Abstract
T-helper type-1 (Th1) and type-2 (Th2) cytokines, respectively, favor T-cell–mediated immunity and defense against intracellular pathogens or antibody-mediated immunity and defense against extracellular pathogens. Here we report that type-1 and type-2 cytokines also exert a regulatory effect on human monocyte survival. Interleukin-12 (IL-12) enhanced survival in long-term (10 days) cultures of adherent monocytes, whereas IL-10 induced death by apoptosis. In short-term cultures (2 days), the Th2 cytokines, IL-10 and IL-4, enhanced apoptosis; however, the Th1 cytokines, IL-12 and IL-2 only showed a reducing effect on monocyte apoptosis in culture conditions that decreased monocyte adhesion leading to increased levels of spontaneous apoptosis; finally, the Th1 cytokine, interferon-γ (IFN-γ), acted in a dose-dependent fashion: At high concentrations, IFN-γ enhanced apoptosis, which is an effect related to IL-10 secretion and reduced by antibodies to IL-10. Th1 cytokines reduced monocyte apoptosis induced by several stimuli: IL-2 reduced apoptosis induced by either IL-10 or high concentrations of IFN-γ, IL-12 reduced apoptosis induced by either the ligation of the Fas (CD95) molecule or γ-irradiation, and IFN-γ (at low doses that did not trigger apoptosis) reduced apoptosis induced by γ-irradiation. These findings suggest that the regulatory role of type-1 and type-2 cytokines on the development of immune responses and inflammatory reactions also involves the regulation of monocyte death by apoptosis.
THE PATTERN OF cytokines secreted by the immune system in response to an infectious pathogen represents a crucial event in determining the nature and outcome of the immune response, including the persistence or elimination of the microbe and the development or absence of inflammatory and immunopathological reactions.1-3 CD4+ T-helper cells differentiate into two main subsets, the T-helper type-1 (Th1) and T-helper type-2 (Th2) cells, which differ in their cytokine secretion pattern and in their effector functions.3-7 Th1 cells secrete interleukin-2 (IL-2), interferon-γ (IFN-γ), and lymphotoxin, whereas Th2 cells secrete IL-4, IL-5, IL-10, and IL-13. Th1 cells promote cell-mediated immunity, including delayed-type hypersensitivity reactions, recruitment and activation of inflammatory macrophages and leukocytes, and cytolytic responses, leading to protection against intracellular microbes; Th2 cells promote optimal antibody-mediated immunity, including the production of IgA and IgE isotypes, eosinophil, and mast cell activation, leading to protection against extracellular microbes; CD4+ T cells can also differentiate into Th0 cells, which secrete various mixtures of both Th1 and Th2 cytokines.3-7 Most of these cytokines can be produced by cells other than CD4+ Th cells, such as differentiated CD8+ T cells and activated accessory cells, including monocytes/macrophages, B cells, natural killer cells, basophils, and mast cells.4,5,8 Three cytokines produced by accessory cells play an essential role in the initiation of a Th1 or Th2 response: IL-12 by allowing the differentiation of naive CD4+ T cells into Th1 cells and the secretion of IFN-γ (which inhibits the differentiation of Th2 cells); IL-4 by allowing the differentiation of CD4+ T cells into Th2 cells; and IL-10 by inhibiting the differentiation of Th1 cells.3,5 9
Monocytes/macrophages are of central importance in the initiation, the development, and the outcome of the immune response.10,11 They play an important role in the presentation to CD4+ T cells of foreign antigens and activation cosignals, which initiate and maintain CD4+ T-cell activation; in the secretion of the IL-12 and/or IL-10 cytokines, which exert opposite effects on the differentiation of CD4+ Th1 cells; and in the effector phase of the immune response, in particular the Th1-mediated destruction of intracellular pathogens and inflammatory reaction.3,5,9-12 Monocytes/macrophages are also a target for type-1 and type-2 cytokines in the immune response.3,11,13 14 Type-1 cytokines, such as IFN-γ, exert strong activation effects on monocyte/macrophage functions, including enhanced major histocombatibility complex (MHC) expression, costimulatory molecule expression, respiratory burst activity, and microbicidal effector functions. In contrast, Th2 cytokines exert inhibitory effects on monocyte/macrophage function and inflammatory response, IL-4 by antagonizing the macrophage activation effect of IFN-γ and IL-10 by suppressing numerous monocyte/macrophage responses including MHC expression, respiratory burst, and cytokine secretion.
Monocytes, the precursor cells for tissue macrophages, have been shown to undergo rapid programmed death by apoptosis in cultures, unless signalled by bacterial products, such as lipopolysaccharides, or inflammatory cytokines, such as IL-1β or tumor necrosis factor, which enhance monocyte survival by reducing apoptosis induction.15,16 Programmed cell death, or apoptosis, is a physiological self-destruction mechanism regulated by signals provided by other cells, characterized by typical morphological features and leading to the removal of the dying cell by macrophages or neighboring cells in a way that prevents the onset of inflammatory reactions.17-22 We have previously reported that type-1 and type-2 cytokines may exert a regulatory role on apoptosis induction in CD4+ Th1 cells.23 24 Here, we have investigated whether the potent regulatory role of Th1 and Th2 cytokines on the development of the immune response and on monocyte/macrophage function may also involve a control on monocyte survival through regulation of programmed cell death or apoptosis.
MATERIAL AND METHODS
Reagents, antibodies, and cytokines.Murine antihuman monoclonal antibodies (MoAbs) used were the agonistic anti CD95 IgM MoAb (CH11), control IgM MoAb (GC323), phycoerythrin-labeled CD14 MoAb (RMO52), fluorescein isothiocyanate (FITC)-labeled HLA-DR MoAb (B8. 12.2), and FITC-labeled CD95 MoAb (UB2) (Immunotech, Marseille, France). Purified azide-free neutralizing rat antihuman IgG1 MoAbs were anti–IL-10 (JES3-9D7) and anti–IL-4 (MP4-25D2; PharMingen, San Diego, CA). Cytokines included recombinant human IL-2 (kindly provided by Roussel-Uclaf, Paris, France), IL-4, IL-10, IL-12 (R & D Systems, Abingdon, UK), and IFN-γ (PharMingen); IL-10 secretion was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Biosource, Camarillo, CA); other reagents were acridine orange dye (Immunotech), YO-PRO-1 dye (Molecular Probes, Inc, Eugene, OR), and Hoechst 33342 dye (Bisbenzimide; Sigma, La Verpillière, France).
Cells and culture conditions.Peripheral blood mononuclear cells were isolated from human heparinized venous blood (CRTS, Lille, France) by Ficoll-Hypaque density gradient centrifugation, and resuspended in RPMI 1640 (GIBCO, Courbevoie, France) supplemented with 20% heat-inactivated fetal calf serum (Boehringer Mannheim, Meylan, France), 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate (GIBCO), and gentamicin (Gentalline, 8 μg/mL; Schering-Plough, Levallois-Perret, France). Monocytes were isolated from peripheral blood mononuclear cells by adherence on culture flask, for 2 hours at 37°C, 5% CO2 . Nonadherent cells were removed by washing, and the adherent cells were cultured at 37°C overnight. After additional washes, the final adherent population contained at least 95% monocytes as determined by flow cytofluorimetry analysis (Epics Profile; Coulter Coultronics, Magency, France) using CD14 MoAbs. Monocytes were cultured in 6-well culture plates (Falcon; Becton Dickinson, Mountain View, CA) at 4 × 105/mL, or in poly–L-lysine (0.01%, Sigma) precoated 8-glass chamber slides (Lab-Tek; NUNC, Naperville, IL), at 1 × 105/mL, as indicated. In some experiments, monocytes (4 × 105/mL) in 6-well culture plates were also irradiated at 10 Gy (Philips RT, France) or treated with the agonistic Fas MoAb CH11 (1 μg/mL).
Apoptosis measurement.Apoptosis in vitro was measured by three different methods: (1) under a fluorescent microscope (Leica, Rueil Malmaison, France) after costaining with YOPRO-1 dye (which only labels apoptotic cells)25 and with Hoechst 33342 dye (bisBenzimide; which labels nuclei from both living and apoptotic cells)25,26; 200 cells were counted in each field, three fields were analyzed for each experimental condition, and percentages of apoptotic cells were the mean of results from analyses of the three fields; (2) by fluorescent microscopy analysis of in situ DNA fragmentation, using the terminal deoxytransferase (TdT)-mediated dUTP nick end labeling (TUNEL) technique,27 as previously described28; briefly, slides were washed once with TdT buffer (GIBCO) and then incubated with 0.5 nmol/L fluorescein-dUTP (Boehringer Mannheim), 5 U TdT (Promega), in 30 μL TdT buffer (Boehringer Mannheim); the slides were then incubated for 30 minutes at 37°C and then washed with phosphate buffered saline, the incorporation of fluorescent nucleotide (dUTP-FITC) at each end terminus of the strand break DNA allowed to visualize apoptotic cells; and finally (3) the percentage of apoptotic cells was quantitated by flow cytofluorimetry analysis after incubation with the acridine orange nuclear dye, as previously described,26 with apoptotic cells representing a characteristic distinct peak of reduced fluorescence intensity and forward scatter below the peak of living cells.
Percentages of cell death prevention, when so mentioned, were expressed as follows24,29: [(apoptosis in stimulated cells) − (apoptosis in stimulated cells with MoAbs or cytokines)/(apoptosis in stimulated cells) − (apoptosis in unstimulated cells)] × 100.
Statistical analysis.Statistical significance P was assessed using the paired Student's t-test.
RESULTS AND DISCUSSION
Opposite effect of IL-12 and IL-10 on long-term survival and differentiation of human monocytes.Freshly isolated peripheral blood human monocytes were cultured for 10 days on poly–L-lysine coated plates to promote monocyte adhesion and differentiation into macrophages. In the sole presence of 20% heat inactivated fetal calf serum, and in the absence of addition of any cytokine, a progressive decrease in the absolute number of surviving monocytes was observed leading to the disappearance of around 90% monocytes and to the differentiation of the 10% surviving monocytes into macrophages (Fig 1). Most of the dying cells were cleared from the cultures, which is consistent with the known role of monocytes/macrophages in the rapid ingestion and clearance of apoptotic cells.18,30 As also shown in Fig 1, addition of the Th1 cytokine IL-12 (400 ng/mL) at the onset of the culture led to an important increase in the absolute numbers of surviving monocytes (30% instead of 10% after 10-day cultures). Addition of the Th2 cytokine IL-10 (400 ng/mL) at the onset of the culture had an opposite effect on monocyte survival. After 1-day treatment, morphological features of apoptosis, such as cytoplasm hypervacuolization, began to be apparent in the monocyte cultures. After 10 days, no differentiation of monocytes into macrophages was detected, and all monocytes appeared as small round apoptotic cells with pyknotic nuclei.17-20 IL-10 also had an obvious preventive effect on the clearance of apoptotic cells, leading to their prolonged persistance in the culture.
Effect of IL-10 and IL-12 on long-term survival and differentiation of monocytes. Monocytes (1 × 105/mL) were cultured for 10 days in poly–L-lysine coated plates either in medium alone or in the presence of IL-10 (400 ng/mL) or IL-12 (400 ng/mL) added at the onset of the culture. Monocytes are shown after 1 day (A), 5 days (B), and 10 days (C) of culture. Monocytes were visualized using hematoxilin-eosin coloration (Original magnification × 350). These results are from one representative experiment out of three.
Effect of IL-10 and IL-12 on long-term survival and differentiation of monocytes. Monocytes (1 × 105/mL) were cultured for 10 days in poly–L-lysine coated plates either in medium alone or in the presence of IL-10 (400 ng/mL) or IL-12 (400 ng/mL) added at the onset of the culture. Monocytes are shown after 1 day (A), 5 days (B), and 10 days (C) of culture. Monocytes were visualized using hematoxilin-eosin coloration (Original magnification × 350). These results are from one representative experiment out of three.
Monocytes, the precursor cells for tissue macrophages, spontaneously undergo apoptosis unless they receive signals that allow their survival and/or differentiation into macrophages. Macrophages are long-lived cells that are resistant to several apoptosis-inducing stimuli, including γ-irradiation and other DNA damaging agents.31 Therefore, it is possible that signals that allow proper differentiation into macrophages play a major role in allowing monocyte survival. In this respect, it is interesting that IL-10 and IL-12, in addition to their effect on monocyte survival, also exerted an effect on monocyte differentiation into macrophages. IL-10 completely prevented the differentiation of monocytes (Fig 1), suggesting the possibility that this inhibitory effect on monocyte differentiation is the major trigger of apoptosis; however, another possibility is that apoptosis induction is the mechanism by which IL-10 prevents further differentiation of monocytes into macrophages. IL-12 also showed an effect on monocyte differentiation, reducing and/or delaying the differentiation of a large proportion of the surviving monocytes into characteristic macrophages (Fig 1). This apparent uncoupling of monocyte survival and differentiation raises interesting questions. One possibility is that IL-12 independently affects both monocyte differentiation and survival; another possibility is that the main effect exerted by IL-12 is the prevention of monocyte death, allowing the survival of monocytes that have not yet achieved their optimal differentiation into macrophages.
Together, these findings suggested that the effects of IL-12 and IL-10 on monocyte survival 10 days after addition of the cytokines were a consequence of a more rapid effect on the regulation of monocyte death by apoptosis. To further investigate this possibility, we explored whether Th1 and Th2 cytokines modulated apoptosis induction in short-term cultures (48 hours) of monocytes.
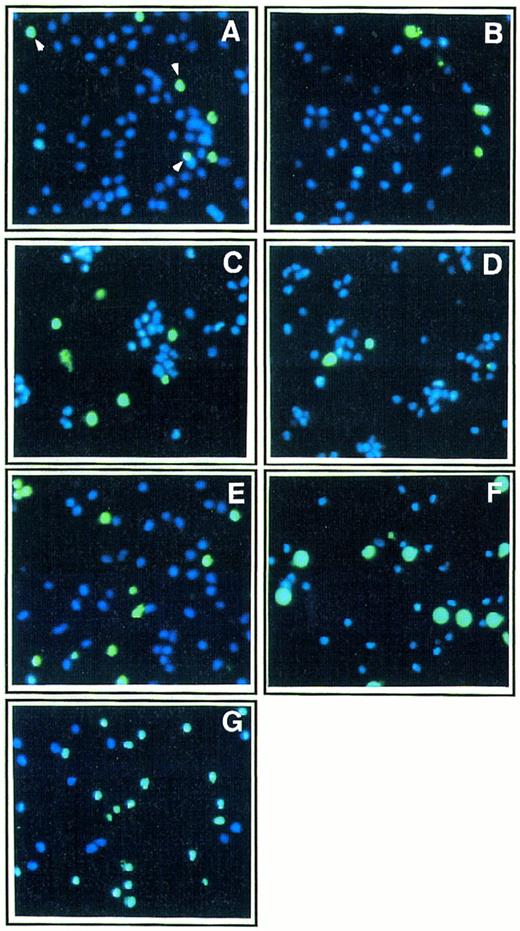
Effect of Th1 and Th2 cytokines on monocyte death by apoptosis.Apoptosis is characterized by typical morphological features including cell shrinkage, nuclear chromatin condensation and fragmentation, and DNA fragmentation.17,18,20,21 As shown in Figs 2 and 3, two different methods were used to explore nuclear changes typical of apoptosis by fluorescence microscopy analysis in short-term cultures of monocytes allowed to adhere on poly–L-lysine coated plates: (1) costaining with the nuclear dye YOPRO-1, which only labels (green fluorescence) cells undergoing apoptosis,25 and with the nuclear dye Hoechst 33342, which labels both living and apoptotic cells (blue fluorescence)26; (2) identification of DNA fragmentation in apoptotic cells using the TUNEL technique.27 In the absence of addition of any exogenous cytokines, the percentage of apoptotic cells in short-term (48 hours) cultures of monocytes in poly–L-lysine coated plates was 18% ± 3.5 (mean ± standard deviation [SD] from three independent experiments). The effect of three major Th1 cytokines, IL-12, IL-2, and IFN-γ, and of two major Th2 cytokines, IL-4 and IL-10, was investigated. As shown in Fig 2 and 3, Th2 cytokines enhanced monocyte apoptosis with IL-10 (400 ng/mL) inducing death in 38% cells, IL-4 (400 ng/mL) in 30% cells, and a mixture of IL-10 and IL-4 in 55% cells.
Effect of Th1 and Th2 cytokines on morphologic features of apoptosis in monocyte culture. Ultraviolet fluorescence microscopic analysis of monocytes (1 × 105 cells/mL) cultured for 48 hours in poly–L-lysine coated plates in the presence of medium alone (A) or either IL-2 (200 IU/mL; B), IFN-γ (10,000 IU/mL; C), IL-12 (400 ng/mL; D), IL-4 (400 ng/mL; E), IL-10 (400 ng/mL; F ), or a mixture of IL-4 (400 ng/mL) and IL-10 (400 ng/mL; G). After the 2-day culture, cells were treated with two fluorescent nuclear dyes, YOPRO-1 (10 μmol/L, for 5 minutes), which stains apoptotic cells in green, and Hoechst 33342, which stains in blue nuclei from both living and apoptotic cells (original magnification × 250); percentages of apoptotic cells were 18% (A), 17% (B), 32% (C), 16% (D), 30% (E), 38% (F ), and 55% (G). Two hundred cells were counted in each field, and results are mean from analyses of three fields for each experimental condition. These results are from one representative experiment out of three.
Effect of Th1 and Th2 cytokines on morphologic features of apoptosis in monocyte culture. Ultraviolet fluorescence microscopic analysis of monocytes (1 × 105 cells/mL) cultured for 48 hours in poly–L-lysine coated plates in the presence of medium alone (A) or either IL-2 (200 IU/mL; B), IFN-γ (10,000 IU/mL; C), IL-12 (400 ng/mL; D), IL-4 (400 ng/mL; E), IL-10 (400 ng/mL; F ), or a mixture of IL-4 (400 ng/mL) and IL-10 (400 ng/mL; G). After the 2-day culture, cells were treated with two fluorescent nuclear dyes, YOPRO-1 (10 μmol/L, for 5 minutes), which stains apoptotic cells in green, and Hoechst 33342, which stains in blue nuclei from both living and apoptotic cells (original magnification × 250); percentages of apoptotic cells were 18% (A), 17% (B), 32% (C), 16% (D), 30% (E), 38% (F ), and 55% (G). Two hundred cells were counted in each field, and results are mean from analyses of three fields for each experimental condition. These results are from one representative experiment out of three.
Effect of Th1 and Th2 cytokines on DNA fragmentation in monocyte cultures. Ultraviolet fluorescence microscopic analysis of monocytes (1 × 105 cells/mL) cultured for 48 hours in poly–L-lysine coated plates in the presence of medium alone (A) or either IL-2 (200 IU/mL; B), IFN-γ (10,000 IU/mL; C), IL-12 (400 ng/mL; D), IL-4 (400 ng/mL; E), IL-10 (400 ng/mL; F ), or a mixture of IL-4 (400 ng/mL) and IL-10 (400 ng/mL; G). After the 2-day culture, in situ DNA fragmentation was assessed by the TUNEL method using FITC-dUTP (see Material and Methods section). Strand break DNA in apoptotic cells were visualized in green (original magnification × 250). These results are from one representative experiment out of three.
Effect of Th1 and Th2 cytokines on DNA fragmentation in monocyte cultures. Ultraviolet fluorescence microscopic analysis of monocytes (1 × 105 cells/mL) cultured for 48 hours in poly–L-lysine coated plates in the presence of medium alone (A) or either IL-2 (200 IU/mL; B), IFN-γ (10,000 IU/mL; C), IL-12 (400 ng/mL; D), IL-4 (400 ng/mL; E), IL-10 (400 ng/mL; F ), or a mixture of IL-4 (400 ng/mL) and IL-10 (400 ng/mL; G). After the 2-day culture, in situ DNA fragmentation was assessed by the TUNEL method using FITC-dUTP (see Material and Methods section). Strand break DNA in apoptotic cells were visualized in green (original magnification × 250). These results are from one representative experiment out of three.
However, in these short-term cultures of adherent monocytes, the Th1 cytokine IL-12 (400 ng/mL) showed no detectable effect on apoptosis induction. The enhancing effect of IL-12 on monocyte survival in long-term cultures (10 days; Fig 1) was, therefore, not related to a detectable preventive effect on monocyte apoptosis induction during the first 2 days of the culture. The Th1 cytokine IL-2 (200 IU/mL) also showed no detectable effect on apoptosis induction in these 2-day cultures. The Th1 cytokine IFN-γ had a more complex, dose-dependent effect: At concentrations ranging from 100 to 1,000 IU/mL, IFN-γ showed no enhancing effect on apoptosis, whereas IFN-γ–mediated monocyte activation, as detected by enhanced expression of surface MHCs, was already observed at concentrations of 100 IU/mL (not shown); at high concentrations (10,000 IU/mL), IFN-γ enhanced apoptosis, which led to the death of 30% of the cells (Fig 2). In contrast to IL-10, which led in 5 days to induction of apoptosis in the whole monocyte population (Fig 1), IFN-γ at high concentrations showed a biphasic effect; after enhancing apoptosis, during the first 48 hours, to a similar extent as IL-10 (Fig 2), IFN-γ allowed prolonged monocyte survival as well as optimal differentiation of the surviving monocytes into macrophages (not shown).
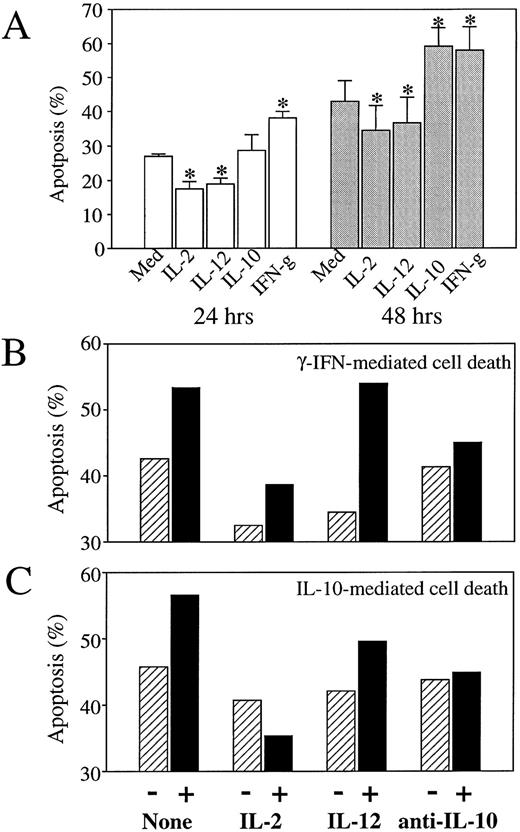
The effect of Th1 and Th2 cytokines on monocyte apoptosis induction was further investigated in short-term cultures (48 hours) in the absence of poly–L-lysine, a condition that decreases the adherence of monocytes and, therefore, reduces their further ability to differentiate into macrophages. In the absence of addition of any cytokine, these culture conditions led to an increase in monocyte death, consistent with the idea that adhesion signals participate in the prevention of apoptosis in adherent cell populations.32 As shown in Fig 4A, spontaneous levels of monocyte apoptosis at 48 hours were 42.9 ± 6.2 in the absence of poly–L-lysine (compared with 18 ± 3.5% in poly–L-lysine coated plates), and were already 27.1 ± 0.55 at 24 hours. In these nonadherent culture conditions, the Th2 cytokines IL-10 (Fig 4A and C) and IL-4 (not shown) and the Th1 cytokine IFN-γ (at the high concentration of 10,000 IU/mL; Fig 4A and B) conserved an enhancing effect on apoptosis induction, whereas the Th1 cytokines IL-12 and IL-2 showed a modest but reproducible and statistically significant reducing and/or delaying effect on spontaneous apoptosis induction (Fig 4A). As shown in Fig 4B and C, IL-2 also exerted a reducing effect on monocyte apoptosis induced by either IFN-γ or IL-10. As also shown in Fig 4B and C, the addition of neutralizing antibodies to IL-10, which suppressed IL-10–mediated enhancement of apoptosis, also reduced apoptosis induced by high concentrations of IFN-γ. A dose-dependent analysis of the effect of IFN-γ indicated that IFN-γ induced IL-10 secretion at concentrations (10,000 IU/mL) that enhance apoptosis, while inducing no IL-10 secretion at concentrations up to 1,000 IU/mL, which do not enhance apoptosis induction (Fig 5); low concentrations of 100 IU/mL even seemed to reduce spontaneous apoptosis, a finding consistent with previous reports.33 These findings suggest that the dose-dependent enhancing effect of IFN-γ on apoptosis induction during the first 48 hours of monocyte culture involves a process of autocrine release of IL-10 in response to IFN-γ.
Effect of IL-2, IL-12, and anti–IL-10 antibodies on IFN-γ and IL-10–mediated monocyte apoptosis. Monocytes (4 × 105 cells/mL) were cultured (in the absence of poly–L-lysine) in the absence or presence of various cytokines or anti–IL-10 neutralizing antibodies; percentages of apoptotic cells were assessed by flow cytofluorimetry analysis using the acridine orange nuclear dye. (A) Monocytes were cultured for 24 hours or 48 hours, as mentioned, in the presence of medium alone or of IL-2 (200 IU/mL), IL-12 (400 ng/mL), IL-10 (400 ng/mL), anti–IFN-γ (10,000 IU/mL). Histograms represent mean ± SD of three independent experiments. *P < .03. (B) Monocytes were cultured for 48 hours in the presence of medium alone (▨) or IFN-γ (10,000 IU/mL; ▪) in the absence (−) or presence (+) of IL-2 (200 IU/mL), IL-12 (400 ng/mL), or neutralizing antibodies to IL-10 (10 μg/mL). Results are from one representative experiment out of two. (C) Monocytes were cultured for 48 hours in the presence of medium alone (▨) or IL-10 (400 ng/mL) (▪) in the absence (−) or presence (+) of IL-2 (200 IU/mL), IL-12 (400 ng/mL), or neutralizing antibodies to IL-10 (10 μg/mL). Results are from one representative experiment out of two.
Effect of IL-2, IL-12, and anti–IL-10 antibodies on IFN-γ and IL-10–mediated monocyte apoptosis. Monocytes (4 × 105 cells/mL) were cultured (in the absence of poly–L-lysine) in the absence or presence of various cytokines or anti–IL-10 neutralizing antibodies; percentages of apoptotic cells were assessed by flow cytofluorimetry analysis using the acridine orange nuclear dye. (A) Monocytes were cultured for 24 hours or 48 hours, as mentioned, in the presence of medium alone or of IL-2 (200 IU/mL), IL-12 (400 ng/mL), IL-10 (400 ng/mL), anti–IFN-γ (10,000 IU/mL). Histograms represent mean ± SD of three independent experiments. *P < .03. (B) Monocytes were cultured for 48 hours in the presence of medium alone (▨) or IFN-γ (10,000 IU/mL; ▪) in the absence (−) or presence (+) of IL-2 (200 IU/mL), IL-12 (400 ng/mL), or neutralizing antibodies to IL-10 (10 μg/mL). Results are from one representative experiment out of two. (C) Monocytes were cultured for 48 hours in the presence of medium alone (▨) or IL-10 (400 ng/mL) (▪) in the absence (−) or presence (+) of IL-2 (200 IU/mL), IL-12 (400 ng/mL), or neutralizing antibodies to IL-10 (10 μg/mL). Results are from one representative experiment out of two.
Dose-dependent effect of IFN-γ on monocyte apoptosis and IL-10 secretion. Monocytes were cultured for 24 hours in the presence of medium alone or of various concentrations of IFN-γ. (A) Each histogram represents the percentage of apoptotic cells in response to a different concentration of IFN-γ assessed by flow cytofluorimetry analysis using the acridine orange nuclear dye. (B) Each circle represents the amount of IL-10 secreted in response to a different concentration of IFN-γ.
Dose-dependent effect of IFN-γ on monocyte apoptosis and IL-10 secretion. Monocytes were cultured for 24 hours in the presence of medium alone or of various concentrations of IFN-γ. (A) Each histogram represents the percentage of apoptotic cells in response to a different concentration of IFN-γ assessed by flow cytofluorimetry analysis using the acridine orange nuclear dye. (B) Each circle represents the amount of IL-10 secreted in response to a different concentration of IFN-γ.
Together, our findings suggest that a Th2 immune response leading to the combined secretion of IL-10 and IL-4, may result in enhanced monocyte death by apoptosis. A Th1 immune response leading to the combined secretion of IL-12, IL-2, and IFN-γ may, in contrast, result in enhanced monocyte survival through the combination of several mechanisms: a long lasting enhancing effect of IL-12 on monocyte survival; a modest reducing effect of IL-2 on spontaneous apoptosis induction; a reducing effect of IL-2 on apoptosis induced by high levels of IFN-γ secretion; and a reducing effect of IL-2 on apoptosis induced by IL-10, secreted by accessory cells such as B cells. These findings also suggest that a truncated Th1 immune response that leads to high levels of IFN-γ secretion in the absence of IL-2 (such as immune effector responses involving mainly activated CD8+ T cells) may result in a reduction in the number of monocytes that will achieve differentiation into activated macrophages. In particular, human immunodeficiency virus (HIV) infection, which leads to elevated levels of IFN-γ and IL-10 secretion with reduced IL-2 and IL-12 secretion,34 35 may provide an environment that may be as detrimental to the survival of monocytes as it is to the survival of CD4+ T cells.
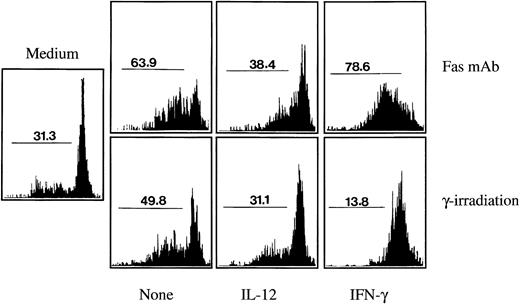
Effect of Th1 cytokines on Fas-mediated and/or γ-irradiation–induced monocyte apoptosis.We explored whether Th1 cytokines may exert a broader effect on monocyte apoptosis induction. In several cell populations, including monocytes, apoptosis can be induced by two different stimuli: the cross linking of the Fas (CD95) receptor molecule by cells expressing the Fas ligand, which can be mimicked using an agonistic Fas antibody; or DNA damage, which can be induced by γ-irradiation.22 36 As shown in Fig 6, in the absence of any death-inducing stimuli and of any cytokine addition, spontaneous monocyte apoptosis after 24 hours culture was around 30%. Antibody-mediated Fas crosslinking increased apoptosis to around 65% and γ-irradiation (10 Gy) to around 50%. As also shown in Fig 6, pretreatment during 1 hour with IL-12 almost completely reduced γ-irradiation and Fas-induced apoptosis to the background levels. Flow cytofluorometry analysis indicated that IL-12 had no effect during 24 hours on the expression of the Fas molecule on the monocyte cell surface (not shown). IFN-γ, at low concentrations that did not induce apoptosis (100 UI/mL), completely prevented apoptosis induction by γ-irradiation, reducing the level of monocyte apoptosis below the background level. However, the effect of IFN-γ on Fas-mediated apoptosis was inconsistent: In cultures of blood-derived monocytes from five different individuals, IFN-γ showed either an increasing effect on Fas-mediated apoptosis in three cases (32.5% mean increase in apoptosis induction) or a reducing effect in two cases (45.2% mean reduction in apoptosis induction).
Effect of Th1 cytokines on monocyte apoptosis induced by Fas crosslinking or by γ-irradiation. Monocytes (4 × 105 cells/mL) were cultured for 24 hours (in the absence of poly–L-lysine) either in the absence of any stimuli (medium), in the presence of an agonistic antibody to Fas (2 μg/mL), or after γ-irradiation (10 Gy). Addition of the Fas MoAb or γ-irradiation was performed either on monocytes incubated with medium alone (None) or on monocytes that had been preincubated for 1 hour with IL-12 (400 ng/mL) or with IFN-γ (100 IU/mL). Percentages of apoptotic cells were assessed by cytofluorimetry analysis using the nuclear dye acridine orange. These results are representative of three independent experiments, except for the effect of IFN-γ on Fas MoAb-induced apoptosis, which was found to vary (either enhancing or reducing apoptosis) in five different experiments (see Results and Discussion section).
Effect of Th1 cytokines on monocyte apoptosis induced by Fas crosslinking or by γ-irradiation. Monocytes (4 × 105 cells/mL) were cultured for 24 hours (in the absence of poly–L-lysine) either in the absence of any stimuli (medium), in the presence of an agonistic antibody to Fas (2 μg/mL), or after γ-irradiation (10 Gy). Addition of the Fas MoAb or γ-irradiation was performed either on monocytes incubated with medium alone (None) or on monocytes that had been preincubated for 1 hour with IL-12 (400 ng/mL) or with IFN-γ (100 IU/mL). Percentages of apoptotic cells were assessed by cytofluorimetry analysis using the nuclear dye acridine orange. These results are representative of three independent experiments, except for the effect of IFN-γ on Fas MoAb-induced apoptosis, which was found to vary (either enhancing or reducing apoptosis) in five different experiments (see Results and Discussion section).
Finally, investigation of monocyte cultures during several days after γ-irradiation suggested that pretreatment with IL-12 had a delaying rather than a preventing effect on apoptosis induction, whereas pretreatment with IFN-γ exerted a long-time preventive effect (not shown).
These findings suggest that IL-12 and/or IFN-γ may increase monocyte survival in the context of the immune or inflammatory tissues in which the monocyte will be attracted to initiate an immune response or to exert an effector function. In particular, Th1 CD4+ T cells have been shown to express the Fas ligand37 and to be able to delete monocyte/macrophages through a Fas-mediated mechanism.38 We have previously reported that IL-12 reduces Fas-mediated apoptosis in CD4+ Th1 cells from HIV-infected persons.24,29 The findings presented here suggest that IL-12 may exert a broader suppressive effect on apoptosis induction by acting on monocytes and CD4+ T cells, allowing an optimal interaction between these cell populations during the development of a Th1 response. The reducing effect of IL-12 and IFN-γ on monocyte apoptosis induced by γ-irradiation may reflect a general mechanism of resistance to DNA damage that could be relevant to inflammation because inflammatory reactions, a hallmark of Th1 effector responses and defense mechanisms,3 lead to the release of reactive oxygen species, which can induce DNA damage.39 The fact that IFN-γ showed a longer lasting preventive effect than IL-12 on monocyte apoptosis induced by γ-irradiation may be because of the fact that IFN-γ leads to monocyte differentiation into macrophages, whereas IL-12 seems to delay such differentiation. Consistent with this possibility, macrophage-colony stimulating factor, another potent differentiation factor of monocytes into macrophages, has been previously reported to allow long-lasting survival of monocytes in response to γ-irradiation.40
Together, our findings suggest that the regulatory role of type-1 and type-2 cytokines on the development and outcome of the immune response may involve a differential control of monocyte survival. In particular, two cytokines that exert a very early role in the orientation of a Th1 or Th2 response, IL-10 and IL-12, appear to exert opposite effects: IL-12 by increasing monocyte survival and IL-10 by enhancing monocyte death by apoptosis. These effects are reminiscent of the opposite effects of IL-12 and IL-10 that we have previously reported on the regulation of CD4+ Th1-cell apoptosis.23,24 29
The molecular mechanisms by which type-1 and -2 cytokines may regulate monocyte and CD4+ T-cell survival and death by apoptosis remain to be investigated. Potential candidates include the phosphatidylinositol-3 kinase,41 the Janus kinases associated with cytokine receptors, and the signal transducers and activators of transcription.42 43 Our preliminary results suggest that Th1 and Th2 cytokines do not act on monocytes by changing the expression of the Bcl-2 survival gene product, but may affect the expression of the p53 protein (data not shown).
The existence of an enhancing effect of type-2 cytokine on monocyte death by apoptosis may provide one of the mechanisms by which type-2 cytokines, and in particular IL-10, exert their anti-inflammatory activities.3,6,7 Type-1 cytokines induce the recruitment of monocytes and their differentiation into macrophages leading to inflammatory reactions, such as delayed type hypersensititvity, that are effective in eliminating intracellular microbes but can also lead to pathological conditions in the form of granulomatous inflammation.3,6,7 Our finding that type-1 cytokines enhance monocyte survival and resistance to apoptosis induced by several stimuli may provide one of the mechanisms by which type-1 cytokines optimize the recruitment and activation of monocytes that will allow the development of the effector phase of the Th1-cell–mediated response. However, once monocytes are fully differentiated into macrophages the nature of the factors required for further suppression or induction of death by apoptosis may change in a developmentally regulated fashion.33
Assessing to what extent type-1 and -2 cytokines may exert their potent role on the orientation of the immune response through a regulatory effect on the death and survival of several immune cell populations may have important implications for the understanding of the immunopathology of infectious and inflammatory diseases as well as for the design of cytokine-based immunotherapeutic approaches.
ACKNOWLEDGMENT
We thank T. Brunner for discussing the manuscript.
Supported by INSERM; Institute Pasteur de Lille; Lille II University Medical School; grants from Agence Nationale de Recherche sur le Sida (ANRS) and Fondation pour la Recherche Médicale to J.C.A.; and an ANRS Fellowship to J.E.
Address reprint requests to J. Estaquier, PhD, INSERM U13, Groupe Hospitalier Bichat-Claude Bernard, 170 Bd Ney, 75877 Paris cedex 18, France.