Abstract
Contact with bone marrow stromal cells is crucial for the normal growth and development of B-cell precursors. We have previously shown that human bone marrow stromal cell tyrosine kinase activity can be activated by direct contact with B-lymphoid cells (J Immunol 155:2359, 1995). In the present study, we show that increased tyrosine phosphorylation of focal adhesion kinase, paxillin, and extracellular-related kinase 2 (or p42 MAP kinase) accounted for the major changes occurring in stromal cell tyrosine phosphorylation after 5 to 10 minutes of contact with the RAMOS B-lymphoma cell line. Although adhesion of B-cell precursors to stromal cells is primarily mediated by very late antigen-4 (VLA-4) and vascular cell adhesion molecule-1 (VCAM-1), VLA-4–deficient and adhesion-deficient RAMOS cells were equally capable of stimulating stromal cell tyrosine phosphorylation. Similar changes in the tyrosine phosphorylation pattern of stromal cells were induced by contact with normal human B-cell precursors and several other B-lineage cell lines. After 5 to 30 minutes of contact with stromal cells, no change in protein tyrosine phosphorylation was detected in RAMOS or normal human B-cell precursors removed from stromal cells. Pretreatment of stromal cells with cytochalasin D abrogated contact-mediated enhancement of stromal cell tyrosine phosphorylation, suggesting that an intact cytoskeleton was essential. These results suggest that B-cell contact activates stromal cell signaling cascades that regulate cytoskeletal organization and transcription, independent of the interaction mediated by VLA-4 and VCAM-1.
THE BONE MARROW microenvironment is a complex milieu of stem cells, developing progenitors of the lymphoid, myeloid, and erythroid lineages, and interstitial cells such as endothelial cells, reticular cells, fibroblasts, and adipocytes.1 In the growth and development of B-cell precursors, interaction with fibroblast-like stromal cells is particularly critical, and B-cell precursors and stromal cells are intimately associated in vivo.2 A variety of culture conditions have been shown to support the survival and limited growth of human B-cell precursors,3-12 and physical contact between B-cell precursors and bone marrow-derived stromal cells was essential for growth of B-cell precursors in most studies. However, the mechanism by which contact facilitates survival and growth of B-cell precursors is not completely understood.
Three different mechanisms for support of B-cell precursors by stromal cells are possible. First, bone marrow stromal cells produce cytokines that potentially affect B-cell growth and development.1 However, the necessity of contact between B-cell precursors and stromal cells indicates that either the critical cytokines are produced in such minute quantities that close proximity of the two cell types is necessary or that the critical cytokines are presented to the B-cell precursors in membrane-anchored form. Second, human B-cell precursors and stromal cells are known to adhere primarily through the interaction of very late antigen-4 (VLA-4; or CD49d/CD29) and vascular cell adhesion molecule-1 (VCAM-1; or CD106) on their respective surfaces.13,14 Cell signaling associated with VLA-4 engagement has been reported for many cell types, including B cells,15-17 and it is possible that signals transduced by B-cell VLA-4 via stromal cell VCAM-1 or the CS-1 domain of fibronectin are a critical factor in B-cell precursor growth and development. Third, it is possible that contact between B-cell precursors and stromal cells influences the function of the stromal cells, enhancing their production of soluble mediators or membrane-expressed molecules that aid in support of B-cell growth and development.
Consistent with this third possibility, we have previously shown that contact with the B-lymphoma cell line RAMOS stimulates stromal cell tyrosine kinase activity and causes enhanced stromal cell production of interleukin-6 (IL-6).18 A CD49d-deficient, and thus VLA-4–deficient, variant of the RAMOS cell line (RAMOS-4c) was similar to wild-type RAMOS in its ability to stimulate stromal cells, indicating that this stimulation is independent of engagement of stromal cell VCAM-1 by RAMOS VLA-4. The identity of the tyrosine phosphorylated substrates, and hence the signaling pathway(s) activated, were not delineated in our prior study.18 We now show that contact with wild-type or VLA-4–deficient RAMOS cells or with normal human B-cell precursors enhances tyrosine phosphorylation of stromal cell focal adhesion kinase (FAK), paxillin, and the mitogen-activated protein (MAP) kinase extracellular response kinase 2 (ERK2). Because RAMOS-4c cells are deficient in adhesion to stromal cells, this enhancement of stromal cell FAK, paxillin, and ERK2 tyrosine phosphorylation after contact is independent of VLA integrin-mediated adhesion.
MATERIALS AND METHODS
Cells.Human bone marrow B-cell precursors and stromal cells were isolated as previously described.19,20 Briefly, human adult bone marrow mononuclear cells from Ficoll-Hypaque interfaces were allowed to adhere to flasks, from which stromal cells were propagated in Ex-Cell 300 medium (JRH Bioscience, Lenexa, KS) supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, and 10% fetal calf serum. Stromal cells were grown to confluence, passaged twice, and then maintained in Ex-Cell 300 without serum for 1 day to 2 weeks before use in signalling experiments. B-cell precursors were isolated from fetal bone marrow nonadherent mononuclear cells by magnetic bead depletion of T cells, myeloid, macrophage, erythroid, and surface IgM+ B cells using monoclonal antibody (MoAb) against CD2, CD33, CD11b, glycophorin A, and IgM, respectively. Resulting cells were 95% CD19+ and approximately 85% surface IgM−. The use of fresh human tissue for the experiments described herein was approved by the University of Minnesota Institutional Review Board Human Subjects Committee. The surface IgM+/IgD−/λ+ RAMOS Burkitt's lymphoma cell line was obtained from Dr Brian Van Ness (Institute of Human Genetics, University of Minnesota, Minneapolis, MN). RAMOS and the B-cell precursor acute lymphoblastic leukemia (ALL) cell line KM-3 were propagated in RPMI 1640 containing 50 U/mL penicillin, 50 μg/mL streptomycin, and 5% fetal calf serum. BLIN-3 and BLIN-4 are CD19+, cytoplasmic μ− ALL cell lines that were established in our laboratory from the bone marrows of two patients with ALL (N. Shah and T.W.L, unpublished data). BLIN-3 and BLIN-4 have been in continuous culture in X-VIVO 10 serum-free medium for 5 months and require bone marrow stromal cells and supplementation with 10 ng/mL IL-7 for growth. Isolation and characterization of the CD49d-deficient (VLA-4−) RAMOS-4c variant has been described elsewhere.18
Antibodies and reagents.Antiphosphotyrosine (4G10), antipaxillin (MAB3060), rabbit antimouse IgG (6170-01), and purified mouse IgG1 (MOPC21) were purchased from Upstate Biotechnology Inc (Lake Placid, NY), Chemicon International, Inc (Temecula, CA), Southern Biotechnology (Birmingham, AL), and Organon Teknika Corp (Westchester, PA), respectively. Rabbit antibodies recognizing human ERK2 (C-14) and FAK (C-903) were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Rabbit anti-FAK (hFAK1), raised against a peptide corresponding to amino acids 556-575 of human brain FAK (or amino acids 708-727 of human T-cell FAK) has been previously characterized.21 Anti–β-tubulin (E7) MoAb was obtained and produced as described elsewhere.22 Horseradish peroxidase-linked donkey antirabbit and sheep antimouse Ig were purchased from Amersham Life Science (Arlington Heights, IL). GST-6His-Elk1(307-428) fusion protein23 bound to glutathione sepharose was the generous gift of Drs Wei Li and Dan Mueller (Department of Medicine, University of Minnesota), with permission from Dr Robert Hipskind (Institut de Genetique Moleculaire, Montpellier, France). All other reagents were purchased from Sigma Chemical Co (St Louis, MO), unless otherwise noted.
Protein kinase assays.Evaluation of stromal cell and B-cell tyrosine phosphorylation was a modification of the protocol previously described.18 Where indicated, stromal cells were pretreated for 30 minutes with 10 nmol/L cytochalasin D at 37°C in serum-free medium. RAMOS cells (5 × 106/mL) or B-cell precursors (7 × 106/mL) were washed once, equilibrated to 37°C, and incubated on stromal cell monolayers for 1 to 30 minutes in a 37°C incubator. The medium containing B cells that had not settled onto stromal cells was aspirated and discarded. B cells adherent to or in contact with stromal cells were then washed off the stromal cells in 1 to 2 mL phosphate-buffered saline (PBS) and collected for independent analysis. Stromal cells were washed an additional three to four times with a 10-second stream of cold PBS delivered from a 500-mL wash bottle until B cells were not detected by light microscopy. Stromal cells incubated in medium lacking B cells were washed using the same procedure. This washing procedure did not visibly disrupt the adherent stromal cell layer. Cells were lysed with freshly prepared ice-cold lysis buffer containing 1% NP40, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 100 μmol/L sodium molybdate, 1 mmol/L Na3VO4 , 2 mmol/L phenylmethylsulfonyl fluoride, 1% aprotinin, 10 μg/mL leupeptin, 10 mmol/L iodoacetamide, 25 μg/mL p-nitrophenyl guanidinobenzoate, and 50 mmol/L Tris-Cl, pH 7.5 (lysis buffer). After 15 to 30 minutes of solubilization, stromal cells were scraped off the dish and collected. All samples were vortexed 15 to 30 minutes and centrifuged for 15 minutes at 14,000g. Supernatants were assayed for protein content by the bicinchonic acid method using bovine serum albumin as a standard. If immunoprecipitation was not performed, lysates were boiled in sodium dodecyl sulfate (SDS) sample buffer (10% glycerol, 1% SDS, 50 mmol/L 2-mercaptoethanol, 1 mmol/L sodium orthovanadate, 50 mmol/L Tris-Cl, pH 6.8) for 10 minutes.
For immunoprecipitations, supernatants were recentrifuged for 15 to 30 minutes at 14,000g before protein assay. Immunoprecipitations were performed by precoating protein A sepharose with primary antibody and then mixing 50 to 100 μL of a washed 1:1 slurry of antibody-protein A sepharose with lysate overnight at 4°C. For immunoprecipitation with antipaxillin, protein A sepharose was precoated with rabbit antimouse IgG before the addition of primary antibody. All immunoprecipitates were washed four times with lysis buffer and eluted by boiling for 10 minutes in 3% SDS, 715 mmol/L 2-mercaptoethanol, 15% glycerol, 0.01% bromophenol blue, and 80 mmol/L Tris-Cl, pH 6.8.
Proteins were separated by SDS-polyacrylamide gel electrophoresis on 10% to 11% acrylamide gels, except for ERK2 immunoprecipitates, which were electrophoresed using a modified gel and buffer recipe designed to maximize resolution between 40 and 50 kD.24 Gels were electrophoretically transferred to nitrocellulose; blocked in 50 mmol/L Tris-Cl, 150 mmol/L NaCl, 0.1% Tween-20, and 2% bovine serum albumin (pH 7.4) for 2 hours to overnight; and then immunoblotted using enhanced chemiluminescence (Amersham Life Science) for detection. Where indicated, blots were stripped by incubation in 2% SDS, 100 mmol/L 2-mercaptoethanol, and 62.5 mmol/L Tris-Cl, pH 6.7, at 50°C for 30 minutes, reblocked, and reimmunostained.
Solid-phase kinase assays were performed essentially as described by Hibi et al25 for cJun. Cells were lysed in 0.1% Triton X-100, 0.3 mol/L NaCl, 1.5 mmol/L MgCl2 , 0.2 mmol/L EDTA, 0.5 mmol/L dithiothreitol (DTT), 0.1 mmol/L Na3VO4 , 20 mmol/L β-glycerophosphate, 10 μg/mL leupeptin, 1% aprotinin, 25 μg/mL p-nitrophenyl guanidinobenzoate, and 25 mmol/L HEPES, pH 7.6, for 30 minutes and centrifuged at 14,000g for 10 minutes. Supernatants were assayed for protein using Coomassie reagent (Pierce, Rockford, IL) and adjusted to 0.05% Triton X-100, 75 mmol/L NaCl, 2.5 mmol/L MgCl2 , 0.1 mmol/L EDTA, and 20 mmol/L HEPES, pH 7.6, without changing the concentration of the remaining constituents (binding buffer). Elk1-GST fusion protein bound to glutathione sepharose (15 μL/sample) and cell lysate (35 μg/sample) were mixed on a rotator overnight at 4°C, washed four times with binding buffer, and incubated for 15 minutes at 37°C in 20 mmol/L HEPES (pH 7.6), 20 mmol/L MgCl2 , 2 mmol/L DTT, 0.1 mmol/L Na3VO4 , 20 mmol/L β-glycerophosphate, 20 μmol/L ATP, and 5 μCi (γ32P)ATP/sample in a volume of 30 μL/sample to allow phosphorylation of Elk1 by bound protein kinases. The kinase reaction was stopped by washing beads three times with binding buffer. Proteins were eluted by boiling for 5 minutes in 3% SDS, 715 mmol/L 2-mercaptoethanol, 15% glycerol, 0.01% bromophenol blue, and 80 mmol/L Tris-Cl (pH 6.8) and electrophoresed through 10% SDS gels. Gels were stained with Coomassie Blue to verify equal amounts of Elk1 in each lane and then dried and autoradiographed.
RESULTS
Enhancement of stromal cell tyrosine phosphorylation by RAMOS cell contact.Our previous data indicated that contact with the B-lymphoma cell line RAMOS enhanced tyrosine phosphorylation of multiple stromal cell proteins and resulted in enhanced secretion of stromal cell-derived IL-6.18 To gain additional insight into the stromal cell signaling pathways activated by B-cell contact, we sought to identify the stromal cell proteins showing consistent increases in tyrosine phosphorylation.
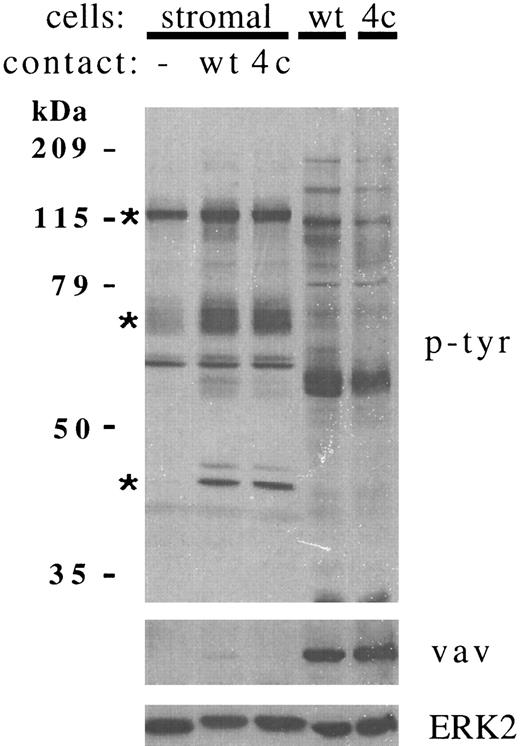
We previously reported that stromal cell proteins of 110 kD and 64-70 kD were constitutively tyrosine phosphorylated, but that phosphorylation was enhanced by contact with RAMOS cells. In further experiments, the larger protein was more accurately estimated at 120 kD. These two proteins, plus a 42-kD protein (which was phosphorylated de novo in most experiments), accounted for the most prominent and reproducible increases in stromal cell tyrosine phosphorylation after RAMOS cell contact (Fig 1, upper panel). These proteins were clearly of stromal cell origin, because tyrosine phosphorylated proteins of similar size were not detected in lysates of the RAMOS cells that had been washed from stromal cells (last 2 lanes). The leukocyte-specific oncoprotein vav26 was readily detected in RAMOS cell lysates but was absent from stromal cells. Detection of vav was therefore a sensitive method used to monitor the presence of residual RAMOS cell proteins in stromal cell lysates. Figure 1 (lower panel) shows the very faint detection of vav in stromal cell lysates after contact with RAMOS-wt, but not RAMOS-4c.
Contact with RAMOS B cells increases tyrosine phosphorylation of multiple bone marrow stromal cell proteins. Stromal cells were incubated in medium alone (−) or in contact with RAMOS-wt (wt) or RAMOS-4c (4c) cells for 5 minutes at 37°C. Cells not in contact were aspirated and discarded. Stromal cells and RAMOS cells were then collected separately, lysed, and electrophoresed as detailed in the Materials and Methods. Twenty-five micrograms of stromal cell or RAMOS protein were electrophoresed in each lane. (Upper panel) Antiphosphotyrosine immunoblotting. (Middle panel) Anti-vav immunoblotting. (Lower panel) Anti-ERK2 immunoblotting. Asterisks indicate the major stromal cell proteins demonstrating consistent increases in tyrosine phosphorylation in greater than 20 individual experiments.
Contact with RAMOS B cells increases tyrosine phosphorylation of multiple bone marrow stromal cell proteins. Stromal cells were incubated in medium alone (−) or in contact with RAMOS-wt (wt) or RAMOS-4c (4c) cells for 5 minutes at 37°C. Cells not in contact were aspirated and discarded. Stromal cells and RAMOS cells were then collected separately, lysed, and electrophoresed as detailed in the Materials and Methods. Twenty-five micrograms of stromal cell or RAMOS protein were electrophoresed in each lane. (Upper panel) Antiphosphotyrosine immunoblotting. (Middle panel) Anti-vav immunoblotting. (Lower panel) Anti-ERK2 immunoblotting. Asterisks indicate the major stromal cell proteins demonstrating consistent increases in tyrosine phosphorylation in greater than 20 individual experiments.
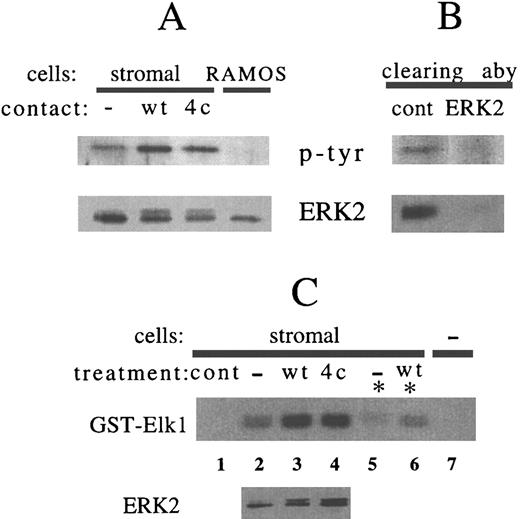
Stromal cell ERK2 is tyrosine phosphorylated after RAMOS cell contact.When stromal cell lysates were immunoblotted for the 42-kD protein ERK2, a change in mobility of stromal cell ERK2 could be detected after RAMOS cell contact (Fig 1, lower panel). Immunoprecipitation of ERK2 also showed that contact with either RAMOS-wt or RAMOS-4c increased tyrosine phosphorylation and slowed the electrophoretic migration of stromal cell ERK2 (Fig 2A). Slower ERK2 mobility correlates with dual phosphorylation on serine and tyrosine, which is necessary for kinase activation.27 28 In most experiments, tyrosine phosphorylated or gel-shifted ERK2 was detected only after contact with RAMOS (Fig 1, lower panel). In some experiments, a portion of ERK2 was constitutively gel-shifted, and this portion was increased after contact with RAMOS (Fig 2A). In a few experiments (3 of 25), a large proportion of stromal cell ERK2 was constitutively phosphorylated and shifted before B-cell contact. In striking contrast, RAMOS cell ERK2 existed entirely as the faster-migrating, dephosphorylated, and inactive form, and this was not altered by contact with stromal cells under these experimental conditions (Fig 2A). Immunoprecipitation with anti-ERK2, but not with a control antibody, removed both ERK2 and the 43-kD tyrosine phosphorylated protein from stromal cell lysate (Fig 2B).
Contact with RAMOS-wt or RAMOS-4c increases phosphorylation of stromal cell ERK2. Stromal cells were incubated in medium alone (−) or in contact with RAMOS-wt (wt) or RAMOS-4c (4c) for 5 minutes at 37°C. Stromal cells and RAMOS cells were collected separately and lysed as detailed in the Materials and Methods. (A and B) Lysates were incubated with antibody-coated protein A sepharose overnight at 4°C. Sepharose beads were sedimented by centrifugation for 10 seconds. The supernatant lysate was removed from selected samples and then immunoprecipitates were washed, eluted, and electrophoresed. (A) Anti-ERK2 immunoprecipitates. RAMOS represents immunoprecipitation of a pool of equal amounts of RAMOS-wt and RAMOS-4c lysate. (B) Supernatant lysates removed from rabbit antimouse IgG (cont) or anti-ERK2 immunoprecipitations of stromal cells after RAMOS-wt contact. (Upper panels) Antiphosphotyrosine immunoblotting. (Lower panels) Anti-ERK2 immunoblotting. (C) Lane 1 (cont) represents control glutathione sepharose (lacking GST-Elk1) incubated with stromal cell lysate. Lanes 2 through 4 represent lysate from stromal cells, stromal cells in contact with RAMOS-wt, and stromal cells in contact with RAMOS-4c subjected to solid-phase kinase assay using GST-Elk1 as a substrate (upper panel) or immunostained with anti-ERK2 (lower panel). For lanes 5 and 6, lysates were precleared by immunoprecipitation with anti-ERK2 (*) before incubation with GST-Elk-1-sepharose. Lane 7 represents Elk1-GST sepharose not incubated with lysate. Autoradiography of γ32P-GST-Elk1 represents 10 minutes of exposure. Results are representative of three (A and B) and two (C) individual experiments.
Contact with RAMOS-wt or RAMOS-4c increases phosphorylation of stromal cell ERK2. Stromal cells were incubated in medium alone (−) or in contact with RAMOS-wt (wt) or RAMOS-4c (4c) for 5 minutes at 37°C. Stromal cells and RAMOS cells were collected separately and lysed as detailed in the Materials and Methods. (A and B) Lysates were incubated with antibody-coated protein A sepharose overnight at 4°C. Sepharose beads were sedimented by centrifugation for 10 seconds. The supernatant lysate was removed from selected samples and then immunoprecipitates were washed, eluted, and electrophoresed. (A) Anti-ERK2 immunoprecipitates. RAMOS represents immunoprecipitation of a pool of equal amounts of RAMOS-wt and RAMOS-4c lysate. (B) Supernatant lysates removed from rabbit antimouse IgG (cont) or anti-ERK2 immunoprecipitations of stromal cells after RAMOS-wt contact. (Upper panels) Antiphosphotyrosine immunoblotting. (Lower panels) Anti-ERK2 immunoblotting. (C) Lane 1 (cont) represents control glutathione sepharose (lacking GST-Elk1) incubated with stromal cell lysate. Lanes 2 through 4 represent lysate from stromal cells, stromal cells in contact with RAMOS-wt, and stromal cells in contact with RAMOS-4c subjected to solid-phase kinase assay using GST-Elk1 as a substrate (upper panel) or immunostained with anti-ERK2 (lower panel). For lanes 5 and 6, lysates were precleared by immunoprecipitation with anti-ERK2 (*) before incubation with GST-Elk-1-sepharose. Lane 7 represents Elk1-GST sepharose not incubated with lysate. Autoradiography of γ32P-GST-Elk1 represents 10 minutes of exposure. Results are representative of three (A and B) and two (C) individual experiments.
The transcription factor Elk1 is a substrate of ERK2.23 GST fusion proteins containing amino acids 307-428 of Elk1 associate with ERK2, allowing isolation of the complex on glutathione sepharose beads. Activity of the associated ERK2 can then be measured in a solid-phase kinase assay in which GST-Elk1 is the substrate. Elk1-associated kinase activity in stromal cells was increased after RAMOS cell contact, and this correlated with the presence of slower-migrating ERK2 in the lysate (Fig 2C, compare upper and lower panels in lanes 2, 3, and 4). Although stress-activated protein kinase (JNK) is also able to associate with and phosphorylate Elk1,23 preclearing of ERK2 from lysates before incubation with Elk1-GST sepharose resulted in greatly reduced kinase activity (Fig 2B, lanes 2 and 3 v lanes 5 and 6), indicating that ERK2 was responsible for the majority of Elk1 phosphorylation.
Stromal cell FAK and paxillin tyrosine phosphorylation is enhanced by RAMOS cell contact.We investigated whether the cytoplasmic tyrosine kinase FAK represented all or part of the increase in stromal cell tyrosine phosphorylation at 120 kD. Immunoprecipitated FAK was constitutively tyrosine phosphorylated in unstimulated stromal cells, but FAK phosphorylation was further enhanced by contact with RAMOS-wt or RAMOS-4c cells (Fig 3A). The increase in FAK phosphorylation was modest (∼2-fold), but statistically significant based on results of multiple experiments (P = .01, P = .08 for contact with RAMOS-wt or RAMOS-4c, respectively, by two-tailed t-test). Contact with either RAMOS-wt or RAMOS-4c was comparable in eliciting this increase (Fig 3B). Tyrosine phosphorylation of FAK was never detected in RAMOS cells removed from contact with stromal cells, even though immunoprecipitation of 1 mg of RAMOS or stromal cell lysate yielded comparable amounts of FAK (Fig 3A, lower panel).
Phosphorylation of stromal cell FAK and paxillin are increased after contact with RAMOS-wt or RAMOS-4c. Stromal cells were incubated in medium alone (−) or in contact with RAMOS-wt (wt) or RAMOS-4c (4c) for 5 minutes at 37°C. Stromal cells and RAMOS cells were collected, lysed, and immunoprecipitated and immunoblotted as detailed in the Materials and Methods. (A) Immunoprecipitation of 1 mg of cell protein/sample with rabbit preimmune IgG (cont) or with h-FAK1 polyclonal antibody (FAK). Preimmune IgG immunoprecipitation was performed on pooled stromal cell lysate. RAMOS indicates FAK immunoprecipitate from pooled lysate of RAMOS-wt and RAMOS-4c that had been washed from stromal cells. (Upper panel) Immunoblotting with antiphosphotyrosine. (Lower panel) Immunoblotting with rabbit anti-FAK C-903. (B) Densitometric comparison of FAK phosphorylation in stromal cells after contact with RAMOS-wt (wt) or RAMOS-4c (4c) normalized to FAK phosphorylation of stromal cells incubated in medium (−) in four separate experiments. Error bars represent the standard error. (C) Immunoprecipitation of 0.6 mg cell protein/sample with antipaxillin. RAMOS indicates paxillin immunoprecipitate from pooled lysate of RAMOS-wt and RAMOS-4c that had been washed from stromal cells. (Upper panel) Immunoblotting with antiphosphotyrosine. (Lower panel) Immunoblotting with antipaxillin. (D) Densitometric comparison of paxillin phosphorylation in three separate experiments (as for FAK in [B]).
Phosphorylation of stromal cell FAK and paxillin are increased after contact with RAMOS-wt or RAMOS-4c. Stromal cells were incubated in medium alone (−) or in contact with RAMOS-wt (wt) or RAMOS-4c (4c) for 5 minutes at 37°C. Stromal cells and RAMOS cells were collected, lysed, and immunoprecipitated and immunoblotted as detailed in the Materials and Methods. (A) Immunoprecipitation of 1 mg of cell protein/sample with rabbit preimmune IgG (cont) or with h-FAK1 polyclonal antibody (FAK). Preimmune IgG immunoprecipitation was performed on pooled stromal cell lysate. RAMOS indicates FAK immunoprecipitate from pooled lysate of RAMOS-wt and RAMOS-4c that had been washed from stromal cells. (Upper panel) Immunoblotting with antiphosphotyrosine. (Lower panel) Immunoblotting with rabbit anti-FAK C-903. (B) Densitometric comparison of FAK phosphorylation in stromal cells after contact with RAMOS-wt (wt) or RAMOS-4c (4c) normalized to FAK phosphorylation of stromal cells incubated in medium (−) in four separate experiments. Error bars represent the standard error. (C) Immunoprecipitation of 0.6 mg cell protein/sample with antipaxillin. RAMOS indicates paxillin immunoprecipitate from pooled lysate of RAMOS-wt and RAMOS-4c that had been washed from stromal cells. (Upper panel) Immunoblotting with antiphosphotyrosine. (Lower panel) Immunoblotting with antipaxillin. (D) Densitometric comparison of paxillin phosphorylation in three separate experiments (as for FAK in [B]).
The cytoskeletal protein paxillin migrates on gel electrophoresis as a broad band around 70 kD, and tyrosine phosphorylation of paxillin has often been associated with FAK activation.29 We therefore investigated whether the increased stromal cell tyrosine phosphorylation detected at 64-70 kD represented paxillin. As for FAK, tyrosine phosphorylation of paxillin immunoprecipitated from stromal cell lysates was enhanced by contact with RAMOS cells (Fig 3C). Lysates of RAMOS cells that had been in contact with stromal cells contained reduced amounts of paxillin compared with stromal cell lysates, and tyrosine phosphorylation was not detected (Fig 3C, far right lane). Tyrosine phosphorylation of stromal cell paxillin was consistently enhanced approximately threefold after contact with either RAMOS-wt or RAMOS-4c (Fig 3D), and this difference was highly significant (P < .001, P = .009 for contact with RAMOS-wt or RAMOS-4c, respectively).
Immunoprecipitation of FAK and paxillin removes major phosphorylated proteins from stromal cell but not RAMOS cell lysate.We further assessed the contribution of FAK and paxillin to the tyrosine phosphorylation profile of stimulated or unstimulated stromal or RAMOS cells by analyzing residual tyrosine phosphorylation in lysates left over from immunoprecipitations (Fig 4). Immunoprecipitation with antipaxillin cleared stromal cell lysates of nearly all the 64-70–kD antiphosphotyrosine immunoreactivity (Fig 4, lanes 2 and 3), indicating that either paxillin or associated proteins of 64-70 kD represent the major phosphorylated proteins of this mobility. Similarly, immunoprecipitation with anti-FAK cleared stromal cell lysates of nearly all the 120-kD antiphosphotyrosine immunoreactivity (Fig 4, lanes 4 and 5). These data indicate that paxillin and FAK represent prominent tyrosine phosphorylated proteins in stromal cells. Consistent with the lack of detection of phosphorylated FAK or paxillin in RAMOS immunoprecipitations, no major changes in antiphosphotyrosine profile occurred in RAMOS lysates after either paxillin or FAK immunoprecipitation (Fig 4, lanes 6 through 8).
Immunoprecipitation with FAK and paxillin antibodies removes major phosphorylated bands from stromal cell, but not RAMOS cell lysate. Stromal cells were incubated in medium alone (−) or with RAMOS cells (+) for 5 minutes at 37°C. Stromal cells and RAMOS cells were collected separately, lysed, and incubated with antibody-coated protein A sepharose overnight at 4°C as described in the Materials and Methods. Sepharose beads were sedimented by centrifugation for 10 seconds. The supernatant lysate was removed, electrophoresed through 10% SDS gels, and immunoblotted with antiphosphotyrosine. Lanes represent original lysate (lane 6) or supernatant lysates removed from preimmune rabbit IgG (lane 1), antipaxillin (lanes 2, 3, and 7) or anti-FAK (lanes 4, 5, and 8) immunoprecipitations. Stromal cells in contact (+) were equal protein amounts pooled from stromal cells incubated with RAMOS-wt or RAMOS-4c. RAMOS cells were equal protein amounts pooled from samples containing RAMOS-wt and RAMOS-4c. Results are representative of three individual experiments.
Immunoprecipitation with FAK and paxillin antibodies removes major phosphorylated bands from stromal cell, but not RAMOS cell lysate. Stromal cells were incubated in medium alone (−) or with RAMOS cells (+) for 5 minutes at 37°C. Stromal cells and RAMOS cells were collected separately, lysed, and incubated with antibody-coated protein A sepharose overnight at 4°C as described in the Materials and Methods. Sepharose beads were sedimented by centrifugation for 10 seconds. The supernatant lysate was removed, electrophoresed through 10% SDS gels, and immunoblotted with antiphosphotyrosine. Lanes represent original lysate (lane 6) or supernatant lysates removed from preimmune rabbit IgG (lane 1), antipaxillin (lanes 2, 3, and 7) or anti-FAK (lanes 4, 5, and 8) immunoprecipitations. Stromal cells in contact (+) were equal protein amounts pooled from stromal cells incubated with RAMOS-wt or RAMOS-4c. RAMOS cells were equal protein amounts pooled from samples containing RAMOS-wt and RAMOS-4c. Results are representative of three individual experiments.
The src family kinases src (56 kD) and fyn (59 kD) can be phosphorylated on tyrosine, are of an electrophoretic mobility consistent with stromal cell tyrosine phosphorylation profiles, and have been reported to play a role in FAK-related signaling pathways.30 31 However, tyrosine phosphorylation of stromal cell fyn was not altered by contact with RAMOS cells (data not shown). Expression of src in stromal cells could not be detected by immunoblotting or by in vitro kinase assays of src immunoprecipitates.
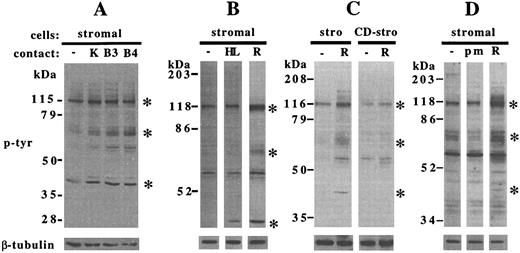
Specificity of stromal cell stimulation.A number of human B-lineage cell lines were able to stimulate comparable (KM3, BLIN-3, BLIN-4, and NALM-6) or more subtle (BLIN-1 and 1E8) increases in stromal cell tyrosine phosphorylation, compared with RAMOS cells (Fig 5A and data not shown). In contrast, contact with the myeloid cell line HL-60 did not alter tyrosine phosphorylation of the 120-kD and 64-70–kD stromal cell proteins in five separate experiments over time courses of 1 to 20 minutes, but did induce relatively weak tyrosine phosphorylation at 43 kD in some experiments (Fig 5B). The slower-migrating form of ERK2 was enhanced by HL-60 contact in the same experiments and comigrated with the 43-kD protein (data not shown), indicating that the increased tyrosine phosphorylation at 43 kD likely represented activated ERK2. Stimulation by contact with RAMOS cells required an intact stromal cell cytoskeleton, because pretreatment of stromal cells with cytochalasin D severely inhibited stromal cell tyrosine phosphorylation (Fig 5C). Contact with intact cell membranes was necessary for tyrosine kinase activation, because incubation of stromal cells with latex microspheres did not enhance tyrosine phosphorylation (Fig 5D). Of note, the microspheres were sufficiently adherent to stromal cells to make them difficult to remove by washing.
Specificity of enhanced tyrosine phosphorylation in stromal cells. Stromal cells were incubated for 5 minutes at 37°C and collected, electrophoresed, and immunoblotted as detailed in the Materials and Methods. (A) Stromal cells incubated in medium alone (−) or after contact with KM-3 (K), BLIN-3 (B3), or BLIN-4 (B4) cells. (B) Stromal cells incubated in medium alone (−) or in contact with HL-60 cells (HL) or RAMOS-4c (R). (C) Untreated stromal cells (stro) or cytochalasin D-treated stromal cells (CD-stro) incubated in medium alone (−) or with RAMOS-wt (R). (D) Stromal cells incubated in medium alone (−), with polystyrene microspheres (pm), or with RAMOS-wt (R). Blots were probed with antiphosphotyrosine (upper panels), stripped, and reprobed with anti–β-tubulin (lower panels). Asterisks indicate the major stromal cell proteins demonstrating increases in tyrosine phosphorylation after RAMOS contact. (A), (B), (C), and (D) are representative of 3, 6, 3, and 1 individual experiments, respectively.
Specificity of enhanced tyrosine phosphorylation in stromal cells. Stromal cells were incubated for 5 minutes at 37°C and collected, electrophoresed, and immunoblotted as detailed in the Materials and Methods. (A) Stromal cells incubated in medium alone (−) or after contact with KM-3 (K), BLIN-3 (B3), or BLIN-4 (B4) cells. (B) Stromal cells incubated in medium alone (−) or in contact with HL-60 cells (HL) or RAMOS-4c (R). (C) Untreated stromal cells (stro) or cytochalasin D-treated stromal cells (CD-stro) incubated in medium alone (−) or with RAMOS-wt (R). (D) Stromal cells incubated in medium alone (−), with polystyrene microspheres (pm), or with RAMOS-wt (R). Blots were probed with antiphosphotyrosine (upper panels), stripped, and reprobed with anti–β-tubulin (lower panels). Asterisks indicate the major stromal cell proteins demonstrating increases in tyrosine phosphorylation after RAMOS contact. (A), (B), (C), and (D) are representative of 3, 6, 3, and 1 individual experiments, respectively.
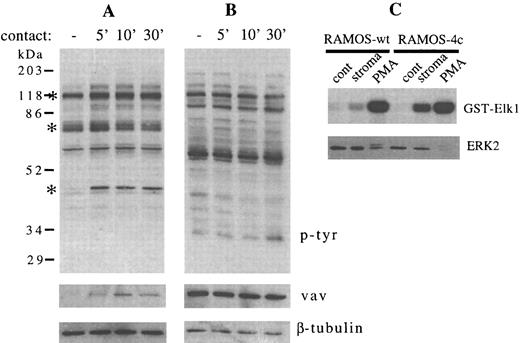
We also asked whether bidirectional cell signaling might be occurring, resulting in changes in RAMOS cell tyrosine phosphorylation that mirrored those occurring in stromal cells after contact. During a time course of 5 to 30 minutes, we detected no change in the overall pattern of RAMOS-wt tyrosine phosphorylation after contact with stromal cells (Fig 6B), although increased tyrosine phosphorylation of the 120-kD, 64-70–kD, and 43-kD stromal cell proteins was detected in the same experiment (Fig 6A). In contrast to this absence of RAMOS-wt protein tyrosine phosphorylation, substantial VLA-4–mediated adhesion of RAMOS-wt is detectable after 30 minutes of contact with stromal cells.18 The delay (1 to 2 minutes) inherent in removal of the RAMOS cells from stromal cells could lead to a decay of kinase signals before lysis of the RAMOS cells. Although little change in ERK2 mobility was seen in this or other experiments (Figs 1, 2A, and 6C and data not shown), there was an increase in Elk1-associated kinase activity in RAMOS-wt or RAMOS-4c cells that had been washed from stromal cells (Fig 6C), indicating that this assay may either be more sensitive, or it may reflect enhanced activity of kinases other than ERK2. PMA treatment of RAMOS cells resulted in increases in both slow-migrating ERK2 and Elk1-associated kinase activity (Fig 6C).
Contact between RAMOS-wt and stromal cells does not change the phosphotyrosine profile of RAMOS-wt, but enhances Elk1-bound kinase activity. Stromal cells and RAMOS-wt cells were incubated at 37°C, collected, lysed, electrophoresed, and immunoblotted as detailed in the Materials and Methods. (A) Stromal cells in medium for 5 minutes (−) or in contact with RAMOS-wt for the times indicated (in minutes). (B) RAMOS-wt cells in medium (−) or in contact with stromal cells for the times indicated (in minutes). (Upper panel) Antiphosphotyrosine immunostaining. (Middle panel) Anti-vav immunostaining. (Lower panel) Anti–β-tubulin immunostaining. Asterisks indicate the major stromal cell protein species demonstrating consistent increases in tyrosine phosphorylation. (C) RAMOS cells were incubated in medium alone (cont), on stromal cells (stroma), or with 20 ng/mL phorbol 12-myristate 13-acetate (PMA) for 5 minutes at 37°C. Lysates were assayed by solid-phase kinase assay (γ32P-GST-Elk1, 3.5 hours of exposure, upper panel) or by immunostaining with anti-ERK2 (lower panel) as detailed in the Materials and Methods. Data in (C) were obtained in the same experiment as Fig 2C. Results are representative of three (A and B) and two (C) individual experiments.
Contact between RAMOS-wt and stromal cells does not change the phosphotyrosine profile of RAMOS-wt, but enhances Elk1-bound kinase activity. Stromal cells and RAMOS-wt cells were incubated at 37°C, collected, lysed, electrophoresed, and immunoblotted as detailed in the Materials and Methods. (A) Stromal cells in medium for 5 minutes (−) or in contact with RAMOS-wt for the times indicated (in minutes). (B) RAMOS-wt cells in medium (−) or in contact with stromal cells for the times indicated (in minutes). (Upper panel) Antiphosphotyrosine immunostaining. (Middle panel) Anti-vav immunostaining. (Lower panel) Anti–β-tubulin immunostaining. Asterisks indicate the major stromal cell protein species demonstrating consistent increases in tyrosine phosphorylation. (C) RAMOS cells were incubated in medium alone (cont), on stromal cells (stroma), or with 20 ng/mL phorbol 12-myristate 13-acetate (PMA) for 5 minutes at 37°C. Lysates were assayed by solid-phase kinase assay (γ32P-GST-Elk1, 3.5 hours of exposure, upper panel) or by immunostaining with anti-ERK2 (lower panel) as detailed in the Materials and Methods. Data in (C) were obtained in the same experiment as Fig 2C. Results are representative of three (A and B) and two (C) individual experiments.
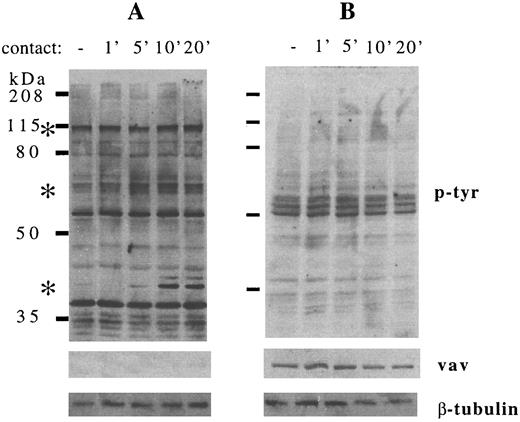
Detection of changes in FAK, paxillin, and ERK2 phosphorylation in stromal cells after B-cell contact is consistent with the possibility that B lymphocytes communicate their presence to bone marrow stromal cells, potentially altering stromal cell function. Because most of these studies were conducted using RAMOS cell lines whose growth is independent of stromal cell contact, we asked whether contact with normal human B-cell precursors was also able to increase stromal cell protein tyrosine phosphorylation and whether the B-cell precursors in turn would be stimulated by contact with stromal cells. B-cell precursors (CD19+/surface IgM−) were isolated from normal human bone marrow and coincubated with stromal cells for 1 to 20 minutes (Fig 7). B-cell precursors removed from stromal cell contact did not show detectable changes in protein tyrosine phosphorylation, after adjusting for the slight differences in protein loading that were detected by vav and β-tubulin immunoblotting (Fig 7B, middle and lower panels). In contrast, the pattern of stromal cell tyrosine phosphorylation stimulated by B-cell precursor contact was similar to that stimulated by RAMOS contact. Phosphorylation of 120-kD, 64-70–kD, and 43-kD proteins, most likely representing FAK, paxillin, and ERK2, was increased after contact with normal human B-cell precursors. In a separate experiment, FACS-purified CD19+CD34+ pro-B cells and CD19+CD34− pre-B cells were both comparable to RAMOS-wt in stimulating stromal cell tyrosine phosphorylation (data not shown).
Contact between normal human B-cell precursors and stromal cells increases tyrosine phosphorylation of multiple stromal cell but not B-cell precursor proteins. Stromal cells and normal human CD10+ CD19+ surface IgM− B-cell precursors were incubated at 37°C, collected, lysed, electrophoresed, and immunoblotted as detailed in the Materials and Methods. (A) Stromal cells in medium for 5 minutes (−) or in contact with B-cell precursors for the times indicated (in minutes). (B) B-cell precursors in medium alone (−) or in contact with stromal cells for the times indicated (in minutes). (Upper panel) Antiphosphotyrosine immunostaining. (Middle panel) Anti-vav immunostaining. (Lower panel) Anti–β-tubulin immunostaining. Asterisks indicate the major stromal cell protein species demonstrating consistent increases in tyrosine phosphorylation. Results are representative of two individual experiments.
Contact between normal human B-cell precursors and stromal cells increases tyrosine phosphorylation of multiple stromal cell but not B-cell precursor proteins. Stromal cells and normal human CD10+ CD19+ surface IgM− B-cell precursors were incubated at 37°C, collected, lysed, electrophoresed, and immunoblotted as detailed in the Materials and Methods. (A) Stromal cells in medium for 5 minutes (−) or in contact with B-cell precursors for the times indicated (in minutes). (B) B-cell precursors in medium alone (−) or in contact with stromal cells for the times indicated (in minutes). (Upper panel) Antiphosphotyrosine immunostaining. (Middle panel) Anti-vav immunostaining. (Lower panel) Anti–β-tubulin immunostaining. Asterisks indicate the major stromal cell protein species demonstrating consistent increases in tyrosine phosphorylation. Results are representative of two individual experiments.
DISCUSSION
In our prior study, we reported that contact with B lymphocytes stimulated increases in human bone marrow stromal cell tyrosine phosphorylation of multiple stromal cell proteins and production of IL-6.18 This stimulation was similar whether stromal cells were in contact with the VLA-4–deficient and adhesion-deficient cell line variant RAMOS-4c or the parental RAMOS cell line. We have now shown that stromal cell tyrosine kinase activation after short-term B-cell contact is cytochalasin D-inhibitable, can be stimulated by contact with a variety of B cells including normal human B-cell precursors, and involves FAK, paxillin, and ERK2 as major substrates. Activation of such a contact-initiated cell signaling cascade in stromal cells may have an influence on B-cell precursors in vivo by enhancing the ability of stromal cells to support B-cell growth and development.
Phosphorylation of FAK and paxillin after activation by various external stimuli has been described in human, mouse, and chicken fibroblasts.32 The most thoroughly studied stimulus for the phosphorylation of these proteins is integrin-dependent adhesion of trypsinized fibroblasts to extracellular matrix proteins, where peak enhancement of phosphorylation occurs on a time scale of hours. In our study, the stromal cells had presumably expressed a layer of extracellular matrix proteins including collagens and fibronectin.33 34 Phosphorylation in focal contacts on the lower surface of the stromal cell due to adhesion to extracellular matrix may account for the observed baseline phosphorylation of FAK and paxillin (Figs 1 and 3). It follows that B-cell contact may therefore activate FAK tyrosine phosphorylation at the upper surface of the stromal cell. The modest twofold increase in FAK phosphorylation after B-cell contact is consistent with constitutive phosphorylation of up to one-half of stromal cell FAK. Increased FAK phosphorylation was maximal at 5 to 10 minutes of contact and was present after contact with adhesion-deficient RAMOS-4c cells.
Several soluble stimuli of fibroblast FAK or paxillin phosphorylation have been identified. These include growth factors such as platelet-derived growth factor35 or IL-329; lipid-acting agents such as lysophosphatidic acid, sphingomyelinase, and sphingosine36-38; and neuropeptides such as bombesin and endothelin.39 To our knowledge, none of these molecules are likely to be localized to B-cell stromal cell contact sites, and there has been no mechanism yet reported for any integrin-independent cell-to-cell stimulation of FAK or paxillin.
The signaling cascades associated with FAK and paxillin tyrosine phosphorylation have similar characteristics regardless of stimulus.32,40 The mitogen-activated protein kinase cascade, including ERK2, can be activated by integrin-mediated signal transduction,41 although this event may be unrelated to changes in cytoskeletal organization.42 In our study, stromal cell ERK2 activity was clearly enhanced by contact with B lymphocytes as indicated by phosphorylation and mobility shift of ERK2 on SDS gels and confirmed by the ability of an Elk1-GST fusion protein to coprecipitate increased kinase activity (Figs 1 and 2). ERK-2 was responsible for the majority of this kinase activity, because preclearing the lysate for ERK2 abrogated the majority of the activity (Fig 2C). However, ERK2 phosphorylation may not be strictly associated with FAK and paxillin phosphorylation, because contact with HL60 cells enhanced tyrosine phosphorylation of 43-kD (presumably ERK2), but not 120-kD or 64-70–kD proteins (Fig 5A). This may imply that ERK2 phosphorylation is more sensitive to cell contact. Alternatively, ERK2 phosphorylation may occur independently of FAK or paxillin phosphorylation. MAP kinases are important in transducing signals to the nucleus through activation of transcription factor families such as AP-143 and NFκB.44 The activation of ERK2 may therefore play a role in stromal cell cytokine expression, such as enhancement of stromal cell IL-6 secretion after B-cell contact,18 because IL-6 transcription can be activated by binding of NFκB45 or AP-146 to consensus binding sites on the IL-6 promoter. Significantly, NFκB may be involved in enhancement of stromal cell IL-6 expression after contact with myeloma cells.47
The nonreceptor tyrosine kinases src and fyn are physically associated with FAK and cluster in areas of focal contact during adhesion.30 31 The role of these kinases in cytoskeletal organization may have more to do with relocalization of kinase activity to focal contacts than with changes in overall fyn or src activity. The lack of change in stromal cell fyn phosphorylation after B-cell contact is consistent with this idea.
Tyrosine phosphorylation of FAK is associated with several cellular events affecting cell shape and cytoskeletal reorganization. Cell spreading; formation of stress fibers, filopodia, or lamellipodia48; cell shape changes associated with cell division49; and membrane ruffling (which may be associated with pinocytosis)40,50,51 have been connected with signaling pathways that include FAK phosphorylation. FAK−/− mouse embryos show defects in migration of germ layers during development,52,53 underscoring the importance of FAK in cell motility. Stromal cells in our system are already fully spread and quiescent in the absence of serum, exhibit a well-developed network of actin fibers, and do not appear to be motile (L.J.J. and T.W.L., unpublished data). RAMOS cells lack VLA-5, which is needed for transmigration through the stromal cell layer.54 It is therefore unlikely that stromal cell spreading or motility or stromal cell displacement due to B-cell transmigration are associated with changes in stromal cell FAK phosphorylation. However, integrity of the stromal cell cytoskeleton is required for signaling, because disruption of the cytoskeleton with cytochalasin D abrogated stimulation of FAK, paxillin, or ERK2 phosphorylation (Fig 5C). Because transmission electron micrographs of stromal cells cocultured with B-cell precursors have demonstrated the presence of filopodia in areas of contact,55 increases in stromal cell FAK phosphorylation observed in our assay possibly represent early steps in establishment of these structures.
VLA-4 expression is clearly important in B-cell development. Some studies have shown that B-cell precursor growth on stromal cells in vitro can be inhibited by VLA-4 antibodies.6,56 Chimeric mice in which ES cells deleted for β1 integrin subunit CD2957 or the VLA-4 α subunit CD49d58 had been injected into RAG-1−/− or RAG-2−/− blastocysts showed a dramatic deficiency of B cells in the bone marrow, blood, and lymphoid organs. Both studies contained data to suggest that deficient migration from the yolk sac to the fetal liver or from the fetal liver to the bone marrow may have been partly responsible for B-cell deficiencies. We have been unable to demonstrate a VLA-4–mediated effect on tyrosine phosphorylation patterns in either RAMOS cells or normal human B-cell precursors after contact with stromal cells for up to 30 minutes, although removal of B cells from stromal cells may have allowed for a decay of tyrosine phosphorylation before the B cells were lysed. Of note, contact with stromal cells did enhance Elk1-associated kinase activity in RAMOS cells using an assay that is potentially more sensitive than monitoring overall phosphotyrosine profiles, although the data shown required 20-fold longer exposure for RAMOS cells (Fig 6C) than for stromal cells (Fig 2C) in the same experiment. Other investigators have reported increased tyrosine phosphorylation of B-cell proteins including FAK,15,16 CD19, and lyn17 in studies involving antibody-mediated cross-linking of VLA-4, which may be a more potent stimulus eliciting more readily detectable phosphorylation. It is also possible that other molecular interactions at the B-cell precursor/stromal cell interface activate B-cell precursor tyrosine phosphatases, thereby making tyrosine phosphorylation events transduced through VLA-4 more transient and less detectable.
In conclusion, contact not involving B-cell VLA-4 has a rapid effect on stromal cells, activating signaling cascades that potentially influence cytoskeletal organization and transcriptional regulation. Although the importance of VLA-4 and VCAM-1 interactions in mediating B-cell precursor/bone marrow stromal cell interactions are well characterized, additional stromal cell molecules that may interact with developing B cells have recently been identified.59 60 Further characterization of additional protein or nonprotein interactions between B cells and stromal cells should facilitate a more thorough understanding of the role of stromal cells in B-cell growth and development within the bone marrow microenvironment.
ACKNOWLEDGMENT
The authors thank Drs Wei Li and Dan Mueller (Department of Medicine, University of Minnesota) for helpful advice and for providing the Elk-1-GST sepharose beads and Bob Hipskind (Institut de Genetique Moleculaire, Montpellier, France) for permission to use the GST-6his-Elk1 (307-428) fusion protein.
Supported in part by National Cancer Institute Grants No. 5F32-CA65057 (to L.J.J.) and R01-CA31685 (to T.W.L.) and by the Leukemia Task Force.
Address reprint requests to Tucker W. LeBien, PhD, Box 806 UMHC, University of Minnesota Cancer Center, 425 E River Rd, Minneapolis, MN 55455.

![Fig. 3. Phosphorylation of stromal cell FAK and paxillin are increased after contact with RAMOS-wt or RAMOS-4c. Stromal cells were incubated in medium alone (−) or in contact with RAMOS-wt (wt) or RAMOS-4c (4c) for 5 minutes at 37°C. Stromal cells and RAMOS cells were collected, lysed, and immunoprecipitated and immunoblotted as detailed in the Materials and Methods. (A) Immunoprecipitation of 1 mg of cell protein/sample with rabbit preimmune IgG (cont) or with h-FAK1 polyclonal antibody (FAK). Preimmune IgG immunoprecipitation was performed on pooled stromal cell lysate. RAMOS indicates FAK immunoprecipitate from pooled lysate of RAMOS-wt and RAMOS-4c that had been washed from stromal cells. (Upper panel) Immunoblotting with antiphosphotyrosine. (Lower panel) Immunoblotting with rabbit anti-FAK C-903. (B) Densitometric comparison of FAK phosphorylation in stromal cells after contact with RAMOS-wt (wt) or RAMOS-4c (4c) normalized to FAK phosphorylation of stromal cells incubated in medium (−) in four separate experiments. Error bars represent the standard error. (C) Immunoprecipitation of 0.6 mg cell protein/sample with antipaxillin. RAMOS indicates paxillin immunoprecipitate from pooled lysate of RAMOS-wt and RAMOS-4c that had been washed from stromal cells. (Upper panel) Immunoblotting with antiphosphotyrosine. (Lower panel) Immunoblotting with antipaxillin. (D) Densitometric comparison of paxillin phosphorylation in three separate experiments (as for FAK in [B]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/4/10.1182_blood.v90.4.1626/3/m_bl_0016f3.jpeg?Expires=1769169744&Signature=QWZZvbMUV5Rtp6g2ZdPG4VJpgzHINCepoZAfloHM7sATrNawCjPwfAlQQtMmH8uREC1SvuIY-MAn2uPMepPuKU7w3N8CeE8ouIXOFxZB9aY5mMrGCeKfbtav492svpIvYYs9OhPciWlE0h2jHl1r9aeVvoL7jTLrr03ZvSjyP0ddM-eP1ir4Evfj5oBWufHshf~ntpdYSM~GeD8X1-G5TaySJh5eGMNANlCTRBQw4KfUZ1WCFiTdwryx52INWayNuHGSfETnN9loMJcbEd~ZEbGYXD7CQ-GkOB4GaORMu9QZK6DwwPYEKcIB~AQWALQTVhvCQ3gcpbTISLuSyyL3Jw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal