Abstract
Multiple myeloma is a very devastating cancer with a high capacity to destroy bone matrix. Matrix metalloproteinases (MMPs) play a critical role in bone remodeling and tumor invasion. In this study, we have investigated the involvement of interstitial collagenase (MMP-1) and gelatinases (MMP-2 and MMP-9) in the biology of multiple myeloma. We show (1) that myeloma cells express MMP-9 and (2) that this expression is not subjected to regulation either by interleukin-6 (IL-6), the major myeloma cell growth factor, or by other cytokines involved in the multiple myeloma cytokine network. In the tumoral environment, we show that bone marrow stromal cells express MMP-1 and MMP-2. Whereas MMP-1 is positively regulated by IL-1β, tumor necrosis factor-α, and Oncostatin M, MMP-2 is not modulated by any of these cytokines. To evaluate whether myeloma cells can modify the bone marrow stromal environment, we have examined these MMP activities in coculture. Interestingly, we have observed an upregulation of MMP-1 and a partial conversion of the proMMP-2 into its activated form. We conclude that the increase of MMP activity produced or induced by myeloma cells in these cocultures could favor bone resorption and tumor invasion. Inhibition of such activities could represent a new therapeutical approach in multiple myeloma.
MULTIPLE MYELOMA (MM) is a B-cell malignancy characterized by the slow proliferation of malignant plasma cells within the bone marrow. It is a very devastating cancer, with a high capacity to destroy bone matrix and trabeculae. Almost all patients with MM have an early excessive bone resorption leading to lytic lesions and sometimes to hypercalcemia.1 The occurrence of bone lesions in MM is linked to an unbalanced bone remodeling associating excessive bone resorption with low bone formation.2 Furthermore, although tumor progression is observed mainly within the bone marrow during the early stages of the disease, extramedullary spreading occurs during the terminal stage of the disease. Finally, recent reports have shown the presence of circulating malignant plasma cells in the peripheral blood of many patients with MM, suggesting that these migrating myeloma cells could be responsible for tumor progression outside the bone marrow.3 4
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases with proteolytic activity for a large range of components of the extracellular matrix. These enzymes have been implicated in the physiologic turnover of extracellular matrix and in bone remodeling as observed in wound healing, angiogenesis, bone resorption, and several pathologic processes such as rheumatoid arthritis and tumor invasion.5-7 Members of the MMP family fall into three classes based on their substrate specificity: collagenases, gelatinases, and stromelysins. Besides these secreted MMPs, transmembrane MMPs (MT-MMP) have been recently described.8 The extracellular activity of MMPs is controlled at the level of gene expression, proenzyme activation, and interaction with specific tissue inhibitors of MMPs (TIMPs). Indeed, all MMPs are synthesized as inactive proenzymes requiring the removal of an 80-acid amino-terminal domain to be activated, and latent or active MMPs interact with their specific inhibitors (TIMPs).6 Finally, the balance of MMPs to TIMPs appears to be critical for the control of proteolysis by MMP.
The interstitial collagenase (MMP-1) is the only protease beside neutrophil collagenase known to initiate the degradation of type I collagen at neutral pH. Collagen I is the major structural protein of bone and osteoid layer, with its degradation being critical for the initiation of bone resorption. After fragmentation by MMP-1, the denatured collagen I becomes a substrate for the gelatinases MMP-2 and MMP-9. Thus, collagenase and gelatinases work in concert to promote bone resorption. The most convincing evidence supporting the involvement of MMPs in bone resorption comes from studies using both synthetic and natural inhibitors of MMPs that prevent bone resorption.10,11 Moreover, MMP-2 and MMP-9 degrade collagen IV, the major constituent of basement membrane, and have been implicated in tumor invasion and metastasis.12-14
The excessive bone resorption and the tumor invasion process inside and outside the bone marrow, which both characterize MM, prompted us to address the potential role of MMPs in this disease. Little is known about the expression of MMPs in the lymphoid lineage. Only the MMP secretion by human T lymphocytes has been investigated.15 Myeloma cells are found in the close vicinity of bone trabeculae, suggesting a strong relationship between tumor cells and their environment. Moreover, previous results have shown that myeloma cells are able to modify the biology of the bone marrow environment.16-18 To further explore the role of MMPs in MM, it appears important to study MMP production both in myeloma cells and in bone marrow stromal cells and then to analyze their regulation when myeloma and stromal cells are cocultured.
MATERIALS AND METHODS
Reagents.Human interleukin-6 (IL-6) was obtained from Sandoz (East Hanover, NJ). Oncostatin M (OSM) and IL-1β were purchased from Genzyme (Cambridge, MA) and Sigma (St Louis, MO), respectively. IL-10 was kindly provided by Schering-Plough (Levallois-Perret, France). Tumor necrosis factor-α (TNF-α) was purchased from Genentech (San Francisco, CA). TGF-β1 was a gift from Dr R. Garrett (San Antonio, TX). Dexamethasone was obtained from Merck Sharp (Paris, France). 1.25-dihydroxyvitamin D3 [1.25(OH)2D3] was a generous gift from Leo Pharmaceutical (Ballerup, Denmark). 4-Aminophenylmercuric acetate (APMA) was purchased from Sigma. B-B4 monoclonal antibody (MoAb) was a gift from Dr J.Wijdenes (Diaclone Research, Besançon, France). CD13 and CD14 MoAbs were obtained from Immunotech (Marseille, France).
Cell lines and culture conditions.The human myeloma cell lines (HMCL) RPMI-8226 and U266 were obtained from American Type Culture Collection (Rockville, MD). The HMCL NCI-H929 was purchased from DSM (Braunschweig, Germany). XG-2, XG-6, and SBN-1 were established in our laboratory.19 20 Cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, and 5 × 10−5 mol/L 2-β mercaptoethanol.
Bone marrow stromal cells (BMSC) culture and preparation of conditioned media.Bone marrow mononuclear cells were isolated by Ficoll-Hypaque density sedimentation. The cells were plated in RPMI 1640 supplemented with 10% FCS and allowed to attach for 3 days, after which the medium is replaced by fresh medium. After 2 or 3 weeks, a confluent adherent cell monolayer was obtained, then after two passages using trypsin/EDTA solution, they were used to study the production of MMPs. Stromal cells (1.5 × 104 cells) were seeded in 96-well plates (Costar, Cambridge, MA) and were harvested after 48 hours of culture media. The cultures were performed in serum-free RPMI 1640, in the presence or absence of cytokines or steroid hormones.
Purification of myeloma cells and preparation of conditioned media.Mononuclear cells were isolated by Ficoll-Hypaque density sedimentation from bone marrow aspirates or peripheral blood from patients with MM or plasma cell leukemia (PCL). The percentage of plasma cells was then determined by morphology in May-Grünwald-Giemsa and checked by flow cytometry using two-color staining with anti-CD38 and anti–B-B4 MoAbs. Only samples with a plasmocytosis superior to 10% were used for the following purification. Adherent cells were removed by allowing mononuclear cells to adhere to a plastic flask for 90 minutes in RPMI 1640 containing 5% FCS at 37°C in 5% CO2 humidified atmosphere. The purification of myeloma cells was performed by an immunomagnetic method using antimouse IgG magnetic beads (Immunotech). This purification was performed in two steps, a first negative step using the CD13 and CD14 MoAbs to remove monocytic and granular cells and a second positive step using the specific plasma cell B-B4 MoAb to select myeloma cells. The purity of myeloma cells was evaluated by standard morphology (May-Grünwald-Giemsa–stained cytospins) and only cell populations with a purity greater than 99% were used for preparation of conditioned media. B-B4+ myeloma cells were seeded in 96-well plates (Costar) at the concentration of 1.25 × 106 cells/mL and media were harvested after 48 or 72 hours. The cultures were performed in serum-free RPMI 1640 in the presence or absence of cytokines or steroid hormones.
Coculture experiments.Stromal cells were seeded in 96-well plates (Costar) at the concentration of 1.5 × 104 cells/well in RPMI 1640 with 5% FCS. After 24 hours, when the cells are confluent, the medium is removed and fresh serum-free medium (100 μL) is added. Myeloma cells at the final concentration of 1.25 × 105 cells/well are added to stromal cells, and supernatants are harvested after 48 hours of coculture.
Gelatin substrate gel zymography.Conditioned media were mixed with sodium dodecyl sulfate (SDS)/sample buffer without reducing agent, and proteins were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 7.5% polyacrylamide gels containing gelatin at 1 mg/mL, as previously described by Heussen and Dowdle,21 and adjusted for minigel format. After electrophoresis, SDS was removed from the gel by an incubation in 2.5% Triton X-100 for 1 hour at room temperature. The gels were then incubated in a buffer containing 50 mmol/L Tris-HCl, 5 mmol/L CaCl2 , pH 7.6, for 24 hours at 37°C. The gels were stained with Coomassie blue R250 (0.25%). Proteolytic activities were evidenced as clear bands against the blue background of stained gelatin. For some experiments, samples were treated by EDTA (10 mmol/L), 1-10 Phenanthroline (10 mmol/L), or APMA (1 mmol/L) for 24 hours at 37°C before zymography.
Determination of MMP-1 level.The concentration of MMP-1 in serum-free conditioned media was determined using an enzyme-linked immunosorbent assay (ELISA; Amersham, Little Chalfont, UK). This assay measures total enzyme present regardless of whether it is free or bound to inhibitor or whether it is in an active form.
RESULTS
Human myeloma cells secrete constitutively MMP-9 but not MMP-2 and MMP-1.We have examined the production of the gelatinases MMP-9 (92 kD) and MMP-2 (72 kD) by purified myeloma cells isolated from bone marrow or peripheral blood samples from patients with MM and PCL, respectively. The samples presenting a plasmocytosis ranging from 10% to 85% were purified by a double immunomagnetic method allowing us to obtain a purity ≥99%. This method is described in detail in the Materials and Methods. These purified myeloma cells were then cultured for 48 hours in serum-free medium, and the conditioned media were assessed by gelatin zymography. A gelatinolytic activity was observed as a band of 92 kD. The results of five patients are shown in Fig 1A. However, a larger number of patients (n = 15) was analyzed and the 92-kD band was always detected. This gelatinolytic activity was totally abolished by EDTA (10 mmol/L) or 1-10 Phenanthroline (10 mmol/L) treatment, indicating that a metalloprotease supports it (Fig 1B). Moreover, a shift in the molecular weight of the MMP-9 characterizing the MMP-9 active form was obtained when samples were treated by APMA, a well-known activator of MMP in vitro. Clearly, no band of 72 kD (MMP-2) was detected, indicating that primary myeloma cells secrete only MMP-9.
Gelatinase expression in human myeloma cells. Gelatin zymography of 48-hour serum-free conditioned media was performed on equal aliquots (15 μL) as described in the Materials and Methods. (A) Lanes 1 through 3 and 5, purified myeloma cells from bone marrow of patients with MM; lane 4, purified myeloma cells from peripheral blood of a patient with PCL. (B) Supernatants of purified myeloma cells were treated for 24 hours by APMA (10 mmol/L; lane 2), EDTA (10 mmol/L; lane 3), or 1-10 Phenanthroline (1 mmol/L; lane 4) at 37°C. (C) Lanes 1 through 3, HMCL NCI-H929, RPMI-8226, and SBN-1; lane 4, primary tumor myeloma cells that engendered SBN-1.
Gelatinase expression in human myeloma cells. Gelatin zymography of 48-hour serum-free conditioned media was performed on equal aliquots (15 μL) as described in the Materials and Methods. (A) Lanes 1 through 3 and 5, purified myeloma cells from bone marrow of patients with MM; lane 4, purified myeloma cells from peripheral blood of a patient with PCL. (B) Supernatants of purified myeloma cells were treated for 24 hours by APMA (10 mmol/L; lane 2), EDTA (10 mmol/L; lane 3), or 1-10 Phenanthroline (1 mmol/L; lane 4) at 37°C. (C) Lanes 1 through 3, HMCL NCI-H929, RPMI-8226, and SBN-1; lane 4, primary tumor myeloma cells that engendered SBN-1.
We have next investigated the gelatinolytic activity secreted by a large panel of HMCL (RPMI-8226, U266, NCI-H929, XG2, XG6, and SBN-1), and only RPMI-8226 presented a proteolytic activity. This activity was shown by a double band of 92 kD and 90 kD (Fig 1C, lane 2). However, for all HMCL, a band of 92 kD was shown after concentration of conditioned media (10- to 20-fold) by centrifugation with a 25-kD cut-off membrane before gel zymography (data not shown). This indicates that HMCL secrete very weak amount of MMP-9. To test the hypothesis that long-term culture of HMCL leads to a decrease of the capacity to produce MMP-9, we have compared the conditioned media of primary myeloma cells (patient SBN-1) and of the cell line (SBN-1) established from these myeloma cells. Whereas fresh myeloma cells produce a significant amount of MMP-9, SBN-1 cell line that had been immortalized almost 1 year ago has no detectable gelatinolytic activity any more (Fig 1C, lanes 3 and 4).
We further looked for MMP-1 production by purified myeloma cells and HMCL. MMP-1 production was assessed by ELISA. As expected, MMP-1 was not detected in the conditioned media of either purified myeloma cells or HMCL (data not shown).
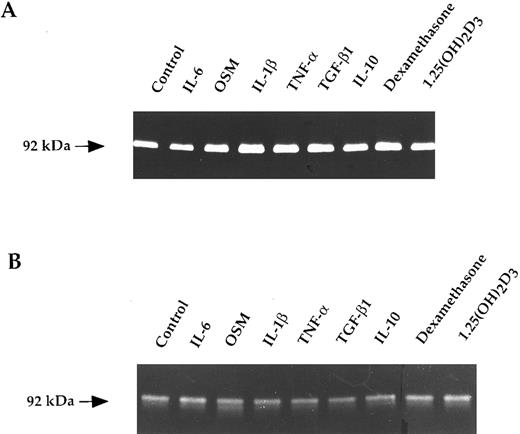
The constitutive secretion of MMP-9 by myeloma cells is regulated neither by cytokines nor by steroid hormones.To define whether the MMP-9 production by myeloma cells was regulated by cytokines and hormones, we have focused our attention on the cytokines and hormones relevant to the biology or the treatment of MM: IL-6, OSM, IL-1β, TNF-α, TGF-β, and IL-10, which are involved in the MM cytokine network; and dexamethasone and 1.25(OH)2D3 , which can inhibit myeloma cell growth.20 22-28 Each cited cytokine (10 ng/mL), dexamethasone (10−6 mol/L), or 1.25(OH)2D3 (10−8 mol/L) was added to myeloma cells for 48 and 72 hours. In addition, for IL-6, a dose response from 5 to 30 ng/mL was analyzed. MMP-9 production by RPMI-8226 cells and purified myeloma cells from five patients were studied by gelatin zymography. The results obtained both on myeloma cells and HMCL indicate that none of the cytokines or hormones tested is able to regulate the MMP-9 production as shown in Fig 2.
Gelatin zymograms of purified myeloma cells (A) and RPMI-8226 cells (B) treated with cytokines and hormones. Myeloma cells (1.25 × 104/100 μL) were untreated (control) or exposed to IL-6 (10 ng/mL), OSM (10 ng/mL), IL-1β (10 ng/mL), TNF-α (10 ng/mL), TGF-β (10 ng/mL), IL-10 (10 ng/mL), dexamethasone (10−6 mol/L), or 1.25(OH)2D3 (10−8 mol/L) for 48 hours. Corresponding culture supernatants (15 μL) were subjected to gelatin zymography.
Gelatin zymograms of purified myeloma cells (A) and RPMI-8226 cells (B) treated with cytokines and hormones. Myeloma cells (1.25 × 104/100 μL) were untreated (control) or exposed to IL-6 (10 ng/mL), OSM (10 ng/mL), IL-1β (10 ng/mL), TNF-α (10 ng/mL), TGF-β (10 ng/mL), IL-10 (10 ng/mL), dexamethasone (10−6 mol/L), or 1.25(OH)2D3 (10−8 mol/L) for 48 hours. Corresponding culture supernatants (15 μL) were subjected to gelatin zymography.
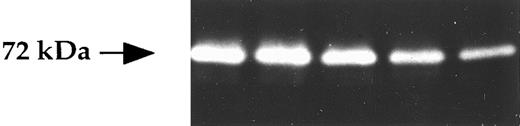
Cells of bone marrow environment secrete MMP-2 and MMP-1 but not MMP-9.BMSC were obtained after long-term culture of bone marrow from myeloma patients, as described in the Materials and Methods. Stromal cell culture supernatants (n = 10) analyzed by gelatin zymography presented a gelatinolytic activity at 72 kD corresponding to the molecular weight of the MMP-2 in its zymogen form (ProMMP-2; Fig 3). No gelatinolytic activity at 92 kD was seen, indicating the lack of detectable MMP-9 production in stromal cells. In conclusion, BMSC secrete only MMP-2, with little variations in the level of production from sample to sample, as shown in Fig 3 for five patients.
Gelatinase expression in BMSC. BMSC (1.5 × 104/100 μL) were cultured for 48 hours and then the corresponding supernatants were studied by gelatin zymograghy.
Gelatinase expression in BMSC. BMSC (1.5 × 104/100 μL) were cultured for 48 hours and then the corresponding supernatants were studied by gelatin zymograghy.
We then assessed the production of MMP-1 in BMSC culture supernatants by ELISA. This assay measures both the proMMP-1 and its active form. All BMSC secrete a detectable level of MMP-1, but the constitutive production of MMP-1 by BMSC varies from patient to patient (4 to 26 ng/mL), as shown in Table 1.
The BMSC secretion of MMP-2 is regulated neither by cytokines nor by steroid hormones.The different cytokines IL-6, OSM, IL-1β, TNF-α, and TGF-β and the steroid hormones dexamethasone and 1.25(OH)2D3 were tested on MMP-2 secretion in BMSC (n = 5) at 48 and 72 hours. As shown in Fig 4, MMP-2 secretion by stromal cells is not subjected to regulation by any of the tested agents.
Gelatin zymogram of BMSC treated with cytokines or hormones. BMSC (1.5 × 104/100 μL) were untreated (control) or exposed to IL-6 (10 ng/mL), OSM (10 ng/mL), IL-1β (10 ng/mL), TNF-α (10 ng/mL), TGF-β (10 ng/mL), dexamethasone (10−6 mol/L), or 1.25(OH)2D3 (10−8 mol/L) for 48 hours. Corresponding culture supernatants (15 μL) were subjected to gelatin zymography.
Gelatin zymogram of BMSC treated with cytokines or hormones. BMSC (1.5 × 104/100 μL) were untreated (control) or exposed to IL-6 (10 ng/mL), OSM (10 ng/mL), IL-1β (10 ng/mL), TNF-α (10 ng/mL), TGF-β (10 ng/mL), dexamethasone (10−6 mol/L), or 1.25(OH)2D3 (10−8 mol/L) for 48 hours. Corresponding culture supernatants (15 μL) were subjected to gelatin zymography.
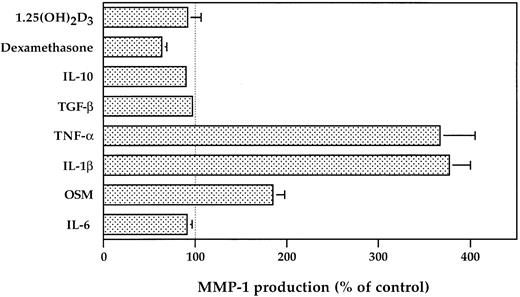
The BMSC secretion of MMP-1 is upregulated by OSM, IL-1β, and TNF-α and downregulated by dexamethasone.IL-6, the major growth factor in MM, has no effect on MMP-1 production, in contrast to OSM, another member of gp130 family cytokine, which has a promotive effect on MMP-1 production with a twofold increase (Fig 5). In addition, we observe that 10 ng/mL of IL-1β or TNF-α is equally potent in enhancing MMP-1 production, with a fourfold increase (Fig 5). A significant inhibitory effect (40% ± 5.5%, P = .01) was obtained by the addition of dexamethasone (10−6 mol/L), which is the steroid of reference used in the MM treatment. We noticed that TGF-β and 1.25(OH)2D3 had no effect on MMP-1 production.
Regulation of MMP-1 production by BMSC treated with cytokines or hormones. BMSC (1.5 × 104/100 μL) were untreated or exposed to IL-6 (10 ng/mL), OSM (10 ng/mL), IL-1β (10 ng/mL), TNF-α (10 ng/mL), TGF-β (10 ng/mL), IL-10 (10 ng/mL), dexamethasone (10−6 mol/L), or 1.25(OH)2D3 (10−8 mol/L) for 48 hours. The MMP-1 level was then measured in the corresponding culture supernatants by ELISA. Three different BMSC have been studied in duplicate, and the results are presented as a percentage of the control.
Regulation of MMP-1 production by BMSC treated with cytokines or hormones. BMSC (1.5 × 104/100 μL) were untreated or exposed to IL-6 (10 ng/mL), OSM (10 ng/mL), IL-1β (10 ng/mL), TNF-α (10 ng/mL), TGF-β (10 ng/mL), IL-10 (10 ng/mL), dexamethasone (10−6 mol/L), or 1.25(OH)2D3 (10−8 mol/L) for 48 hours. The MMP-1 level was then measured in the corresponding culture supernatants by ELISA. Three different BMSC have been studied in duplicate, and the results are presented as a percentage of the control.
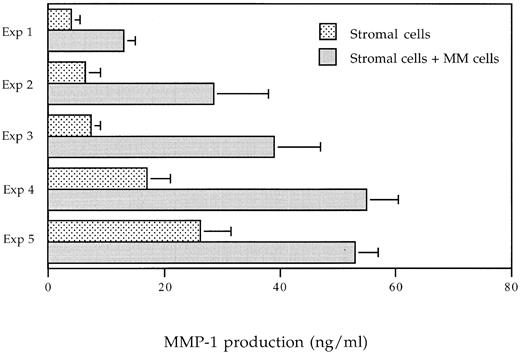
MMP-1 production of BMSC is upregulated in coculture system with myeloma cells.To investigate whether myeloma cells are able to modify the production of MMPs by BMSC, we have analyzed the release of MMP-1 either in monocultures of purified myeloma cells or stromal cells and in cocultures of both cells. BMSC have been allowed to adhere for 24 hours before purified myeloma cells were added, and then after 48 hours of culture, conditioned media were harvested and used for the MMP-1 determination. Ten different coculture experiments were performed and five of them are shown in Fig 6. In all experiments, myeloma cells are able to trigger a strong increase of the MMP-1 production. As shown in Fig 6, the stimulation factor ranges from 2 to 5.25. These results demonstrate the upregulation of MMP-1 production by BMSC in response to myeloma cells. These higher amounts of bioavailable MMP-1 lead to an increase of the capacity to degrade collagen I within the bone marrow.
MMP-1 production in coculture experiments. Stromal cells were seeded in 96-well plates (Costar) at a concentration of 1.5 × 104 cells/100 μL/well. Myeloma cells (1.25 × 105 cells/well) were added to stromal cells, and supernatants were harvested after 48 hours of coculture. MMP-1 level was evaluated by an ELISA. Five differents experiments are shown.
MMP-1 production in coculture experiments. Stromal cells were seeded in 96-well plates (Costar) at a concentration of 1.5 × 104 cells/100 μL/well. Myeloma cells (1.25 × 105 cells/well) were added to stromal cells, and supernatants were harvested after 48 hours of coculture. MMP-1 level was evaluated by an ELISA. Five differents experiments are shown.
ProMMP-2 secreted by BMSC is converted to its active form in coculture system with myeloma cells.We next investigated whether modifications in the production or activation of gelatinases occurred in our coculture system. Figure 7 shows gelatin zymography of conditioned media of purified myeloma cells alone, stromal cells alone, and coculture of both cells. Interestingly, in all of these experiments (n = 10), besides the gelatinolytic activity observed for each cells cultured alone, an additional band of 62 kD is detected. This clearly indicates that the proMMP-2 (72 kD) constitutively secreted by stromal cells is converted into active form of 62 kD. In addition, in some coculture experiments, the gelatinolytic activity at 92 kD is slightly increased in comparison to the one observed in tumor cells alone. These results show that myeloma cells are able, on one hand, to convert the proMMP-2 into the active form and, on the other hand, to induce activity of MMPs within the bone marrow.
Zymography analysis of coculture media. Stromal cells were seeded in 96-well plates at a concentration of 1.5 × 104 cells/100 μL/well. Myeloma cells (1.25 × 105 cells/well) were added to stromal cells, and supernatants were harvested after 48 hours of coculture. Lane 1, monoculture of BMSC; lane 2, coculture of BMSC and myeloma cells; lane 3, monoculture of myeloma cells. Three different experiments are shown.
Zymography analysis of coculture media. Stromal cells were seeded in 96-well plates at a concentration of 1.5 × 104 cells/100 μL/well. Myeloma cells (1.25 × 105 cells/well) were added to stromal cells, and supernatants were harvested after 48 hours of coculture. Lane 1, monoculture of BMSC; lane 2, coculture of BMSC and myeloma cells; lane 3, monoculture of myeloma cells. Three different experiments are shown.
DISCUSSION
In MM, although several cytokines have been involved in the excessive bone resorption and in tumor expansion, these complex processes remain incompletely elucidated.22-27,29,30 To further understand these processes, we have studied the involvement of MMPs in the biology of MM. MMPs have been shown to contribute to destructive process in different pathologies such as rheumatoid arthritis and osteoarthritis and to be involved in tumor progression.5 31 To our knowledge, our current study is the first one to document a role of MMPs in MM.
We have found that myeloma cells constitutively expressed MMP-9 and that this production is not subjected to regulation by the cytokines and hormones that are relevant to the biology and the treatment of MM. Similar constitutive expression of MMP-9 was observed in tonsil B-cell populations (unpublished data) and has been described in normal T lymphocytes,15 suggesting that MMP-9 expression in myeloma cells is not related to malignancy. Although quantitative differences in the pattern of MMP-9 expression from patient to patient have been observed, a larger number of patients should be necessary to accurately determine whether a correlation with the agressivity of the disease could be found. Whereas all primary myeloma cells express MMP-9, only one HMCL of six tested secretes a detectable level of MMP-9. The comparison of MMP-9 level produced by the SBN-1 cell line and by primary cells at the origin of SBN-1 suggests that MMP-9 expression decreased after cell culture passages. This observation finds a counterpart in studies showing that metastatic transformed rat embryo cells passaged in vitro released no detectable MMP-9, in contrast to tumor explants that expressed a detectable level of MMP-9.32 However, we cannot exclude the possibility that the decreased expression of MMP-9 reflects a selection of the subpopulation lacking MMP-9 secretion during immortalization process.
We have shown that BMSC from patients with MM constitutively expressed MMP-1 and MMP-2, this observation being in agreement with the documented production of MMP-1 and MMP-2 by fibroblasts and osteoblasts.33,34 Our present study shows that only the pattern of MMP-1 production was modulated by the cytokines involved in the biology of MM. Indeed, we have analyzed the effect of two myeloma cell growth factors (IL-6 and OSM) sharing the same transducer chain gp130. Interestingly, we noticed that OSM, in contrast to IL-6, stimulated the MMP-1 production. One possible explanation for this difference of regulation by IL-6 and OSM can be due to the absence of the IL-6–specific receptor (gp80) on BMSC. We confirm this hypothesis by showing that BMSC do not express gp80 (unpublished data). Moreover, IL-1β and TNF-α were equally potent in increasing MMP-1 production. The regulation induced by IL-1β and TNF-α on MMP-1 production and the lack of regulation by these cytokines on MMP-2 production are in agreement with what was reported for other cell types and with the specificities of the respective promoter of MMP-1 and MMP-2 genes. Indeed, whereas MMP-1 gene has a 12-O-tetradecanoyl phorbol 13-acetate (TPA)-responsive sequence that is a binding site for the nuclear factor AP-1; MMP-2 gene lacks this responsive element.35,36 We confirm that dexamethasone, a potent antitumoral agent in MM, induced a strong inhibition of MMP-1 production. These results are consistent with the notion that IL-1β and TNF-α upregulate and dexamethasone downregulates AP-1 activity that is the limiting factor in MMP-1 expression.36
In MM, it has been clearly established that myeloma cells interact with the bone marrow environmental cells and thereby modify their functions. In the present report, studies of MMP production in coculture experiments of myeloma cells with stromal cells provide evidence that myeloma cells are able to increase MMP-1 production and to induce MMP-2 activation in stromal cells. Secondarily, in some coculture experiments, a weak upregulation of MMP-9 is noticed. These data strongly suggest that an important increase of MMP activity occurs within the bone marrow in the vicinity of myeloma cells.
The amount of proMMP-1 appears critical for the final capacity to resorb matrix, because it was reported that, in MMP-1 transgenic mice, the synthesis of the latent form of collagenase is sufficient to cause extracellular matrix degradation.37 It is likely that the increase of the MMP-1 amount observed in our coculture experiments reflects an enhanced capacity to degrade collagen I. Moreover, it is interesting to note that the conversion of proMMP-2 into its active form will actively contribute to the final collagenolytic activity by achieving the degradation of collagen I initiated by MMP-1. It is now well documented that MMP-2 activity directly modulates tumor invasion.14 Therefore, we can hypothesize that the activation of MMP-2 favors the spreading of myeloma cells inside and outside the bone marrow. The mechanisms responsible for the upregulation of MMP activity remain to be elucidated. It will be especially important to investigate the balance between MMPs and TIMPs and to determine whether MT-MMP, which is known as one of the major activators of proMMP-2,8,38 is involved in our model. However, our preliminary data indicate that cellular contact between BMSC and MM cells is not required for the activation of MMP-2. These data are not in favor of the implication of the MT-MMP. However, its role cannot be totally excluded because a secreted form of MT-MMP has been described.39
In the present report, we have demonstrated the active role of bone marrow environment for the final MMP activity, confirming the importance of the interactions between tumor cells and environment as it was previously shown for the IL-6 production.16-18 Finally, the increased activity of MMP provides evidence that MMPs are involved in the biology of MM and is relevant to the major characteristics observed in vivo in patients with MM: the excess of bone resorption and the tumor spreading occurring at the terminal stage of the disease outside the bone marrow.
At the present time, our data showing production MMP-9, activation of MMP-2, and induction of MMP-1 by human myeloma cells are of major interest for the management of patients with MM, with the purpose being to limit bone resorption and tumor spreading. In fact, some inhibitors of MMPs are now available and two different types of pathologies have already been the target for the use of these MMP inhibitors: metastatic cancer and disease associated with articular cartilage and bone destruction. Several recent studies report the efficiency of inhibitors in animal models of arthritis leading to a dramatic suppression of cartilage and bone destruction.10 In cancer, an MMP inhibitor (Batimastat) has been shown to be effective in a variety of murine tumor metastatic models and is now used in human cancer clinical trials.40 41 Thus, our findings showing that MMP activity is enhanced in MM suggest that the inhibition of this activity could represent an interesting therapeutical approach in MM. We could expect that this therapeutical approach can target two major deleterious effects of MM: bone destruction and tumor spreading.
ACKNOWLEDGMENT
The authors thank M. Etrillard for technical assistance and C. Ville for secretarial assistance.
Supported by Grant No. 6285 from the Association pour la Recherche sur le Cancer. S.B. was supported by the Association pour la Recherche sur le Cancer.
Address reprint requests to Martine Amiot, PhD, INSERM U463-Oncogénèse Immunohématologique, Institut de Biologie, 9, quai Moncousu 44035 Nantes cedex 01, France.