procedure, as sensitive in situ staining suggests LC lack CD80/CD86.74 Similarly, LC isolated from mice lack CD80/CD86.358 Culture of human blood DC,169,292,359,360 human LC,170,357,361 and mouse LC358,362 leads to relatively rapid induction of CD80/CD86. Finally, interstitial DC isolated from rat liver173 likewise appear to lack CD80/86.
The stimuli that control relative CD80/CD86 expression on DC are being clarified. GM-CSF induced both CD80 and CD86 on mouse LC, whereas IFN-γ only upregulated CD86.358 IL-4 induced both molecules on mouse LC.363 Curiously, in vitro generation of CD34+-derived DC-like cells induced CD80 before CD86360 but, in all other contexts, CD86 expression precedes that of CD80. Crosslinking of MHC class II molecules on DC may provide a retrograde signal upregulating CD80/CD86 expression. In contrast, IFN-γ inhibits CD80 expression on mouse LC363 and IL-10 has been reported to prevent CD80/86 expression on LC363 and spleen DC.287 IL-10 suppresses the LC-induced MLR.331 Similar inhibition of DC but not Mo induction of CD86 occurs with IL-10.364
Although both CD80 and CD86 deliver a strong costimulatory signal via CD28,354 their effect via CTLA-4 (after activation it moves from the cytoplasm to T-lymphocyte surface) is unclear. Blocking CD86 inhibits the MLR to a greater extent than blocking CD8025 and CD86 blockade reduces DC primary Ag specific responses.292
CD40
The CD40 molecule is a member of the TNF receptor (TNF-R) family. Its ligand CD40L, a TNF-α–like molecule, is expressed on T lymphocytes within a few hours of their activation.365 The CD40L on T lymphocytes provides an essential costimulatory signal for CD40+ B lymphocytes. The CD40 molecule was described on human tonsil DC,46 and subsequently noted to be present in low density on resting human blood DC26,51 and LC.39 It is rapidly upregulated after a brief period of tissue culture. In humans the cytokines IL-1, IL-3, GM-CSF, and TNF-α increase CD40 expression, whereas IL-2, IL-4, IL-6, IL-12, and IFN-γ26 do not. CD40 is upregulated on CD34+-generated DC.366 An analysis of human CD40:CD40L interactions suggests two functions for the CD40 molecule on DC.26 Firstly, retrograde signaling via CD40 was the most potent stimulus identified to date for upregulating CD80/CD86. Secondly, despite this effect, soluble CD40L and CD40-Ig inhibit DC stimulation of allogeneic T lymphocytes,26 suggesting that the DC CD40-CD40L T-lymphocyte interaction provided an additional costimulatory signal. Significant additional inhibitory activity occurred when both CTLA-4Ig (blocking CD80/CD86) and CD40-Ig were used to block a DC stimulated MLR.
HSA/CD24
An MoAb able to block costimulator function was shown to recognize HSA367 and costimulatory activity was attributed to HSA on mouse LC.172 Mouse HSA transfectants stimulated T lymphocytes and double-transfection studies inferred HSA and CD80 had synergistic costimulatory activity.368 The human homologue CD24 is not present on DC, and similar experiments produced no evidence to support the view that human CD24 has costimulator function.369
OX40L
OX40, another member of the TNF-R family,370,371 is present on activated peripheral CD4+ and a subset of activated CD8+ T lymphocytes. The OX40L is found on EBV-transformed B lymphocytes372 and CD40L-stimulated B lymphocytes.373 It costimulates OKT3, phytohemagglutinin (PHA), or phorbol 12-myristate 13-acetate (PMA) activated T lymphocytes372 and it seems likely that the OX40L:OX40 binding contributes to DC:T-lymphocyte interactions.
4-1BBL
4-1BB is expressed on activated thymocytes and T lymphocytes.374-376 PHA activation of T lymphocytes induces 4-1BB within a few hours.377 The 4-1BBL has been identified on LPS-activated BM MØ and on anti-Ig activated B lymphocytes.375,378 Transfectants expressing 4-1BBL costimulate CD3 triggered T lymphocytes and mimic the effect of antibody crosslinking 4-1BB on T lymphocytes.376,379 4-1BBL is inducible on B-cell lymphomas and acts as an effective costimulatory molecule.380 Again, it seems likely that DC express 4-1BBL, if not 4-1BB, as well.
CD6 Ligand
Crosslinking studies suggests that CD6 can function as an accessory protein in T-lymphocyte activation.381,382 A ligand for CD6, ALCAM (activated leukocyte adhesion molecule), has been identified.382 ALCAM has 5 Ig-like external domains and mediates cell adhesion to CD6. It is not yet known whether this ligand pairing provides a costimulatory interaction. However, as ALCAM is induced on activated Mo, whether it is expressed or not on DC is a relevant question, particularly as CD6 antibodies can influence the autologous MLR.383 384
SLAM Ligand
A novel 70-kD glycoprotein designated SLAM (CDw150) has recently been described as yet another member of the Ig superfamily, which has limited similarity with CD48 and LFA-3.385 SLAM is constitutively expressed on peripheral blood, memory T lymphocytes, and induced on naive T lymphocytes after activation. It has recently been identified on blood DC and upregulates with activation.113 Monovalent antibody bound to SLAM enhanced Ag-specific T-lymphocyte proliferation and cytokine production.
MHC Signals
Complete or partial activation of the responding T lymphocyte has the potential to provide molecular feedback by T-lymphocyte membrane molecules or secreted products, ie, cytokines (Fig 4). The DC MHC/peptide:TCR/CD4 or CD8 T-lymphocyte interaction has been suggested to provide a retrograde signal to DC after cognate recognition of Ag by the TCR.16 Data have accumulated to suggest the cytoplasmic portion of MHC molecules, notably class II, mediate intracellular signaling events in other cells281 386 and preliminary data suggest that class II signaling upregulates CD80/CD86 on Mo. The effect of crosslinking MHC class II molecules on DC costimulator molecule expression is under investigation.
Cytokines and Chemokines
The issue as to which of the many immunologically important cytokines (reviewed elsewhere) are expressed by DC remains a somewhat vexed one. Conflicting data have arisen because the purity of some DC preparations may have allowed significant cytokine release from contaminating populations. Even more relevant, different states of differentiation/activation, particularly DC phagocytosis/pinocytosis, may profoundly influence DC cytokine production.
IL-1α and IL-1β
For a long time, IL-1 was credited (inappropriately) with providing a second signal from Mo or MØ as APC for primary immune responses.
Findings in the mouse suggested that DC did not produce IL-1387 and raised the alternative possibility that DC themselves (notably in the thymus) were responsive to IL-1 and there was an indirect effect of IL-1 on DC-T lymphocyte priming. Neither IL-1α nor IL-1β is produced in significant amounts by human tonsil or blood DC as assessed by immunolabeling, bioassays, or enzyme-linked immunosorbent assay (ELISA) analysis.16,268,388 RT-PCR analysis shows limited IL-1α and β transcripts in both resting blood16,389 and tonsil16 DC, with the latter being more obvious. It should be emphasized that, as yet, these more sensitive studies (which have the same DC purity caveats) have not demonstrated IL-1 secretion or function. Despite this, it is possible that DC within tissues produce IL-1. Murine LC are thought to produce IL-1β but show differential stimuli dependent release in culture.390 Some data in humans implies that in vitro–generated DC and stimulated blood DC and LC may produce IL-1.366,389,391 Stimuli such as phagocytosis315 or phorbol ester/calcium ionophore366 may be required to induce IL-1 secretion by DC. It is also clear DC produce large quantities of IL-1R antagonist mRNA, but the functional outcome of this is unknown.16
TNF-α
TNF-α secretion by mouse DC has not been studied in detail, although human studies suggest there are low levels of TNF-α mRNA in blood389,392 and tonsil DC.16 No biologically detectable TNF-α392 was secreted, but this may vary with the stimuli used. DC-like cells generated from CD34+ cells produce TNF-α when stimulated via CD40.366 TNF-α antiserum inhibited a DC stimulated allo-MLR,392,393 but this neutralized the TNF-α produced by activated T lymphocytes, which acts as a significant autocrine factor.392 Lymphotoxin (TNF-β) has limited expression in DC at the mRNA level389 but the significance of this is unclear.
GM-CSF
IL-6
Mouse LC produce IL-6394 and DC are thought to be the major source of IL-6 in the mouse LN. Northern blot analysis failed to detect IL-6 mRNA388 but RT-PCR detects small amounts in human blood DC16,389 and larger amounts in tonsil DC.16 Although IL-6 protein was not obviously produced by blood DC,388 viral exposure induces IL-6 secretion.395 High numbers of DC used as APC are associated with IL-6 production and allogeneic cytotoxic T-lymphocyte responses are blocked by IL-6 antibody.396
IL-7
IL-7 is a pleiotropic cytokine397 that has an important role in the differentiation of B lymphocytes, the maturation of thymocytes,398,399 and as a growth factor for mature T lymphocytes.400,401 Highly purified CD4+ as well as CD8+ T lymphocytes proliferate in response to IL-7 and a comitogen.402 The expression of IL-7 in human CD83+ blood DC was reported to be variable at the mRNA level.389 Sorg et al403 have shown that activated CMRF-44+ DC but not freshly isolated DC express IL-7 mRNA. Moreover, purified CD14+ Mo failed to express IL-7. An IL-7–neutralizing MoAb was shown to inhibit DC stimulation of an allo-MLR.
IL-10
Intriguing RT-PCR data suggest that DC may produce IL-10, which may further regulate T-lymphocyte responses.389
IL-12
IL-12 consists of two chains, a p40 protein and a p35 protein, which are both required for activity. Mouse DC coordinate secretion of the p40 and p35 components (p40 alone may inhibit) to produce functional IL-12339,404 and IL-12 production by human DC has also been described.405 Microparticle adsorbed versus soluble Ag may activate IL-12 mRNA synthesis.315 At least in vitro, the production of IL-12 by DC may drive the bias toward a TH1 response so often observed experimentally. Intriguing data suggest that human TH2 clones can induce DC IL-12 and that their cytokine profile was influenced by the APC.406
IL-15
IL-15 has no homology with IL-2, but the two cytokines share a number of biological activities, presumably as a result of their shared receptor γ chain.407 IL-15 is produced by several cell types, whereas IL-2 is derived primarily from T lymphocytes. This, and the wide distribution of the IL-15Rα chain, suggests IL-15 has a different physiological role to IL-2. IL-15 is produced by Mo, MØ,408 and Mo-DC. Blood-derived DC have been shown to induce IL-15 in RNA and protein.409
IFN
Other Cytokines
No biologically active IL-2 is produced by DC.16 Low-level IL-3 transcripts have been found by some investigators389 and not others (unpublished). Neither IL-4 nor IL-5 mRNA was detected by RT-PCR in CD83+ cells, a feature which distinguishes DC from Mo.389 Equivocal IL-11 mRNA levels were reported in CD83 + blood DC.389 IL-13 was not found by RT-PCR.389
Chemokines
DC may well produce several of these low molecular weight compounds, which facilitate the migration of leukocytes. MIP1α and MIP1β mRNA have been reported in CD83+ blood DC.389 Their production by LC has been reported391 and MIP1α is produced by CD34+-generated DC.366 At least some mRNA for RANTES and MCP-1 may also be present in blood DC.389
Recent RT-PCR analysis of CD83+ DC indicated that they produce significant amounts of IL-8 mRNA,389 but IL-8 secretion has yet to be established. DC-like cells generated from CD34+ progenitors secrete IL-8 after CD40 ligation.366
Novel chemokines produced by DC are being investigated.
Cytokine Receptors
DC growth, differentiation, migration, and functional Ag processing and presentation are all regulated by cytokines. The expression of cytokine receptors on DC is likely to determine many of these events16,410 and again the data suggest their density varies according to the state of DC differentiation/activation.411
IL-1R
The type I and the type II IL-1R both bind IL-1α and β as well as the IL-1R antagonist, but the two receptors use different signal transduction pathways. Direct binding of IL-1 to mouse LC (∼500 sites/cell) with nearly an order of magnitude less binding to spleen DC has been shown.410 Purified human blood and tonsil DC express mRNA for type I but not type II IL-1R.16 Direct labeling of IL-1R with Cdw121α and β MoAb has recently confirmed their expression on human LC.411 IL-1α and Il-1β induce CD40 on human blood DC.26
TNFR
The type I TNFR (55 kD) and the type II TNFR (75 kD) differ in their intracellular portions, suggesting different signaling pathways. Studies on human blood DC have confirmed mRNA and surface expression of both types.392 Ryffel et al412 demonstrated type II TNFR on thymic DC using histological techniques.
Functionally, it is clear that TNF-α influences DC growth and differentiation,209 prolongs the viability of isolated DC,413 induces CD40,26 and probably upregulates APC function.392 Furthermore, TNF has been implicated as providing a stimulus for LC migration in mice230 255 and as this effect was achieved using human TNF-α, it suggests a type II receptor interaction.
GM-CSF Receptor (GM-CSFR)
The presence of GM-CSFR on DC was anticipated given the effects of this cytokine on DC phenotype, migration and growth (In Vitro Cultivation of DC and DC Lines).17,38 Mouse DC have been shown to have GM-CSFR by direct binding studies (∼3,000 sites/cell)410 and RT-PCR analysis has shown GM-CSFRα mRNA in human blood and tonsil DC.16 Direct staining of blood DC with CDw116 MoAb also identified GM-CSFR.389 The majority of LC express the GM-CSFRα chain but only 15% of CD1a cells expressed the GM-CSFRβ chain, although the latter upregulated in culture.411
IL-2R
The IL-2Rα (CD25) was not detected on human tonsil DC46 but has been detected on blood DC,51 although the IL-2Rβ chain was not detected.389 Again these differences may reflect the stage of cell differentiation. IL-2R were induced by culture of murine LC414 and by GM-CSF on rat lymphatic DC.81 IL-2Rα was induced on cultured DC by CD40L.366 Despite these findings no functional effects of IL-2 on DC have yet been described.
TGF-R
Other Cytokine Receptors
DC do not express the M-CSFR410,413 or respond to M-CSF or G-CSF,413 although a neonatal-skin–derived LC line may do so.415 An RT-PCR analysis found no evidence of IL-4R on human DC,389 which is surprising given the effects of IL-4 on DC precursor growth and differentiation. IL-6R and gp130 were detected on a subset of human LC.411 IFNR have been described on a subset of LC.411 A number of studies suggest DC respond to IFN-γ by inducing MHC class II Ags, ICAM-1,416 CMRF-44,22 CD80, and CD86.26
Chemokine Receptors
T-Lymphocyte Responses
The circumstantial evidence suggests DC are capable of costimulating a wide range of T-lymphocyte responses. Antigen type, route of administration, the overall cytokine milieu, and the genetic background (this has a profound influence on TH1v TH2 responses) will influence that response.
The Ag source and the relevant peptide binding to DC MHC class I or II molecules selects CD4+ or CD8+ and T-lymphocyte responders. A second contribution may come from the DC itself, via the type and balance of costimulator molecules expressed,417 eg, CD80 v CD86418 or DC secreted cytokines (IL-7, IL-12, etc), which drive either a CD4 (TH1 /TH2 ) or CD8 (TC1 /TC2 ) lymphocyte response.406 419 Thirdly, the external environment, ie, other cell types and the cytokine milieu, may influence the resulting phenotype of a DC-stimulated T-lymphocyte response, either directly or indirectly via the plethora of DC-surface receptors. Local microenvironmental influences appropriate to gut, respiratory tract, or skin may well differ.
In vitro data emphasize that DC drive strong CD4+, predominantly TH1 , responses. This may reflect in vitro bias, particularly of the MLR and perhaps other test Ag systems. The use of DC exposed to PPD to immunize mice results in a predominant TH1 response, ie, IL-2 and IFN-γ without IL-4, when the T lymphocytes primed in vivo are retested in vitro.284,285 An interesting difference was noted when the 19-kD immunodominant protein of M tuberculosis was used as an alternative: the T-lymphocyte response induced limited IL-2 but no IFN-γ production.285 Tetanus toxoid induces a TH1 response in vitro,149 yet this is an Ag that in vivo produces a strong antibody response and IL-4 secretion, ie, a TH2 response from previously sensitized lymphocytes. The production of IL-12 by DC339,404 or by other cells, eg, MØ, may be an important factor in this bias toward DC-directed TH1 responses. Other cytokines apart from IL-4, eg, IL-1, may be produced by DC and direct TH2 responses. Human DC are clearly capable of stimulating both TH1 and TH2 clones in vitro420 and the ultimate response may well reflect a balance between T-lymphocyte IL-4 and DC IL-12 production.406
The strong bias toward TH1 responses is less evident in vivo and the route of DC Ag administration is relevant. The IV administration of Ag-pulsed DC induced an antibody response (TH2 -like), whereas SC administration induced a DTH (TH1 ) response.285,421 This may relate to the production of IL-10 from the spleen.422 Both IL-4 and IFN-γ, indicative of a TH0 response, were produced when mice were injected IV with DC pulsed with KLH.423 However, myoglobulin or human gammaglobulin pulsed DC administered IV stimulated IgG2a production, the antibody isotype associated with the TH1 phenotype.291 Recent in vivo data obtained using DC pulsed with tumor Ags suggest that these DC preparations result in strong cellular (including cytotoxic responses) as well as humoral responses (see later). It has been suggested that mucosal-derived DC generate a preferential TH2 response.424
IN VITRO CULTIVATION OF DC AND DC LINES
Production of DC in vitro has provided cells for basic scientific studies and for therapy. The divergent approaches adopted for growing DC and the problems inherent in identifying DC require some scientific caution.
Normal DC and DC-Like Cells
Several cellular sources have been used to generate DC.
BM
GM-CSF–generated mouse BM cells resemble DC morphologically and have most but not all the phenotypic characteristics of DC.20,21,284 The ability of these cells when injected in vivo to migrate and function as DC is most encouraging.82 Several groups have added IL-4 to GM-CSF in order to produce mouse DC,202,203,425-428 but this may enhance the nonspecific stimulation of T lymphocytes.425 In vitro flt-3 ligand contributes to mouse lymphoid (and it is anticipated myeloid) DC production.304
The allostimulatory activity of human BM84 makes the functional assessment of the progeny of cultured human BM using the MLR problematic. It appears that committed CD14+ Mo/MØ within human BM are not good primary APC.44,84 It is a significant question as to whether the in vitro conditions, including cytokines, induce such activity in differentiating Mo/MØ. Indeed, there is evidence that CD14+ cells differentiated in vitro from BM precursors acquire APC activity not seen in fresh human BM.84 213
Young et al127,212,213 have suggested that SCF, GM-CSF, and TNF-α provided optimal conditions to allow at least a significant percentage of DC to grow in liquid culture. Assessment of the cells produced suggests approximately 10% to 15% are differentiated human DC. Overall yields are of the order of 106 cells from 104 CD34+ BM cells after 3 to 4 weeks. Flt3 ligand may increase the number of DC from human BM progenitors.217 The presence of MØ may suppress DC development in culture.
Cord Blood CD34+-Selected Blood Progenitors
Caux et al101 described the culture of CD34+ cord blood cells in GM-CSF and TNF-α to produce a population of CD1a cells (5% to 15% of the progeny) with marked allostimulatory activity. Yields were approximately 107 CD1a cells from 106 CD34+ cells at 2 weeks. Santiago-Schwarz et al429 have described similar results. It appears that IL-3 can substitute for GM-CSF in this system.163 A further report366 explored the use of additional CD40L stimulation and provided a more detailed phenotype, indicating some heterogeneity in the resulting cell population. This complex cell population, which includes DC, can process soluble Ag and prime naive T lymphocytes.430 Other groups have followed suit using similar or modified conditions.218,431-433 The inclusion of SCF for the first 5 days increased cell yields approximately fivefold (day 12 yields were approximately 10-fold starting CD34+ cells).433 The resulting populations were heterogenous but late addition of IL-4 (day 12) induced CD1a expression and increased the allostimulatory activity of the cells. Strobl et al434 used SCF, GM-CSF, TNF-α, and TGFβ1 to generate CD1a+ progeny from CD34+ cord blood cells and showed that TGFβ1 would substitute for serum in this system.
Mobilized Peripheral Blood CD34+ Progenitors
Mobilized CD34+ cells have been used as another source of progenitors for attempts to grow DC. Bernhard et al431 used GM-CSF to culture CD34+ progenitors producing 30% to 60% CD1a+ cells within a 20- to 40-fold expanded total cell population at day 15. Mackensen et al432 added GM-CSF and IL-4 to a cocktail of SCF, EPO, IL-1β, IL-3, and IL-6, thereby generating a high proportion (45%) of CD1a+ cells. Siena et al218 used SCF and flt-3 ligand to supplement GM-CSF and TNF-α culture of CD34+ mobilized cells. Yields were of a similar order (4 × 107 cells from 106 CD34 cells) with 33% to 55% CD1a+ cells in the progeny. A recent study210 raised the possibility that CD34+ cells showed commitment to epithelial-based CD1a+ or CD1a− nonepithelial DC populations: expression of the skin homing receptor defined a CD1a+ CLA+ LC precursor.
Blood DC and Their Precursors
Markowicz and Engleman17 established that human blood DC (and their precursors) obtained by Percoll gradient separation and phenotypic selection could be maintained in GM-CSF for 4 to 6 weeks. The cells formed clumps in fluid phase culture and after several days developed allostimulatory cells with a DC morphology.
PBMC (blood Mo)
Sallusto and Lanzavecchia149 cultured adherent human PBMC in GM-CSF and IL-4 or GM-CSF and TNF-α and found potent APC populations emerged. Elegant studies established that the GM-CSF and IL-4–generated APC could process and present soluble Ag to tetanus toxoid-specific clones. Conversely, GM-CSF– and TNF-α–generated APC were less effective at processing specific Ag but had equivalent allostimulatory capacity. Romani et al435 also used GM-CSF and IL-4 to generate DC-like progeny from adherent PBMC. The resulting cells contained a significant proportion of DC-like cells (3 to 8 × 106 cells from 40 mL blood at 5 to 7 days). The experiments did not clarify from which component of PBMC the APC were derived.
Recent experiments suggest that peripheral blood Mo prepared by a variety of methods may differentiate into potent allostimulatory cells.75,223,347,436,437 Particular cytokine combinations, most including IL-4, which suppresses Mo development220 and downregulates CD14 expression,125 may drive Mo to DC differentiation. IL-10438 and TNF-α149 may control the balance between Ag uptake capacity and the upregulation of costimulator activity. Although these Mo-DC have functional attributes not unlike DC, they require testing in Ag-specific assays.
Induction of CD83 has been reported on Mo cultured in similar conditions.225 The CMRF-44 MoAb has also proved useful for analyzing the progeny of cytokine cultured PBMC (Fig 1D). Optimal concentrations of initial GM-CSF, IL-4, and subsequently TNF were evolved and the activity of the CMRF-44− phenotype Mo− DC were shown to be superior Ag specific APC (in preparation).
A concern in many of these experiments is that the starting PBMC preparations include both Mo and DC or their precursors and the progeny may be heterogeneous. Two significant phenotypic differences apply to in vitro cytokine-generated DC. Cord blood–derived CD1a+ cells are CD16+366 and CD11b is strongly expressed on CD1a+ cells generated from mobilized CD34+ cells.432 These observations, plus the paucity of CMRF-44 and CD83 staining in certain circumstances (in preparation), suggests that many of these cells retain certain Mo/MØ features.
LC and Tissue DC
The technical requirements for their preparation and the low yield make it unlikely that epidermal LC will be a future source of DC for therapy. Although dermal DC migrate readily from skin explants, this source is unlikely for practical reasons.
To summarize, a unifying approach might use a cytokine mixture to encourage initial hematopoietic precursor proliferation before adding a mixture of DC differentiating agents 4 days later. Antigen loading might then be initiated and further factors used to optimize DC costimulator activity before therapeutic administration. The resulting cells are heterogeneous, both morphologically and phenotypically. Nonetheless, they include significant numbers of functional APC, some of which are likely to be DC.431 Studies to verify their capacity to migrate are needed.
DC-Derived (transformed) Cell Lines
The availability of well-characterized DC lines is essential to enhance progress.
Transformation of Normal DC
Some putative mouse DC lines have been described,439,440 but their DC origin has not been widely accepted, nor has further data with these accrued. Significant progress has been made since using retroviral transformation of murine DC preparations. O'Neill and Ni441 transformed mouse spleen cells and obtained cell lines, which had several characteristics of DC. Transformation of murine progenitor MØ and LC has generated cell lines with antigen-presenting characteristics of DC.442 Superinfection of GM-CSF–transduced mouse BM cells with c-myc and c-raf oncogenes produced 33D1 and DEC-205+ DC clones, which have been cultured for 6 months.443
LC-like lines have been generated from neonatal mouse skin. These have a block in differentiation preventing MHC class II molecule upregulation and significant costimulation,415 which could be modulated by changing the cytokine environment. Attempts to generate LC lines in humans have also focused on neonatal skin.
DC Malignancies
It would be most curious if DC did not undergo spontaneous malignant change.
Some attempt has been made to ascribe a DC phenotype to a few examples of acute myeloid leukemia444 445 but this is difficult, whilst the phenotype and antigen-presenting activity of cytokine-treated Mo/MØ remains unclear. Even though the French-American-British (FAB) M4/M5 and possibly FAB MO AML subgroups may include some leukemias of DC origin, their definitive recognition will depend on the use of the new DC markers.
The bcr-abl translocation is found in blood DC of CML patients (in preparation) and in Mo-DC derived from CML patients.446 These cells have normal APC function.
Histiocytosis is a rare condition predominantly affecting children.447 Class I histiocytosis, defined by CD1 staining, is probably a malignancy of LC origin and a case labeled with both the CD83 and CMRF-44 MoAb further emphasized the association (unpublished). Some lines derived from Histiocytosis X material are undergoing further characterization. An association between LC histiocytosis, Hodgkin's disease (HD), and non-Hodgkin's lymphoma is well documented.448 The related “indeterminate cell proliferations” present with either solitary or multiple cutaneous nodules. Although morphologically and phenotypically similar to LC histiocytosis, they lack the characteristic Birbeck granules but are S100+ and CD1+.449
IDC tumors have also been described but are extremely rare with less than 20 reported cases.450,451 These resemble proliferations of LC but lack both Birbeck granules and CDI.450
The malignant cells in HD, RSC/HC, have several intriguing similarities to DC, despite the fact that they do not resemble DC morphologically. The possibility that these cells might be derived from IDC was raised some time ago and reinforced recently by phenotypic comparisons between RSC/HC and human DC.452,453 The origins of RSC/HC remains uncertain despite evidence that a proportion of selected cases have a clear B-lymphoid origin.454 However, quite a strong argument can be made that at least a proportion of RSC/HC are DC related, which would fit with most biological features of this disease. Recent analyses suggesting that the CD83454 and p55191 Ag are expressed on most HC adds weight to the hypothesis. HC also have potent APC activity, as do certain HD cell lines.455 Although the origin of some of the latter are hotly disputed, one cell line, L428, has been shown to have a unique molecular constitution,456 potent allostimulatory activity,455 and a cell-surface phenotype which includes the CMRF-44, CD83, CD80/CD86, and CD40 Ags.25,169 454-456
DC IN DISEASE PROCESSES
The difficulty in identifying DC has been limiting but the CD83, CMRF-44, and CMRF-56 reagents to track DC have encouraged new clinical studies.
Infectious Disease
HIV
Patients infected with HIV lose the ability to mount primary immune responses and this may, in part, be due to reduced DC function.457,458 MØ secondary antigen presenting function is unaffected in HIV+ subjects. An original report documenting HIV within LC by in situ hybridization has been confirmed by observing direct LC infection using MØ-trophic HIV strains.459 Approximately 1% of LC in acquired immunodeficiency syndrome (AIDS) patients are likely to be infected.460
Some workers originally maintained that DC in vitro did not support productive human immunodeficiency virus (HIV) infection and were not a reservoir of infection.461,462 However, careful analyses showed that rapid replication of the virus occurred in a T-lymphocyte syncytium surrounding the DC.463 Others have produced data to suggest DC (identifying the cells becomes an issue) are infected directly.457,464 DC appear to host LTR retroviral transcriptional control elements310 capable of sustaining HIV replication. DC lack the Sp-1 transcription factor essential for HIV replication; however, this is provided in the DC-T lymphocyte syncytium.195 DC can bind HIV and infect CD4+ T lymphocytes while activating them,465 emphasizing that the interaction between these two cell types is critical.
The reduced APC function may result from reduced T-lymphocyte feedback (see earlier) as DC in HIV-infected patients present satisfactorily to normal syngeneic T lymphocytes.460 An influence of HIV on DC migration has also been postulated.460 DC can generate cytotoxic T-lymphocyte responses to HIV Ags123,297,298 and when infected produce IFN-α.132
Other Clinical Viral Infections
Mycobacterial Infection
Other Bacteria, Protozoal, and Fungal Infections
Clearly DC present a range of Ags from different organisms, eg, Chlamydia296 Leishmania,48,317,318 Candida52 in vitro to T lymphocytes and for in vivo responses. Although major changes in DC numbers, localization, and function might be expected in these infections, the inability to monitor DC readily has prevented further clinical observations. However, a recent study467 showed that dermal DC but not LC engulf Borrelia burgdorferi (Bb), the organism associated with Lyme disease, and present Bb Ags.
There are no data as yet relating to DC numbers, viability, trafficking, etc in different clinical infections.
Immunodeficiency
There have been several reports of deficient APC function in primary immunodeficiencies, including hyper IgM syndrome.468 No DC specific defect has emerged as yet but deficiencies in MHC class II Ag expression and CD40L expression (hyper IgM syndrome) are likely to profoundly affect DC function.
Autoimmune Disease
It is no surprise that DC are also the most efficient APC for endogenous self Ags.338 Certainly, DC are capable of generating strong autologous responses in vitro — this is generally assumed to reflect exogenous Ags but may reflect activation of truly autoreactive clones — the specificities of those autoreactive clones have not been tested. Known autoantigens, eg, myelin basic protein or thyroid extract, may be incubated with DC and these will initiate autoimmune disease in mice.469 Experimental models of diabetes in mice suggest that APC may trigger autoimmunity by initiating a local or systemic response to an infective agent.470 Enhanced cluster formation by DC from nonobese diabetic (NOD) mice has been reported471 and DC incriminated in the islet pathology, which evolves in BB rats.472 In contrast, transfer of DC from pancreatic LN but not DC from other sources inhibited the development of autoimmune disease in NOD mice,473 perhaps by reinforcing tolerance.
Kabel et al474 have studied DC in autoimmune disease and demonstrated histological changes in the tissues, which suggest DC may contribute to the presentation of autoantigens. Some evidence that autoantigens on DC may switch off immune responses has come from human studies showing that thyroglobulin exposed DC reduce antithyroglobulin antibody production in vitro.475
DC may also prolong or exacerbate local immune-based inflammatory reactions such as occur in arthritis. Increased numbers of DC-like cells have been described in chronic arthritic synovial fluid296,476-480 and in adjacent synovial membranes.481,482 Most work relates to rheumatoid arthritis but similar changes are also seen in chronic arthritis of other etiologies. Recent data suggest the DC in chronic arthritis do not have a fully activated phenotype and may express relatively low levels of the CD83/CMRF-44 DC differentiation/activation Ags (in preparation). Even more intriguing is the observation that they do not, despite originating from an inflammatory environment, have high levels of the CD80/86 costimulator molecules.479,480 This does not appear to be due to the IL-10 in synovial fluid and may be an indirect effect on DC as a result of T-lymphocyte feedback triggered by exogenous TGF-β.483
The concept that vaccination with in vitro DC stimulated autoreactive clones may generate yet further “anti-idiotypic” control of an autoimmune response is a challenging prospect for therapy. So, too, are thoughts of fixing DC in a “tolerizing state” for reinforcing self tolerance.
Allergy and Hypersensitivity
LC are intimately involved in contact allergen sensitization261 and appear to have an affinity for certain metals and haptens. Some blood DC and LC express IgE receptors (see Cell Surface and Other DC-Associated Molecules) and may be capable of initiating the late phases of type I hypersensitivity. They may also be involved in the immunological hyper-reactivity of psoriasis364,484 and atopic dermatitis.466 DC appear in the early stages of the inflammatory infiltrate and appear to be located in dermal clusters in psoriasis but appear in diffuse infiltrates in atopic dermatitis.466
The probability that DC within the respiratory tract contribute to inflammatory and immunological events, triggering airway hypersensitivity and allergen-induced asthma, is being investigated. Holt et al245,485 have shown that DC have a substantial turnover in the respiratory tract, influenced markedly by inflammatory mediators. Interaction with aerosolized Ag leads to DC migration, Ag priming of T-lymphocyte responses, and in turn a sensitized T-lymphocyte infiltrate.485 This generates further inflammatory mediators. An inhibitory effect of lung MØ (mediated by nitric oxide) on APC function251 is noted in these systems.
DC in Malignancy
The biology of DC in malignant disease is currently arousing great interest. Animal data from Muller et al486 suggests that skin carcinogenesis is associated with a paucity of DC. There is a large body of literature alluding to the effects of UV radiation on murine DC.487 This appears to reduce DC antigen-presenting function, probably via a direct effect on protein synthesis. DC are associated with tumors in mice; however, further mouse data have called into question whether DC recruited into skin tumors indicate a host immune response to tumor.488
Human LC are likewise sensitive to UV effects.489 UV light decreases numbers in the skin490 and affects DC function,357 blocking upregulation of costimulator molecules. Various correlations have been proposed in human skin cancers. LC were reported to be increased around in situ and early invasive melanomas.491 With progression, LC numbers near deeply invasive melanomas decrease.491 In other skin tumors, increased LC infiltration appears to be associated with increasing tumor differentiation.492 Basal cell carcinoma associated DC do not show increased CD80/86 expression and in one case appeared to be poor APC compared with blood DC.493 Increased LC numbers in the cutaneous T-cell lymphomas suggested improved survival.494 Reduced LC numbers (perhaps via an indirect mechanism) were reported in the skin over breast cancer compared with distant sites.495
There is a steadily increasing body of literature correlating DC or LC numbers (detected by S100 staining) in or adjacent to tumors with their prognosis. Increased numbers are associated with better outcomes in cervical carcinoma, colorectal adenocarcinoma,496 gastric carcinoma,497 nasopharyngeal carcinoma,498 lung adenocarcinoma,499 thyroid,500 and prostate cancer.501
Whether the DC observed within malignant tissue take up tumor associated Ags (TAA) and migrate centrally to initiate effective T lymphocyte responses is unclear. It appears that splenic APC from tumor-bearing mice retain TAA and are capable of immunizing against fibrosarcomas in mice. However, they only acquire sufficient Ag or costimulator capacity late in the disease course when the host is already overwhelmed.502 Gabrilovich et al503 showed that spleen DC function in tumor-bearing mice was reduced. Defective DC function assessed by MLR and reduced ability to generate p53 specific CTL was noted. This was associated with reduced MHC Ag expression on DC. Tumor cell supernatants were shown to suppress the ability to generate functional DC from BM but not to affect the function of already mature control DC.427 Recent data suggest that vascular endothelial growth factor (VEGF ) produced by human carcinoma lines can block DC hematopoiesis in vitro.504
Tumors might frustrate normal DC physiology as yet another mechanism of avoiding immunosurveillance. Studies show that few DC are recruited into the cellular infiltrate of renal cell carcinoma (RCC) and only a small proportion of these have the phenotype (CD83+ CMRF-44+) of mature activated DC. Those that do are located near lymphoid aggregates.190 Significantly, the few CMRF-44+ DC isolated from RCC had APC activity whilst the CMRF-44− DC did not stimulate in an allo-MLR. This, given the MLR-stimulating activity of similar isolated LC and dermal DC, argues in favor of RCC suppression of DC function. Additional data238 indicate that few DC are recruited into prostate carcinomas and these too lack an activated phenotype. Further data are accruing to suggest this phenomenon also occurs in breast and bowel carcinoma.
DC AND TRANSPLANTATION
DC may sensitize for allogeneic responses either (1) directly, by presenting preformed MHC ± minor Ags, or (2) indirectly by processing and presenting MHC alloantigens presumably as peptides.14 UV irradiation of blood products abrogates MHC allosensitization, presumably through an effect on DC.505 Cyclosporin A may affect DC activity506 and unstimulated interstitial DC are steroid sensitive.14
Solid-Organ Transplantation
Soon after interstitial DC were identified it was suggested that these might be the passenger leukocytes which contributed to the strong primary allograft reaction.6,9,11,12 Formal proof that DC in heart and kidney allografts contributed to the immunogenicity of a graft was then obtained.13,14 Treatment of mouse pancreatic islet507 and thyroid508 grafts with antibodies binding DC and C before transplantation prolonged their survival. Despite other expectations, allografted thymic DC appear to be immunogenic in mice.509 The situation with liver allografts in these models is less clear, because this organ appears to have special properties.173 Depletion of donor kidney DC in clinical practice appears to have some clinically beneficial effect510 but maximum efficacy will require very effective DC depletion.
Recipient T lymphocytes cluster with DC in rat allografts, implying direct sensitization of the host's immune response. Transplant studies in mice suggested that donor DC did not exit as expected into lymphatics but some at least migrated via the bloodstream to the spleen.262 This is more consistent with central sensitization rather than intragraft lymphocyte activation. Graft alloantigens are undoubtedly also processed by recipient DC and indirect responses contribute to ongoing anti-allograft responses. Recipient DC entering and trafficking through the donor organ may contribute to this process, as may antigenic material carried directly via blood and lymph to central LN.
The interstitial DC within solid organs are not fully activated/differentiated as regards APC function.24,173 Therefore, it is theoretically possible that these cells mediate a tolerogenic effect in certain circumstances. DC that are treated with UV or heat511 to destroy their Ag-presenting potential, yet retain alloantigenic activity, do have an immunosuppressive effect. Mouse BM-derived DC progenitors apparently prolong the survival of heart allografts.512 Recent data imply that donor leukocytes emigrate from long-term kidney and liver donors and provide low-level chimerism of the recipient.308 Whether these cells represent donor-organ DC or the end progeny of donor hematopoietic progenitors is unknown.49 Therefore, it is not possible to distinguish whether this phenomenon of low-level donor chimerism has an immunosuppressive effect akin to other chimeric/tolerance models229 or whether it is a unique effect attributable to the donor-derived DC. It is possible that in certain circumstances donor tissue–derived DC never achieve normal costimulatory activity, particularly if T-lymphocyte responsiveness is compromised by immunosuppression.
Blood and BM Transplantation
There is little direct experimental data. CD34+ cells stimulate an allo-MLR84 and human BM contains DC-like cells.44 Therefore, direct priming by graft APC of residual host T-lymphocyte immune response, which survives the myeloablative/immunosuppressive conditioning before stem cell transplantation, may contribute to stem cell graft failure/rejection. Fractionation of CD34+ cells to avoid allostimulatory activity, while retaining stem cell reconstituting activity (CD34+ HLA-DR−), might be considered to avoid this.
Animal experiments suggest significant myeloablative treatment removes most, but not all, recipient DC.7 It is relevant that DC inhabit all the sites commonly associated with acute graft-versus-host disease (GVHD). These DC may contribute to direct donor T-lymphocyte allosensitization to minor Ags and prime for acute GVHD as predicted by recent in vitro studies.513 Their function514 in clinical BMT can now be examined to identify DC and define their activation/costimulation status. Clustering of donor T lymphocytes around recipient residual DC might be observed as part of the GVH reaction — activated DC-T lymphocyte association is rare in normal skin (Fig 1I).
The indirect presentation of recipient minor histocompatibility Ag via donor-derived APC may be the main route of GVHD sensitization. The cytokine storm after conditioning515 would be predicted to maximize DC migration and Ag-presenting activity. The reconstitution of DC after autologous stem cell grafting appears to be relatively prompt in animals,7 but it has yet to be studied in human autografts. The reconstitution of LC in humans after HLA-matched sibling BMT takes several weeks, but by 1 year the skin DC are all of donor type.514 Now that methods are available, we are studying DC reconstitution in the blood of HLA-matched sibling allografts.
Control of DC Ag presentation during allografting procedures has immense therapeutic potential as witnessed by the suppressive effects of CTLA-4-Ig and CD40-Ig on organ graft rejection and GVHD.516
DC VACCINATION AND IMMUNOTHERAPY
Clinical hematologists cannot fail to be intrigued by current investigations using DC as cellular therapy. As “nature's adjuvant,” DC may be the ideal vehicle for immunization against infectious agents, malignant tissue, and even autoreactive lymphocytes. Investigators are constructing cellular/molecular vaccines, expressing GM-CSF, costimulator molecules, and TAA for tumor therapy. Some of these approaches, eg, GM-CSF, may recruit DC into tumors517 and others, eg, naked DNA vaccination, may be ways of delivering Ag into host DC.518 Well-defined autologous, Ag-loaded, DC preparations may prove to be the fastest and safest means of vaccinating patients. Clearly there is much to be learned: the type of DC preparation (particularly as lymphoid DC may tolerize) and how to expose them to Ag in vitro (or in vivo) requires considerable experimentation. It is salutary to realize the dramatic differences that DC activation/differentiation state, Ag preparation (protein or peptide) Ag modification, dose of DC and Ag, route of administration, host milieu, host genotype, etc may have on outcome. Furthermore, the possibility that myeloid DC may in certain circumstances suppress475,512 519 rather than enhance immune responses should be remembered.
Infectious Agents
DC have been used in vitro to present bacterial and viral Ags to T lymphocytes. Herpes simplex peptide derivatized with a triacyl tail and delivered to DC by liposomes was more effective than virus alone.286 Strong influenza virus–specific cytotoxic responses can be induced in vitro by using virus-infected DC,520 but depletion of Mo was necessary to remove an inhibitory effect. Peptide rather than whole virus may be better for DC-generated influenza responses.290 DC-generated responses to HIV proteins have been obtained in vitro.123,297,298 521
An impression has emerged that vaccination with Ag pulsed DC in animals is as efficacious, if not considerably more so, than conventional adjuvant vaccines.15 290 DC are likely to improve responses to bacterial or viral vaccines, purified proteins or peptides, but no formal clinical comparison has been undertaken yet. A large number of variables need to be addressed before an optimal DC-based vaccination program might be contemplated (eg, with HIV-derived peptides).
Malignant Disease
The immune response is capable of focusing on TAA which include (1) viral Ags in malignancies with a viral etiology; (2) tumor-specific Ags such as recombined or mutated oncoproteins and abnormally glycosylated molecules, or potentially (3) tumor-lineage–specific autoantigens. Responses may be directed against the malignant cells themselves or perhaps TAA presented by the stroma.
Viral Ags
Donor-derived cytotoxic T lymphocytes are active against post-BMT EBV+ lymphomas. Murine responses to HPPV have been produced in vitro and in vivo.
Tumor-Specific Ags
Some oncogenic proteins, which are unlikely to be subject to downregulation as a result of immuno-selective pressure, make ideal TAA. For example the bcr-abl fusion protein resulting from the 9; 22 translocation in CML and the PML-RARα fusion protein resulting from the 15; 17 translocation in promyelocytic leukemia, are potential leukemia-specific Ags. Likewise, mutations in oncogenes, eg, ras or tumor-suppressor genes, eg, p53 produce potential TAA, albeit with only 1 to 2 amino acid differences from the normal protein. The Ig idiotypes produced by non-Hodgkin lymphoma (NHL) cells are specific tumor markers, as are the Ig paraproteins produced in multiple myeloma. In some instances, the oncogenic changes may create a TAA on a malignant cell by indirect means. Thus, the mucin molecule, MUC-1, is abnormally glycosylated in breast, pancreatic, ovarian, and prostatic cancers as well as multiple myeloma, thereby exposing a repeated antigenic protein sequence.
Tissue-Specific Ags
Several Ags are sufficiently tissue restricted to allow an autoimmune response to be efficacious, eg, MAGE, tyrosinase in melanomas, prostate-specific Ag, or prostate-specific membrane Ag (PSMA) in prostate cancer. The CD33 Ag might also be a sufficiently highly restricted myeloid lineage autoantigen for limited (possibly pretransplant) autoreactive therapy. Finally, overexpression of p53 wild-type protein in tumors may be another target autoantigen.
DC Priming With TAA In Vitro
Studies showed that both mouse spleen DC and LC could present tumor Ag and generate specific T-lymphocyte responses.522
It was by no means certain that the human TCR repertoire would contain receptors for TAA peptide-MHC complexes, but the early data are encouraging. Thus, bcr-abl specific T lymphocytes respond specifically to CML cells and DC incubated with bcr-abl peptides.341 DC also generate primary responses to bcr-abl peptides in vitro. Mo-DC obtained from CML patients generated CML-specific cytotoxic activity.446 Tyrosinase-transfected DC generated specific T-lymphocyte responses523 and DC present PMSA with some specificity in vitro.524 Finally, in vitro sensitization to the HER neu 2 breast cancer Ag has been reported using CD34+ generated DC.431
DC Vaccination With TAA In Vivo
The T-lymphocyte responses described to date against known TAA and a host of as yet undefined TAA are often weak. Further, in any one individual with cancer, the host immune response to TAA has, by definition, failed. Therefore, reliable means of generating significant T-lymphocyte responses to TAA must be evolved, hence the interest in clinical DC immunotherapy. The data,190 suggesting that tumors circumvent DC in vivo, also argue in favor of this approach.
In vivo data to suggest DC are potent immunotherapeutic agents when pulsed with TAA are accumulating rapidly (Table 2). Early experiments suggested spleen APC (probably DC) from tumor-bearing mice and normal APC were capable of inducing immunity in normal mice to a subsequent tumor challenge.502 Skin-derived APC exposed to tumor lysate were also effective at vaccination against tumor challenge.525 Fascinating data obtained in a rat sarcoma model suggested mesenteric-derived lymphatic DC exposed in vivo or in vitro to tumor could protect against tumor inoculation.263 A novel strategy using DC fused to tumor cells appeared to protect against subsequent tumor inoculation in mice.526
In Vivo Immune Responses to Tumors Induced by DC-Induced Anti-tumor Immune Responses In Vivo
| Species . | Tumor . | DC Preparation . | Tumor AG . | Vaccination . | Outcome . | Author (reference) . |
|---|---|---|---|---|---|---|
| . | . | . | . | (Pre/post) . | . | . |
| Mouse | MCA-induced sarcoma (McSa-1) | Spleen DC | Sarcoma cells | 18 Days post IV | Some dose-dependent prolonged survival | Knight et al 1985519 |
| 0 Day IV | All died | |||||
| Mouse | Fibrosarcoma (CSAIM) | T-depleted spleen cells | Tumor membranes | 5 Weekly pre SC | Reduced tumor growth | Shimizu et al 1991502 |
| Mouse | MCA-induced fibrosarcoma (S1509a) | Unseparated epithelial cells (%LC) | Tumor lysate | 7 Days pre SC | Reduced tumor growth | Grabbe et al 1991525 |
| Mouse | Lymphoma (BCL1) | Splenic low density (BSA) | IgM idiotype | 10 Days pre IV | 23/28 Treated alive | Flamand et al 1994535 |
| 5 Days Ig IV | 2/20 Untreated alive | |||||
| Mouse | C3 sarcoma HPV-16 | Splenic | HPV-16 E7 peptide | Days pre IV | Tumor protection | Ossevoort 1995536 |
| Mouse | Lewis lung-(3LL) carcinoma | BM generated (GM-CSF/IL-4) | MUT-1 peptide | 0-7 Days post IV or SC | No tumor growth | Mayordomo et al 1995202 |
| C3 sarcoma-HPV16 melanoma-OVAM-05 | HPV16-E7 peptide OVA peptide | 7, 15, 21 Days post | Established tumor regression | |||
| Mouse | MCA-induced fibrosarcoma (MCA-205) mammary adenocarcinoma (TS/A) | BM generated (GM-CSF/IL-4) | Acid-eluted tumor peptides | 4-7 Days post IV (multiple) | Reduced tumor growth | Zitvogel et al 1996426 |
| C3 embryonal fibroblasts-HPV 16 | ||||||
| Mouse | Melanoma (B16-OVA) | BM generated (GM-CSF/IL-4) | OVA peptide | 0, 7 Days pre SC | No tumor growth | Celluzzi et al 1996203 |
| Mouse | LBSN retroviral transformed fibroblast (F1-B gal) mastocytoma (P815-B gal) | BM generated (GM-CSF) transduced DC line D2SC/1 | B gal protein | 10-12 & 5-7 Days pre IP | No tumor growth | Paglia et al 1996537 |
| Mouse | Thymoma (EL-4-OVA) | BM generated (GM-CSF) | OVA peptide | 8 Days pre IV/IP | 5/6 relapse (27 tumor cells) | Porgador et al 1996529 |
| 5/6 free (107 tumor cells) | ||||||
| Mouse | 3T3 Cells-human p53 mutant (D459) | BM generated (GM-CSF/IL-4) | p53 Mutant peptide | 8 Days then every 4-5 days (post) | Reduced tumor growth | Gabrilovich et al 1996427 |
| Mouse | MCA-induced fibrosarcoma (natural p53 mutant) | BM generated (GM-CSF/IL-4 or TNF) | p53 Mutant peptide | 7 & 14 Days pre IV | Reduced tumor growth | Mayordomo et al 1996428 |
| 7 & 14 Days post IV | Tumor regression or inhibition | |||||
| Mouse | MC38 adenocarcinoma | BM generated (GM-CSF)/MC38 fusion cells | MC38 fusion cell | 14 & 28 Days pre SC or IV | No tumor growth | Gong et al 1997526 |
| 4 & 18 Days post IV | 24/25 No metastases at 28 days | |||||
| Rat | Sarcoma (HSNic) | Mesenteric lymphatic DC | In vivo loading via PP | 5 Weeks pre IP vaccination for each | 9/16 Tumor free | Gyure et al 1987263 |
| In vitro loading | 0/5 Tumor free (reduced tumor growth) | |||||
| Human | Non-Hodgkin's lymphoma | Blood DC | Ig idiotype | 0, 1, 2 L 5-6 mo (+14 day protein boost) post SLC | T-lymphocyte response. Possible tumor response | Hsu et al 1996532 |
| Human | Prostate cancer | GM/IL-4 Mo-DC | Prostate-specific membrane antigen | 4-6 Weekly (≤4 IV infusions) | Possible T-lymphocyte response | Murphy et al 1996533 |
| Species . | Tumor . | DC Preparation . | Tumor AG . | Vaccination . | Outcome . | Author (reference) . |
|---|---|---|---|---|---|---|
| . | . | . | . | (Pre/post) . | . | . |
| Mouse | MCA-induced sarcoma (McSa-1) | Spleen DC | Sarcoma cells | 18 Days post IV | Some dose-dependent prolonged survival | Knight et al 1985519 |
| 0 Day IV | All died | |||||
| Mouse | Fibrosarcoma (CSAIM) | T-depleted spleen cells | Tumor membranes | 5 Weekly pre SC | Reduced tumor growth | Shimizu et al 1991502 |
| Mouse | MCA-induced fibrosarcoma (S1509a) | Unseparated epithelial cells (%LC) | Tumor lysate | 7 Days pre SC | Reduced tumor growth | Grabbe et al 1991525 |
| Mouse | Lymphoma (BCL1) | Splenic low density (BSA) | IgM idiotype | 10 Days pre IV | 23/28 Treated alive | Flamand et al 1994535 |
| 5 Days Ig IV | 2/20 Untreated alive | |||||
| Mouse | C3 sarcoma HPV-16 | Splenic | HPV-16 E7 peptide | Days pre IV | Tumor protection | Ossevoort 1995536 |
| Mouse | Lewis lung-(3LL) carcinoma | BM generated (GM-CSF/IL-4) | MUT-1 peptide | 0-7 Days post IV or SC | No tumor growth | Mayordomo et al 1995202 |
| C3 sarcoma-HPV16 melanoma-OVAM-05 | HPV16-E7 peptide OVA peptide | 7, 15, 21 Days post | Established tumor regression | |||
| Mouse | MCA-induced fibrosarcoma (MCA-205) mammary adenocarcinoma (TS/A) | BM generated (GM-CSF/IL-4) | Acid-eluted tumor peptides | 4-7 Days post IV (multiple) | Reduced tumor growth | Zitvogel et al 1996426 |
| C3 embryonal fibroblasts-HPV 16 | ||||||
| Mouse | Melanoma (B16-OVA) | BM generated (GM-CSF/IL-4) | OVA peptide | 0, 7 Days pre SC | No tumor growth | Celluzzi et al 1996203 |
| Mouse | LBSN retroviral transformed fibroblast (F1-B gal) mastocytoma (P815-B gal) | BM generated (GM-CSF) transduced DC line D2SC/1 | B gal protein | 10-12 & 5-7 Days pre IP | No tumor growth | Paglia et al 1996537 |
| Mouse | Thymoma (EL-4-OVA) | BM generated (GM-CSF) | OVA peptide | 8 Days pre IV/IP | 5/6 relapse (27 tumor cells) | Porgador et al 1996529 |
| 5/6 free (107 tumor cells) | ||||||
| Mouse | 3T3 Cells-human p53 mutant (D459) | BM generated (GM-CSF/IL-4) | p53 Mutant peptide | 8 Days then every 4-5 days (post) | Reduced tumor growth | Gabrilovich et al 1996427 |
| Mouse | MCA-induced fibrosarcoma (natural p53 mutant) | BM generated (GM-CSF/IL-4 or TNF) | p53 Mutant peptide | 7 & 14 Days pre IV | Reduced tumor growth | Mayordomo et al 1996428 |
| 7 & 14 Days post IV | Tumor regression or inhibition | |||||
| Mouse | MC38 adenocarcinoma | BM generated (GM-CSF)/MC38 fusion cells | MC38 fusion cell | 14 & 28 Days pre SC or IV | No tumor growth | Gong et al 1997526 |
| 4 & 18 Days post IV | 24/25 No metastases at 28 days | |||||
| Rat | Sarcoma (HSNic) | Mesenteric lymphatic DC | In vivo loading via PP | 5 Weeks pre IP vaccination for each | 9/16 Tumor free | Gyure et al 1987263 |
| In vitro loading | 0/5 Tumor free (reduced tumor growth) | |||||
| Human | Non-Hodgkin's lymphoma | Blood DC | Ig idiotype | 0, 1, 2 L 5-6 mo (+14 day protein boost) post SLC | T-lymphocyte response. Possible tumor response | Hsu et al 1996532 |
| Human | Prostate cancer | GM/IL-4 Mo-DC | Prostate-specific membrane antigen | 4-6 Weekly (≤4 IV infusions) | Possible T-lymphocyte response | Murphy et al 1996533 |
A peptide specific for the rat Lewis lung cancer is a potent vaccine and causes regression of metastases in mice.527 In a further report, three different tumor models were investigated in mice using viral antigenic target (HPV-16 sarcoma), ovalbumin target (OVA-melanoma), and unmodified Lewis lung cancer.202 Vaccination with Ag pulsed, GM-CSF/IL-4–generated, BM-derived DC produced dramatic tumor responses. Subsequent reports203,426-428,528 529 have confirmed the efficacy of these BM generated “DC preparations” in a variety of mouse tumor models (see Table 2).
Acid-eluted tumor peptides also provide sufficient TAA to immunize against tumors426 and the resulting immune responses are highly specific,426,502 both of which augur well for clinical application. So too does the superiority of DC vaccination over Freunds incomplete adjuvant.428 However, many of the Ags used are transfected viral Ags or highly immunogenic proteins and the generality of the phenomenon with other tumor models needs to be confirmed. A model in which DC tumor vaccination in mice did not appear to be successful519 might be helpful.
The fact that patients with breast cancer respond to MUC-1 Ag530 and mice are protected against human breast cancer implants when vaccinated with oxidized mannan MUC-1531 suggests that this Ag, incubated with DC, may become a new treatment modality for this disease. Clinical studies are in progress using melanoma Ag. Perhaps bcr-abl peptide vaccination could be contemplated in appropriate HLA-typed CML patients either after IFN or autologous BMT has reduced the leukemic load or in patients with early relapse after allogeneic BMT. Percoll gradient, immunoselected DC have been used with tumor Ig idiotype in a phase 1 study to vaccinate patients with low-grade NHL.532 Significant T-lymphocyte and antibody responses to the Ig idiotype were detected, as were some clinical changes suggesting a tumor response. In another phase 1 study, autologous DC pulsed with PSMA-derived peptides were infused into patients with prostate cancer without ill effects and perhaps some effect on T-lymphocyte reactivity to PSMA.533
Once variables regarding DC source, Ag preparation, Ag exposure, and route of administration are clarified, further phase I clinical trials will be appropriate. Incubation with antibody,149 incorporation with pH sensitive liposomes,286 triacylation of peptide,286 oxidized mannan conjugation,531 or fusion constructs with hsp70534 may all improve Ag/peptide loading into DC. The timing of Ag exposure to human blood DC preparations may be critical (submitted) and serum should be avoided because it contains peptidases.342 Once the technology is optimized, the use of DC to generate effective tumor-specific immune responses may become the third modality of anticancer treatment.
CONCLUSION
DC should now be recognized as unique subsets of leukocytes. Myeloid-derived DC have a unique ability to induce primary immune responses, but a lymphoid progenitor–derived DC with different properties has been described. It is unclear how much differentiation of Mo into DC occurs in vivo compared with the differentiation of Mo-DC induced by cytokines in vitro. DC show the essential properties required of APC: migration, Ag uptake, processing, and presentation of Ag and costimulation of lymphocytes. The MoAb, CD83, CMRF-44 and CMRF-56, are proving of value for studying human DC and a routine DC blood count based on CMRF-44 staining is now available (in preparation). The molecular events controlling DC function are not yet fully understood and their transcriptional control of critical genes will be an important new area. Surveillance DC respond to danger signals and initiate central migration and upregulation of Ag-presenting activity. The control of DC costimulation function is such that Ag-specific recognition and T-lymphocyte reciprocal signaling may render the DC Ag-presenting function effectively Ag specific. As such myeloid DC, as well as lymphoid DC, may contribute to central and peripheral tolerance. Better control of DC antigen-presenting function may improve BMT procedures and allow optimization of DC as “nature's adjuvant” for cancer immunotherapy.
NOTES ADDED IN PROOF
Mouse BM-derived DC have been shown to phagocytose and process viable bacteria for presentation of both MHC class I and class II defined epitopes to T lymphocytes.538
Novel CC chemokine receptors have been identified on human DC539,540 which bind the recently described CC chemokine MIP-3α. MIP-3α is chemotactic for CD34+-derived DC and T lymphocytes but is inactive on monocytes. CCR6 is expressed on CD34+-derived DC but not Mo-DC,540 further emphasizing the distinction necessary between these cell preparations. A new CC chemokine (DC-CK1) has been identified recently using an Mo-DC cDNA library.541 DC-CK1 which is detected in IDC by in situ hybridization has the most interesting property of preferentially attracting naive (CD45RA+) T lymphocytes. Another CC chemokine, macrophage-derived chemokine (MDC), is expressed by Mo-DC and induces their migration.542
The presence of DC in human cord blood which was predicted on the basis of its allostimulatory potential has been confirmed (unpublished). Preliminary data suggest that cyclophosphamide/G-CSF–mobilized peripheral blood stem cells may contain relatively reduced numbers of blood DC (in preparation). This intriguing result may partially explain the lower-than-expected GVHD result that has been reported with allogeneic peripheral blood stem cell grafts.
Exciting data have been published linking the recruitment of DC into tumors with immune-mediated tumor regression in flt-3 ligand–treated mice.543 This was accompanied by a significant nonimmunologically mediated effect on tumor growth. First reports on phase I studies in humans suggest flt-3 ligand may be useful for mobilizing DC for cancer immunotherapy.544 Recent experiments describing a transient state of functional unresponsiveness after priming with DC pulsed with the P815AB TAA peptide, reversible by IL-12 administration,545 emphasize the need for careful development of clinical protocols.
ACKNOWLEDGMENT
I am grateful to colleagues who contributed unpublished data to assist with writing this review. Dr G.J. Clark had major input into the preparation of the text and Dr D.B. Fearnley provided invaluable assistance. The constructive criticism of Dr M.A. Baird, Dr A. Heiser, Dr M. Kato, A.B. McLellan, Dr R.V. Sorg, Dr W.N. Patton, Dr A.B. Troy, and Dr S. Vuckovic was invaluable and greatly appreciated. S. Banks provided the considerable secretarial support required to prepare this review.
The Haematology/Immunology/Transfusion Medicine Research Grants have been supported by the New Zealand Health Research Council, Canterbury Medical Research Foundation, Cancer Society of New Zealand, Lottery Health Research Council, McClelland Trust, Leukemia and Blood Foundation of New Zealand, Loch Maben Trust, University of Otago, and others.
Address reprint requests to Derek N.J. Hart, MD, D Phil, Haematology/Immunology/Transfusion Medicine Research Group, Canterbury Health Laboratories, PO Box 151, Christchurch, New Zealand.

![Fig. 1. The morphology of human (see text for description) DC. (A) Fresh “lineage negative” blood DC, isolated without a period of tissue culture, using immunoselection.57 (May-Grünwald-Giemsa [MGG], original magnification [OM] × 1,433). (B) CMRF-44 sorted, Nycodenz gradient purified cultured blood DC77 MGG, OM × 1,433). (C) Tonsil low-density cultured DC stained with anti–HLA-DR. The veils and dendritic processes are more obvious in these preparations (OM × 1,433). (D) Mo-derived DC preparation stained with CMRF-44 using an immunoenzyme (brown, Peroxidase-DAB) technique (in preparation) (OM × 1,433). (E and F ) Fresh “lineage negative” blood DC clustered with CD4+ purified autologous T lymphocytes in the presence of staphylococcal entertoxin A (SEA) and MGG stained. The DC is stained in another cluster (F ) for the costimulator molecule CD86 using an immunoenzyme (Alkaline Phosphatase-Fast Blue) technique (OM × 1,197). (G) EM appearances of a CMRF-44–positive cultured blood DC. Note the mitochondria endosomes and lysosomal vacuoles (OM × 17,000). (H) DC in the interstitial tissues of rat heart identified by anti-MHC class II staining (immunofluorescence) (OM × 479). (I) Dermal CMRF-44+ DC (red, Peroxidase-AEC) and T lymphocytes (blue, Alkaline Phosphatase-Fast Blue) within a section of normal skin adjacent to a hair follicle (OM × 479). (J) Lymph node interfollicular (T lymphocyte) area containing CMRF-44+ IDC (brown, Peroxidase-DAB) compared with CD14 Mo and CD20 B lymphocytes (blue, Alkaline phosphatase-Fast Blue) (OM × 143). (K) Lymph node interfollicular region with CMRF-44+ IDC (blue, Alkaline phosphatase-Fast Blue) showing nuclear labeling for the transcription factor Rel B (brown, Peroxidase-DAB) (OM × 1,197). Bar = 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3245/5/m_bl_0060f1.jpeg?Expires=1769091577&Signature=Ap8r~aD2RHys9ksN3Wo7yeARvZd2qMrjlfdADg-rVa2y9G-HarMPBvdoYUtkVHJprd2EruvXnSDtteM1Xt7r6cxWPXGGV9dyQt6~u2HrtL9gQwbScCoXXwMIW5SGJtkeW4SgUGGAueCIakPSsQPgWeT-LFHroegDBJgHpLVxF7uEOsegBaGwL0-Ua50xGBF7G4x~czprCPA5oRapLL4hJq9dIYmza9Yele2kZZacxWVmt32IlRYrnxJbOZ5DKJhBp-ln0MkzfkFXsmzH-89VDx8MmaGDdHeq~w25W2MBVJsUZhg0bUCmoib~83dE3sMqTY8Pb8BiJq5-JiDzleQGqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
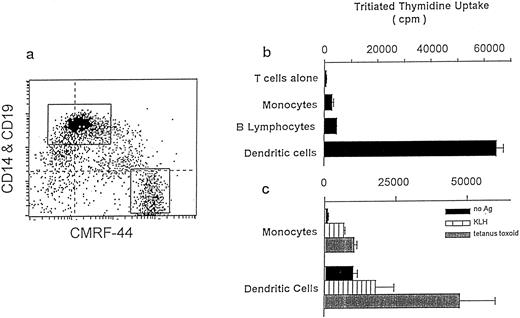
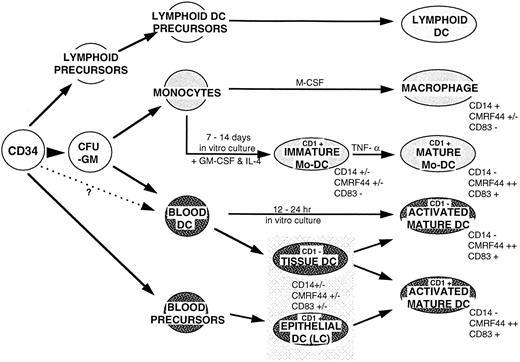

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal