Abstract
The precise role of the endogenous immune system in modulating cancer development remains unclear. Tumor cells are generally thought to be nonimmunogenic because they are of ‘self’ origin. However, tumor-reactive lymphocytes can be isolated from patients with many types of cancer. It is unclear what role these lymphocytes play and why they fail to protect the host. Using a murine B-cell leukemia/lymphoma (BCL1) model, we showed the development of a vigorous antitumor T-cell response in the tumor-susceptible host. Specific T-cell responses against BCL1 developed as early as day 4. However, the nature of this nonprotective response is different from the protective response produced in a major histocompatibility complex–matched tumor-resistant host. Susceptible hosts developed a T helper 2 (Th2)-dominant response, whereas resistant hosts developed a Th1-dominant response to BCL1. Cytolytic activity against BCL1 developed in both resistant and susceptible hosts, but in the susceptible host, this response was weaker and delayed compared with that in the resistant host. Thus, tumor susceptibility does not necessarily mean the absence of an antitumor immune response. Rather, the nature of the antitumor immune response is critical in determining clinical outcome.
LYMPHOID MALIGNANCIES represent a unique class of cancer because these tumor cells are of immune origin and, thus, have immunomodulatory potential.1-3 As malignant immune cells exist in the same compartments as normal immune cells, contact with tumor (idiotype)-reactive precursor cells is almost a certainty. Lymphocytes express unique proteins, immunoglobulin molecules in B cells and T-cell receptors in T cells, which may serve as tumor-specific antigens. B cells constitutively express major histocompatibility complex (MHC) class II molecules and present their idiotypes in context of self MHC class I and II molecules.4 Thus, the inability of the endogenous immune response to control a lymphoid malignancy in the cancer host may not be because of the lack of tumor-specific antigens or access to the antigens. Idiotype-reactive T cells may be systematically modulated and suppressed by the malignant lymphocyte for it to proliferate successfully.
Development of a polarized T-cell response depends in large part on the antigen presenting cell (APC). Recently, antigen presentation by B cells has been shown to preferentially induce T helper 2 (Th2) cytokines, such as interleukin-4 (IL-4) and IL-10, in CD4+ T cells.5 Transformed B cells may themselves act as antigen-presenting cells and drive idiotype-reactive precursor T cells to the Th2 phenotype. In addition, B cells may modulate expression of costimulatory molecules (B7-1 and B7-2), secrete immunosuppressive cytokines (IL-10 and TGF-β), and compete for proliferative cytokines. Malignant B cells may exploit some or all of these immunomodulatory mechanisms to subvert the developing antitumor immune response and escape destruction. Furthermore, when Th2 cells are induced, they secrete IL-4 and IL-5, which can serve as growth factors for malignant B cells and enhance tumor growth.6 7 Clearly, malignant B cells present an array of potential interactions with the host immune system to facilitate their survival and growth.
We have studied a spontaneous B-cell leukemia/lymphoma model (BCL1)8 of BALB/c origin to understand the interactions between tumor cells and the endogenous immune system. This is an aggressive tumor cell line that is uniformly fatal when injected (as few as 100 cells) into BALB/c mice.8 Using this model, we have traced the development and phenotype of BCL1-reactive T cells in BALB/c. For comparison, we also traced the development of the anti-BCL1 immune response in B10.D2 mice, a MHC-matched strain that is resistant to BCL1.
MATERIALS AND METHODS
Animals and cells.Male BALB/c (H-2d) and B10.D2 (H-2d) mice, 8 to 12 weeks old, were obtained from and maintained at the Stanford Research Animal Facility. BCL1 is a spontaneous B-cell leukemia/lymphoma cell line of BALB/c origin.9 It expresses IgM, λ Ig, and class I and II MHC molecules on the cell surface.9 It also expresses B7-1 and B7-2 (data not shown). These cells were maintained by serial passage in BALB/c mice.
Antibodies and cytokines.Recombinant murine (rm)IL-12 was a kind gift from Dr S. Wolfe of Genetics Institute (Cambridge, MA). The rabbit antiserum antiasialo GM1 was purchased from Wako Chemicals (Dallas, TX). Anti-CD3 monoclonal antibodies (MoAbs) were purchased from PharMingen (San Diego, CA). Anti–BCL1-Id MoAbs were purified from hybridoma 6A5.1 (a kind gift from Dr E. Vitetta, University of Texas, Dallas). MoAbs anti–IL-4 (11B11 [ATCC HB-188]) and anti–IFN-γ (XMG1.2) were prepared from hybridomas grown as ascites in pristane-primed BALB/c nu/nu mice. They were purified with protein G-Sepharose columns, dialyzed against phosphate-buffered saline (PBS), and sterile filtered before use.
Injection of mice with cytokines and MoAbs.All injections were done intraperitoneally and diluted in sterile PBS. rmIL-12 was injected at 0.1 mg/mouse daily starting with day 1. 11B11 and XMG1.2 were injected at 0.5 mg/mouse on days 1, 6, 13, 20, 27, and 34. Anti-natural killer (NK) (rabbit antiserum antiasialo-GM1) was used at 20 mL/mouse on days 1, 4, 9, 14, 19, 24, 29, and 34.
Enrichment of splenic T cells.At indicated timepoints, animals were sacrificed (by CO2 narcosis) and their spleens were removed under sterile conditions. Splenocytes were released into single-cell suspensions by pressing against fine nylon mesh, then washed with sterile RPMI at 4°C. Live cells were counted using a hemacytometer under dye exclusion. B cells were removed by passing the splenocyte suspensions through nylon wool columns, then incubated on plates coated with antimurine Ig MoAbs. Nonadherent cells were collected, washed, and counted. A portion of each sample was analyzed by fluorescence-activated cell sorting (FACS) analysis; only samples shown to be >85% T cells (CD3+) were used.
Proliferation.A total of 0.5 × 106 T-enriched splenocytes were cocultured with 0.25 × 106 (2:1) irradiated target cells in RPMI supplemented with 10% human serum. Cocultures were performed in 96-well plates and done in triplicates. At 5 days, wells were pulsed with 3H-thymidine for 16 hours, then harvested with a plate harvester. Proliferative responses are expressed as proliferative index (stimulated counts per minute [cpm]/unstimulated cpm).
Cytolytic activity.The JAM test for cytolytic activity is described in detail elsewhere.10 Briefly, 10 × 106 splenic T cells were cocultured with 5 × 106 irradiated BCL1 (8,000 rads) in each experiment. After 5 days, these cells were harvested and washed. Fresh BCL1 cells were labeled with 3H-thymidine for 4 hours, then washed and counted. Effector cells were placed onto 96-well plates starting at a ratio of 40:1 and serially diluted. Ten thousand labeled BCL1 cells were then added into each well as targets. After 4 hours of incubation, plates were harvested with a plate harvester, and membrane-bound 3H was measured using a scintillation counter. This reflects intact DNA, which comes from nonapoptotic cells and, thus, is used as the inverse of cytotoxic activity. Percent cytotoxicity is calculated as % apoptosis = (control cpm − test cpm)/(immediate cpm).
Quantitation of cytokine secretion.Purified splenic T cells were cocultured with irradiated BCL1 at 2:1, 0.5 × 106 T and 0.25 × 106 BCL1 per well. Cocultures were done in quadruplicates and their supernatants were pooled. Supernatants were collected at 24 hours for IFN-γ quantitation, or 5 days for IL-10 quantitation. For IL-4 quantitation, supernatants were removed at 5 days, and the cells were washed and then given fresh media containing 0.1 μmol/L ConA. After an additional 24 hours, the supernatants were collected for IL-4 quantitation. All supernatants collected were immediately frozen at −80°C and measured in batches. Quantitation of secreted cytokines was done by enzyme-linked immunosorbent assay (ELISA), with results normalized to known standard curves. ConA-stimulated supernatants were also assayed for IFN-γ, and it was found that the addition of ConA did not enhance BCL1-specific secretion of this cytokine (data not shown).
A portion of the ELISA assays were performed using the Cytoscreen immunoassay kits (BioSource, Camarillo, CA). The remainder of the assays were done using coating MoAbs R4-6A2 (T. Mosmann) for IFN-γ, BVD4 (M. Howard, DNAX, Palo Alto, CA) for IL-4, 2A5 (PharMingen, San Diego, CA) for IL-10 and biotinylated detection MoAbs XMG1.2 (T. Mosmann, University of Alberta, Edmonton, Canada) for IFN-γ, BVD6 (M. Howard) for IL-4, and SXC.1 (M. Howard) for IL-10.
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total RNA was isolated using TRIzol reagents (GIBCO-BRL, Life Technologies, Gaithersburg, MD) according to the manufacturer's protocol. After quantitation, 2 μg of the RNA was reverse transcribed using the murine Moloney leukemia virus (M-MLV) reverse transcriptase (GIBCO-BRL). The reactions were performed as follows: 9 μL of RNA, 2 μL of pd(N)6 (200 ng/mL; Pharmacia Biotech, Piscataway, NJ), and 1 μL of prime RNAse inhibitor (5′ → 3′; Boulder, CO) was added to each tube and incubated at 65°C for 1 minute, then 10°C for 3 minutes. The following were then added to each tube as a 13-μL mix: 5 μL of 5X first strand buffer (250 mmol/L Tris-chloride [Tris-Cl], 375 mmol/L potassium chloride [KCl], 15 mmol/L magnesium chloride [MgCl2]), 2 μL of 0.1 mmol/L dithiothreitol (DTT), 1 μL of 25 mmol/L deoxynucleotide triphosphate mix (Pharmacia Biotech), 1 μL ribonuclease inhibitor (GIBCO-BRL), 1 μL of prime RNAse inhibitor, 1 μL of M-MLV (200 U/μL), 2 μL of diethyl pyrocarbonate (DEPC)-treated water. The mixtures (total volume 25 μL) were incubated at 37°C for 60 minutes and at 90°C for 5 minutes. The resulting cDNA samples were stored at −20°C until used for polymerase chain reaction (PCR).
For PCR reactions, 5 μL of each cDNA sample was added to a reaction tube with the following reagents added as a 45-μL mix: 4 μL of deoxynucleotide triphosphate mix (2.5 mmol/L), 5 μL of 10X PCR buffer (200 mmol/L Tris-HCl and 500 mmol/L KCl), 3 μL of 50 mmol/L MgCl2 , 5 mL of each primer (10 pmol/μL), 0.25 μL of Taq DNA Polymerase (GIBCO-BRL), and 22.75 μL of ultrapure water. The reactions were run in a Perkin Elmer-Cetus DNA Thermal Cycler (Norwalk, CT) under the following conditions: 94°C for 3 minutes, 35 cycles at 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute. The amplification primers for hypoxanthine-guanine phosphoribasyl transferase (HPRT), IL-4, IL-10, and IFN-γ (synthesized by Pan Facility, -S t a n f o r d , C A ) w e r e u s e d a s p u b l i s h e d 1 1 : H P R T s e n s e , -5′-GTAATGATCAGTCAACGGGGGAC-3′; HPRT antisense, 5′-CCAGCAAGCTTGCAACCTTAACCA-3′; IFN-γ sense, 5′-TGGAGGAACTGGCAAAAGGATGGT-3′; IFN-γ antisense, 5′-TTGGGACAATCTCTTCCCCAC-3′; IL-4 sense, 5′-ACGAGG-T C A C A G G A G A A G G G A C G C C A T G C A - 3 ;pr ; I L - 4 a n t i s e n s e , --5 ;pr - T C A T T C A T G G A G C A G C T T A T C G A T G A A T C C - 3 ;pr ; I L - 1 0 -sense, 5′-GGACAACATACTGCTAACCGACTC-3′; IL-10 antisense, 5′-AAAATCACTCTTCACCTGCTCCAC-3′. The predicted amplification products (HPRT, 214 bp; IL-4, 241 bp; IL-10, 257 bp; IFN-γ, 336 bp) were verified by gel electrophoresis.
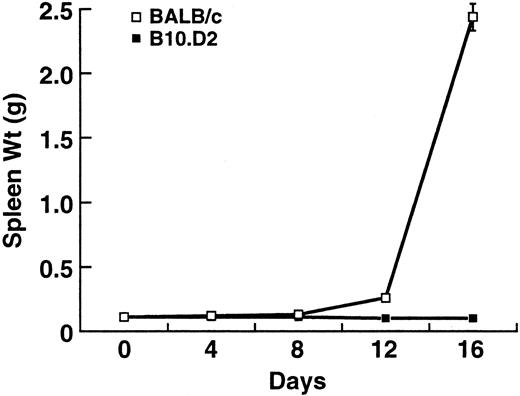
Progression of splenic enlargement in BALB/c and B10.D2 after BCL1 inoculation. Values represent the mean ± standard deviation (SD) of three separate experiments with at least three mice at each timepoint.
Progression of splenic enlargement in BALB/c and B10.D2 after BCL1 inoculation. Values represent the mean ± standard deviation (SD) of three separate experiments with at least three mice at each timepoint.
RESULTS
Effects of BCL1 in BALB/c and B10.D2 mice.Injection of 1 × 106 BCL1 cells into healthy BALB/c (8 to 12 weeks old) mice results in massive splenomegaly and death in approximately 4 weeks. In addition, these mice appear scruffy and cachectic. In contrast, B10.D2 mice, which are MHC-matched (H-2d) but minor histocompatibility antigen (mHC)-mismatched with BALB/c, are resistant to BCL1. They do not develop splenomegaly (Fig 1) and appear completely normal before sacrifice.
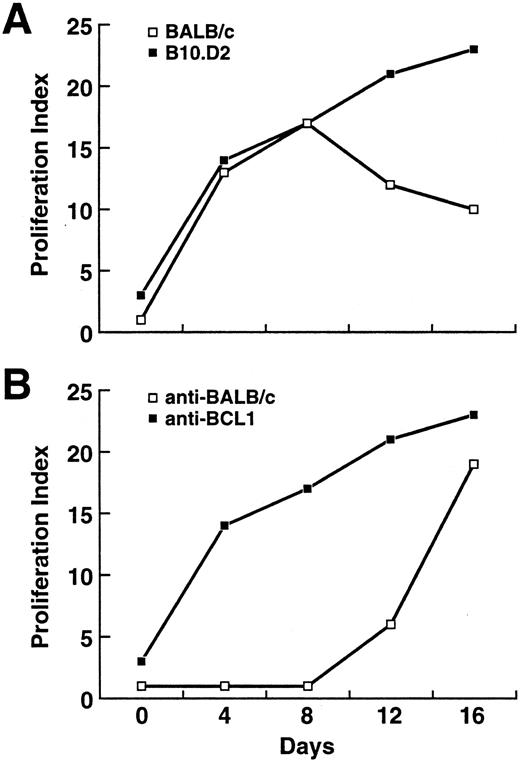
Proliferation of T cells against BCL1.To trace the early development of any immune responses against BCL1 in these two strains, BALB/c and B10.D2 mice were killed at days 0, 4, 8, 12, and 16 after BCL1 injection. Their splenic T cells were enriched for further analysis. When cocultured with irradiated (8,000 rads) BCL1, splenic T cells from B10.D2 proliferated significantly as early as day 4 after BCL1 injection (Fig 2A). Splenic T cells from BALB/c proliferated to nearly the same extent at days 4 and 8, but their proliferation against BCL1 decreased by days 12 and 16, whereas that of B10.D2 continued to increase (Fig 2A). Thus, BCL1-reactive precursor T cells exist in both B10.D2 and BALB/c mice, and they were efficiently activated by BCL1. However, after day 8, BCL1-reactive T cells began to disappear in BALB/c mice.
(A) Development of anti-BCL1 reactivity in BALB/c and B10.D2. Values are taken from a representative experiment from three separate experiments and represent the mean ± SD of triplicate measurements. (B) Proliferation of B10.D2 T cells against BCL1 and BALB/c splenocytes. Splenic T cells from BCL1-inoculated B10.D2 mice were isolated and cocultured with irradiated BCL1 (8,000 rads) or BALB/c splenocytes (3,000 rads).
(A) Development of anti-BCL1 reactivity in BALB/c and B10.D2. Values are taken from a representative experiment from three separate experiments and represent the mean ± SD of triplicate measurements. (B) Proliferation of B10.D2 T cells against BCL1 and BALB/c splenocytes. Splenic T cells from BCL1-inoculated B10.D2 mice were isolated and cocultured with irradiated BCL1 (8,000 rads) or BALB/c splenocytes (3,000 rads).
Because BALB/c and B10.D2 differ at a number of mHC antigens, these may serve as the basis of allorecognition. To evaluate whether the anti-BCL1 response in B10.D2 was mainly because of recognition of mHC differences (BALB/c background) expressed on BCL1, splenic T cells from BCL1-injected B10.D2 were also cocultured with irradiated (3,000 rads) BALB/c splenocytes. Proliferation against irradiated BALB/c splenocytes did not begin until after day 8 (Fig 2B), suggesting that the early proliferation against BCL1 in B10.D2 is primarily against BCL1-specific antigens, but later mHC antigens are also recognized.
Development of cytolytic activity against BCL1.Development of specific cytolytic activity against BCL1 was assessed using the JAM test.10 Splenic T cells from B10.D2 showed significant anti-BCL1 cytotoxicity (>30% peak) by day 4 (Fig 3), which continued to increase to a peak of >50% specific cytotoxicity at day 12. Splenic T cells from BALB/c also developed cytolytic activity against BCL1, but the cytotoxicity was delayed and weaker compared with that in B10.D2 (Fig 3). In BALB/c, there is a lag between onset of proliferation (day 4) and cytolytic activity (day 8) against BCL1, whereas in B10.D2, proliferation and cytotoxicity against BCL1 developed concurrently (day 4).
Development of specific cytolytic activity against BCL1 in BALB/c and B10.D2. Specific cytolytic activity against BCL1 was measured by the JAM test.10 Peak specific lysis was obtained from an effector:target ratio of 40:1. Values are taken from a representative experiment from three separate experiments and represent the mean ± SD of triplicate measurements.
Development of specific cytolytic activity against BCL1 in BALB/c and B10.D2. Specific cytolytic activity against BCL1 was measured by the JAM test.10 Peak specific lysis was obtained from an effector:target ratio of 40:1. Values are taken from a representative experiment from three separate experiments and represent the mean ± SD of triplicate measurements.
Patterns of cytokine production in response to BCL1.The phenotypes of the BCL1-reactive T cells were evaluated by examining the cytokines they secreted by ELISA. In response to irradiated BCL1, splenic T cells from B10.D2 secreted high levels of IFN- γ, which peaked at day 8 after BCL1 injection (Fig 4A; IFN-γ). BALB/c T cells secreted little or no IFN-γ. In contrast, BALB/c T cells secreted IL-4 (Fig 4B; IL-4) and IL-10 (Fig 4C; IL-10) in response to BCL1, both of which also peaked at day 8. Unirradiated BCL1 cells secrete IL-10 but no detectable IL-4 or IFN-γ, and irradiated BCL1 cells secrete no detectable amounts of these cytokines (Fig 4D). These patterns are consistent with a Th1-dominant response against BCL1 in the tumor-resistant strain B10.D2 and a Th2-dominant response in tumor-susceptible strain BALB/c.
Cytokine secretion in response to coculture with BCL1. (A) IFN-γ, (B) IL-4, (C) IL-10, and (D) cytokines secreted by irradiated and nonirradiated BCL1. Values are taken from a representative experiment from three separate experiments and represent the mean ± SD of triplicate measurements.
Cytokine secretion in response to coculture with BCL1. (A) IFN-γ, (B) IL-4, (C) IL-10, and (D) cytokines secreted by irradiated and nonirradiated BCL1. Values are taken from a representative experiment from three separate experiments and represent the mean ± SD of triplicate measurements.
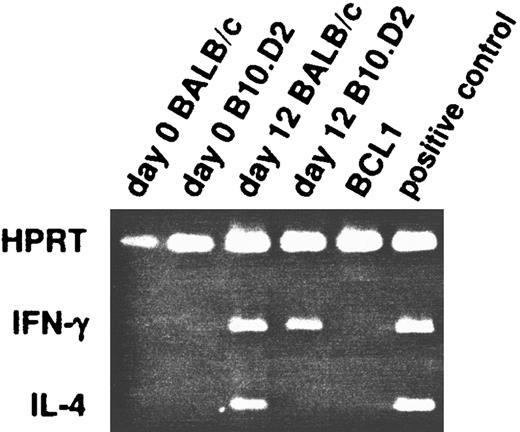
We also assessed production of mRNA for these cytokines by RT-PCR. At days 0 and 12, BALB/c and B10.D2 splenic T cells were lysed with TRIzol and their mRNA extracted for analysis in RT-PCR. On day 0, neither IFN-γ or IL-4 mRNA is detected in splenic T cells from either strain (Fig 5). On day 12, IFN-γ but not IL-4 mRNA is detected in B10.D2 T cells, consistent with the secreted cytokines detected by ELISA. However, mRNA for both IL-4 and IFN-γ are detected in BALB/c T cells on day 12, although the predominant cytokine secreted is IL-4. Because RT-PCR is a very sensitive method, this suggests that either the polarized BCL1-reactive Th2 populations in BALB/c are really heterogeneous,12 or intermediate cells exist that produce both IL-4 and IFN-γ.
Detection of mRNA production by RT-PCR in untreated mice. At day 0 and 12 after BCL1 inoculation, splenic T cells from BALB/c and B10.D2 mice were isolated and their RNA extracted for analysis. They were probed for the presence of IL-4 and IFN-γ messages in RT-PCR. HPRT was used as positive control.
Detection of mRNA production by RT-PCR in untreated mice. At day 0 and 12 after BCL1 inoculation, splenic T cells from BALB/c and B10.D2 mice were isolated and their RNA extracted for analysis. They were probed for the presence of IL-4 and IFN-γ messages in RT-PCR. HPRT was used as positive control.
Manipulation of cytokines and BCL1 progression.Because the development of a Th2 dominant anti-BCL1 response appears to correlate with disease susceptibility, we attempted to alter the developing anti-BCL1 immune response in BALB/c and B10.D2 by manipulating the Th1/Th2 balance. These manipulations included attempting to favor Th1 development in BALB/c with recombinant murine IL-1213 and MoAbs against IL-4,14 11B11, or to favor Th2 development in B10.D2 with MoAbs against IFN-γ,14 XMG1.2. Because natural killer (NK) cells may play a prominent role in the response against BCL1, we also treated both BALB/c and B10.D2 mice with antibodies against NK cells, antiasialo GM1. Each mouse was challenged with a lethal dose of 10,000 BCL1 cells.
Daily injections of rmIL-12 starting at day 1 (group 2) led to a modest but statistically significant prolongation of survival after BCL1 challenge in BALB/c mice (Table 1). In contrast, 11B11 (group 3) had no protective effect. Somewhat surprisingly, the protective effect of rmIL-12 was nullified when 11B11 was coadministered (group 4). Anti-NK antiserum accelerated death after BCL1 inoculation in BALB/c considerably (group 6; P = .0001). This effect was balanced by rmIL-12 because mice injected with both (group 5) had no difference in survival compared with the control mice. Injection of either XMG1.2 (anti–IFN-γ) MoAbs (group 8) or anti-NK antiserum (group 9) had no effect on survival in B10.D2.
Effects of Cytokine Manipulations on Survival After BCL1 Challenge in BALB/c and B10.D2
| Mouse . | Group . | Treatment . | Survival (days) . | P Value . |
|---|---|---|---|---|
| BALB/c | 1 | none | 35.8 ± 1.3 | |
| 2 | rmIL-12 | 45.2 ± 7.0 | .018 | |
| 3 | 11B11 | 36.4 ± 1.8 | .565 | |
| 4 | rmIL-12 + 11B11 | 37.6 ± 6.9 | .581 | |
| 5 | rmIL-12 + anti-NK | 37.6 ± 5.4 | .487 | |
| 6 | anti-NK | 29.4 ± 1.7 | .0001 | |
| B10.D2 | 7 | none | >100 | |
| 8 | XMG1.2 | >100 | ||
| 9 | anti-NK | >100 |
| Mouse . | Group . | Treatment . | Survival (days) . | P Value . |
|---|---|---|---|---|
| BALB/c | 1 | none | 35.8 ± 1.3 | |
| 2 | rmIL-12 | 45.2 ± 7.0 | .018 | |
| 3 | 11B11 | 36.4 ± 1.8 | .565 | |
| 4 | rmIL-12 + 11B11 | 37.6 ± 6.9 | .581 | |
| 5 | rmIL-12 + anti-NK | 37.6 ± 5.4 | .487 | |
| 6 | anti-NK | 29.4 ± 1.7 | .0001 | |
| B10.D2 | 7 | none | >100 | |
| 8 | XMG1.2 | >100 | ||
| 9 | anti-NK | >100 |
All mice in each group were injected with a lethal dose of 10,000 BCL1 intraperitoneally on day 0. P values represent comparisons against group 1 (control group). Values represent mean ± standard deviation of 10 mice in each group. This is a representative experiment of two separate experiments.
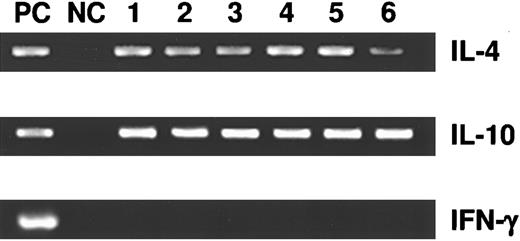
Production of cytokines by splenic T cells in treatment groups 1 through 6 was assessed by RT-PCR. On day 21, three mice from groups 1 through 6 were killed. Their splenic T cells were purified by anti-CD4 magnetic beads (Dynal, Lake Success, NY) and mRNA was isolated, reverse-transcribed, and then probed for IL-4, IL-10, and IFN-γ. Regardless of cytokine and/or MoAb treatments, all mice in groups 1 through 6 produced only IL-4 and IL-10 messages, but no IFN-γ, consistent with Th2-dominant responses (Fig 6).
Detection of mRNA production by RT-PCR in cytokine-treated BALB/c mice (groups 1 to 6). At day 21 after BCL1 inoculation and cytokine treatments, splenic T cells were isolated and their RNA extracted for analysis. They were probed for the presence of IL-4, IL-10, and IFN-γ messages in RT-PCR. A plasmid containing the cDNA for all three cytokines served as the positive control (PC). Negative control (NC) lanes were without template DNA.
Detection of mRNA production by RT-PCR in cytokine-treated BALB/c mice (groups 1 to 6). At day 21 after BCL1 inoculation and cytokine treatments, splenic T cells were isolated and their RNA extracted for analysis. They were probed for the presence of IL-4, IL-10, and IFN-γ messages in RT-PCR. A plasmid containing the cDNA for all three cytokines served as the positive control (PC). Negative control (NC) lanes were without template DNA.
DISCUSSION
Successful manipulation of the immune system for cancer therapy requires a thorough understanding of the endogenous immune response to tumor cells and why the response failed in the first place. Certain malignancies, particularly lymphoid, are significantly more common in the immunocompromised host.15 This supports a role for the endogenous immune system in controlling cancer development. However, the response is clearly not perfect because immunocompetent individuals also develop these malignancies. Other evidence for the ability of the intact immune system to resist certain malignancies include occasional “spontaneous” regression of cancer16 and long-term survival (>10 years) of patients with known metastases.17
Current paradigm holds that tumor cells are poorly immunogenic. Thus, the solution has been to enhance immunogenicity of cancer cells. We previously observed that transient immunosuppression could further accelerate the growth dynamics of BCL1 in BALB/c mice (P. Lee, manuscript submitted). This suggests that an anti-BCL1 immune response developed in BALB/c, which slowed the proliferation of the tumor cells but ultimately did not protect the host. Thus, tumor recognition does not necessarily lead to a protective antitumor immune response for the host.
The question remains as to why the host immune response succeeds in controlling transformed cells in some instances, but fails in others. Even if the response ultimately fails, as in the case of BCL1 in BALB/c, it may not be because of simply the lack of tumor recognition. The crucial issue may not only be whether tumor recognition occurs, but also the magnitude and nature of the response that develops. In lymphoid malignancies, transformed lymphocytes have immunomodulatory mechanisms that may allow them to misdirect the developing host antitumor response.
Using a murine BCL1 model, we sought to determine if an antitumor immune response develops in the tumor-bearing host and, if so, why this response fails to protect the host. BCL1 is a flexible model that allows us to trace the development of the antitumor immune response from early timepoints. Moreover, studying the anti-BCL1 response in B10.D2, an MHC-matched strain that is resistant to BCL1, offers an important comparison to understand the magnitude and time course of a response that successfully eliminates the tumor.
Our results show that BCL1-reactive T cells exist and are efficiently stimulated in BALB/c, and somewhat surprisingly, to a similar extent as in B10.D2 at early timepoints. Furthermore, cytolytic activity against BCL1 also develops in BALB/c. But here, differences begin to emerge between BALB/c and B10.D2. Development of anti-BCL1 cytotoxicity is delayed and weaker in BALB/c compared with B10.D2. With time, proliferation and cytotoxicity against BCL1 disappear in BALB/c, despite continued proliferation of the tumor cells. This suggests that BCL1-reactive T cells are eventually eliminated or “silenced” in BALB/c.
We then analyzed the phenotype of the anti-BCL1 T-cell responses in BALB/c and B10.D2 by assessing the cytokine patterns produced. In response to BCL1, BALB/c T cells secrete IL-4 and IL-10, but no IFN-γ, consistent with a Th2-dominant response. In contrast, B10.D2 T cells secrete the opposite pattern: high levels of IFN-γ and no IL-4 or IL-10, consistent with a Th1-dominant response. We also detected the production of mRNA for these cytokines using RT-PCR. Of note, BALB/c T cells produce mRNA for both IL-4 and IFN-γ on exposure to BCL1, although the predominant secreted cytokine is IL-4. This suggests the possibility that a minority of BCL1-reactive T cells in BALB/c mice develop into the Th1 phenotype. These cells may be responsible for driving the weak, transient cytolytic response against BCL1. More importantly, this observation raises the hope that, with appropriate immunomodulation, this minor Th1 subpopulation may be driven to dominate and lead to a protective response.
The targets of the anti-BCL1 immune responses in BALB/c and B10.D2 remain an important question. It is unclear whether the basis of the Th1-dominant response in B10.D2 is because of recognition of BCL1-specific antigens or mHC differences on BCL1. Appearance of an anti-BCL1 response before an anti-BALB/c response in B10.D2 (Fig 2B) suggests that both sets of antigens are seen. Ultimately, a tumor-specific antigen may also be considered an mHC antigen. The critical trigger becomes the number of potential antigens and their density of expression on the cell surface.18
Given that a Th2-dominant response appears in the susceptible animals, whereas a Th1-dominant response is observed in the resistant animals, we attempted to favor the development of a Th1-dominant response in BALB/c. The approaches we used included recombinant murine IL-1213 and MoAbs against IL-4,14 11B11. Exogenous rmIL-12 led to only a modest but statistically significant prolongation of survival in BALB/c mice challenged with BCL1. It is not surprising that the use of a single cytokine, such as IL-12, led to incomplete protection because an orchestrated antitumor immune response likely requires a number of cytokines acting in sequence. In the Leishmania model, a Th2-dominant response has been successfully shifted to Th1 in BALB/c with MoAbs against IL-4,14 11B11. In our model, we were unable to induce a Th1 response (measured by RT-PCR) nor to show a protective effect with 11B11. The immunoregulatory events surrounding Th1/Th2 differentiation associated with tumor cells appear to be more complex than in Leishmania major infections. BCL1 appears to be a much more powerful and resistant inducer of Th2 responses in BALB/c than Leishmania.
NK cells are another important mediator of antitumor activity. Treatment of BALB/c mice with anti-NK antiserum accelerated death after the BCL1 challenge, suggesting that, although ultimately nonprotective, NK cells nonetheless have some effect on BCL1 in slowing their initial proliferation. Because the protective effects of IL-12 may be mediated via stimulation of NK activity, we coadministered rmIL-12 and anti-NK antiserum. Interestingly, this group had survivals intermediate to that of the rmIL-12 alone and anti-NK alone groups and similar to controls. This is consistent with the predominant effect of rmIL-12 being NK-mediated. Surprisingly, the protective effect of rmIL-12 was also nullified with 11B11. Possible explanations for this observation include 11B11 interfering with rmIL-12 binding, inhibiting NK activity, or that the protective effects of rmIL-12 are somehow IL-4 mediated.
Taken together, our data suggest that the lack of tumor recognition is not the only reason why the host immune response fails in the cancer patient. A developing antitumor response may be misdirected to a nonprotective state by the tumor cell itself, ie, immune deviation,19 especially tumor cells with inherent immunomodulatory capacity such as malignant lymphoid cells. Induction of a nonprotective antitumor immune response may be a prerequisite to clinical cancer development in lymphoid malignancies. By secreting immunomodulatory cytokines (ie, IL-10) and modulating surface accessory molecule expression, the tumor cell may play an active role in shaping the developing immune response to evade immune destruction. The nonprotective response is eventually overwhelmed and disappears despite continued proliferation of the tumor cells. When tumor-reactive lymphocytes are not detectable in the cancer host, it may be that they have already been eliminated or “silenced” by the tumor cells.
A better understanding of the mechanisms by which tumor cells may modulate the host immune system may lead to novel approaches to manipulate host-tumor interactions to favor the development of a protective antitumor immune response. Furthermore, development of a protective antitumor immune response as the immune system recovers after chemotherapy may be the reason why some patients do not relapse. If so, assessing the presence and nature of an antitumor immune response after treatment may be of considerable prognostic value. Immune manipulations before or after cytoreduction from chemotherapy to favor development of a protective antitumor immune response may be a promising clinical approach in oncology.
ACKNOWLEDGMENT
We thank Dr Samuel Strober for kindly providing the BCL1 cells and Genetics Institute for providing rmIL-12. We also thank Drs Mark Davis, Ted Zimmer, and Luis Soares for valuable discussions.
This work was supported by Grant #JFRA-446 from the American Cancer Society. P.P.L. was supported by K08 CA72976-01. D.U. was supported by R01 AI26322 and K07 AI01026. A.E.M. was supported by HD07249 and ST32 AI07290.
Address reprint requests to Nelson J. Chao, MD, Bone Marrow Transplant Program, Duke University Medical Center, 25101A Morris Building, Box 3961 DUMC, Durham, NC 27710.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal