Abstract
Factor XI is a plasma glycoprotein that is required for contact activation initiated fibrin formation in vitro and for normal hemostasis in vivo. In preparation for developing a mouse model of factor XI deficiency to facilitate investigations into this protease's contributions to coagulation, we cloned the complementary DNA for murine factor XI, expressed the protein in a mammalian expression system, and compared its properties with human recombinant factor XI. The 2.8-kb murine cDNA codes for a protein of 624 amino acids with 78% homology to human factor XI. Both recombinant murine and human factor XI are 160 kD homodimers comprised of two 80 kD polypeptides connected by disulfide bonds. Murine factor XI shortens the clotting time of human factor XI deficient plasma in an activated partial thromboplastin time assay, with a specific activity 50% to 70% that of the human protein. In a purified system, murine factor XI is activated by human factor XIIa and thrombin in the presence of dextran sulfate. Murine factor XI differs from human factor XI in that it undergoes autoactivation slowly in the presence of dextran sulfate. This is due primarily to murine factor XIa preferentially cleaving a site on zymogen factor XI within the light chain, rather than the activation site between Arg371 and Val372. Northern blots of polyadenylated messenger RNA show that murine factor XI message is expressed, as expected, primarily in the liver. In contrast, messenger RNA for human factor XI was identified in liver, pancreas, and kidney. The studies show that murine and human factor XI have similar structural and enzymatic properties. However, there may be variations in tissue specific expression and subtle differences in enzyme activity across species.
FACTOR XI is the zymogen of a plasma serine protease that activates factor IX by limited proteolysis.1 The importance of this protein in normal hemostasis is demonstrated by the hemorrhagic diathesis associated with congenital factor XI deficiency in humans, dogs, and cattle.2-4 The physiologic mechanisms by which factor XI is activated and the contribution this protease makes to normal hemostasis are presently the topic of much debate. Activated factor XII (factor XIIa), thrombin, and activated factor XI (factor XIa) have all been shown to activate human factor XI in vitro under various conditions5-11; however, the relevance of these observations to coagulation in vivo remains to be determined. Furthermore, while it is generally agreed that factor XIa contributes to hemostasis by activating factor IX, it is not clear if this step is required for initial thrombin formation or for consolidation of hemostasis after initial formation of the fibrin clot through the extrinsic (factor VIIa/tissue factor) pathway.11-15
An animal model of factor XI deficiency would greatly facilitate investigations into the role of this protein in normal and pathologic coagulation. Although factor XI deficiency has been reported in Holstein cattle and sporadically in dogs,3 4 there are currently no animal models of factor XI deficiency that are easily adapted to a laboratory setting. In preparation for developing a murine model of factor XI deficiency (factor XI “knockout” mouse) to address this problem, we have cloned the complementary DNA (cDNA) for murine factor XI and expressed murine and human factor XI in a mammalian cell culture system. The murine and human cDNAs and the recombinant factor XI proteins were used to compare the structural homology and relative activities of the two proteins, as well as to examine the expression of factor XI messenger RNA in different tissues. Murine and human factor XI are structurally very similar and have relatively similar enzymatic activities. Unlike human factor XI, the murine protein will not undergo autoactivation in the presence of dextran sulfate. Factor XI mRNA was identified in liver tissue from both species, however, expression was also noted in human pancreas and kidney tissue.
MATERIALS AND METHODS
Materials and Reagents
Molecular biology reagents.A murine liver cDNA library in Lamda Zap vector was a gift from R. Wetzel (Washington University, St Louis, MO).16 Human factor XI and prekallikrein cDNAs were gifts from D. Chung and E. Davie (University of Washington, Seattle).17,18 Human factor XII cDNA was a gift from R. MacGillvary (University of British Columbia, Vancouver, Canada).19 Multitissue polyA RNA northern blots for human and murine tissues and polyA enriched RNA from murine liver and pancreas were purchased from Clonetech (Palo Alto, CA).
Tissue culture.The 293 transformed human embryonal kidney cell line was purchased from American Tissue Type Collection, Rockville, MD (ATCC CRL 1573). Dulbecco's modified Eagle medium (DMEM) and G418 (geneticin) were from GIBCO/BRL, Gaithersburg, MD and Cellgro complete media was from Fisher Scientific (Pittsburgh, PA). Soy bean trypsin inhibitor (SBTI), lima bean trypsin inhibitor (LBTI), and aprotinin were from Sigma Chemicals, St Louis, MO.
Proteins and reagents for protein purification and Western blots.Benzamidine, heparin agarose, nitroblue tetrazolium (NBT), and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) were purchased from Sigma Chemicals. S-sepharose fast flow resin and a Superose-12 chromatography column were from Pharmacia Biotech (Piscataway, NJ). Rabbit polyclonal antiserum against human factor XI was raised using standard techniques,20 and IgG was purified from serum by staphylococcal protein A chromatography. Purified human factors XIIa, IX, and X were purchased from Enzyme Research Laboratories (South Bend, IN). Human thrombin was prepared as previously described.21 Recombinant factor VIII was obtained from Baxter/Hyland (Glendale, CA).
Plasmas and reagents for clotting assays.Pooled normal human plasma, human factor XI, and factor IX-deficient plasmas were from George King Biomedical (Overland Park, KS). Normal murine plasma in 0.38% sodium citrate was from Pel-Freez Biologicals (Rogers, AK). Thrombosil aPTT reagent was from Ortho Diagnostic (Raratan, NJ).
Chromogenic substrates.Substrates S-2366 and S-2765 were from DiaPharma Group (Malmo, Sweden).
Buffers and solutions.20× SSC is 3.0 mol/L sodium chloride, 0.3 mol/L sodium citrate, pH 7.0. 20× SSPE is 3.0 mol/L sodium chloride, 0.2 mol/L sodium phosphate monobasic, 20 mmol/L EDTA, pH 7.4. 50× Denhardt's solution is 1% Ficoll (Type 400, Pharmacia), 1% polyvinylpyrrolidone, and 1% bovine serum albumin (BSA).
Miscellaneous reagents.BSA, rabbit brain cephalin, and dextran sulfate (average molecular weight 500,000 daltons) were from Sigma Chemicals.
Isolation of the murine factor XI cDNA.A total of 500,000 plaques from the murine liver cDNA library were screened using a full-length human factor XI cDNA probe labeled with 32P-dATP using a random hexamer primer method.22 Two clones (λMFXI-1 and λMFXI-2) that hybridized to this probe were obtained and were purified through two successive plaque purification steps. Sequencing of the cDNA clones was performed using a Sequenase version 2.0 DNA sequencing Kit (Amersham Life Sciences, Arlington Heights, IL). The sequence of the murine clones was compared with the published cDNA sequences for human factor XI17 and murine prekallikrein23 using a MacVector software package (International Biotechnologies, New Haven, CT).
Expression and purification of recombinant murine and human factor XI.Full-length cDNAs for human and murine factor XI were ligated into the EcoRI cloning site of a mammalian expression vector (pJVCMV24 ) containing the cytomegalovirus promoter. Two hundred and ninety-three cells (5 × 107) were cotransfected with 40 μg of factor XI/pJVCMV and 2 μg of plasmid RSVneo, which contains a gene conferring resistance to neomycin.24 Transfection was performed by electroporation using an Electrocell Manipulator 600 (BTX, San Diego, CA). Transfected cells were grown in DMEM with 5% fetal bovine serum and penicillin/streptomycin for 24 hours and then switched to the same medium containing 500 μg/mL of G418. Media was exchanged every 48 hours. G418 resistant clones were transferred to 24-well tissue culture plates on day 10 to 14 of selection, and culture supernatants were tested for factor XI activity in a modified activated partial thromboplastin time assay (described below). Clones expressing the highest level of recombinant protein were expanded in 2-L roller bottles. After reaching confluence, cells were washed with phosphate-buffered saline and 200 mL of Cellgro complete serum free media supplemented with 10 μg/mL SBTI, LBTI, and aprotinin was added to each roller bottle. Conditioned media was supplemented with benzamidine to 5 mmol/L and stored at −20°C.
Four liters of conditioned media was dialyzed against 50 mmol/L sodium acetate, pH 5.2, 250 mmol/L NaCl, 1 mmol/L EDTA and loaded onto a 100-mL S-sepharose fast-flow cation exchange column. No factor XI activity was detected in the flow through by clotting assay (described below). The column was eluted with a 1-L linear NaCl gradient (250 to 1,000 mmol/L), and factor XI containing fractions were pooled and dialyzed against 25 mmol/L Tris-HCl, pH 7.4, 100 mmol/L NaCl (Tris-buffered saline [TBS]). Dialysate was loaded onto a 10-mL heparin agarose column equilibrated with TBS and eluted with a 100-mL linear NaCl gradient (100 to 1,000 mmol/L). Factor XI containing fractions were pooled and concentrated to a final volume of 500 μL using an Amicon ultrafiltration cell (Amicon, Beverly, MA). The preparation was then passed over a Superose-12 gel filtration column and 500 μL fractions were collected. Samples of each fraction were run on a 10% polyacrylamide-sodium dodecyl sulfate (SDS) gel under nonreducing conditions according to the method of Laemmli,25 followed by staining with Coomassie brilliant blue. Fractions with pure factor XI were pooled and concentrated as above, dialyzed against TBS, and then stored at −70°C. Protein concentration was determined by measuring absorbance at 280 nm using an extinction coefficient (1%) for factor XI of 13.1.
Plasma assay for factor XI activity.Conditioned serum-free media and fractions from purification procedures were screened for factor XI activity by a modified activated partial thromboplastin time (aPTT) assay. Sixty microliters of human factor XI-deficient plasma was mixed with 60 μL of the solution to be assayed and 60 μL of Thrombosil aPTT reagent. The mixture was incubated for five minutes at 37°C, 60 μL of 25 mmol/L CaCl2 was added, and the time to fibrin clot formation was determined using a Dataclot 2 fibrometer (Helena Laboratories, Beaumont, TX). To determine the specific activity of human and murine recombinant factor XI preparations, proteins were diluted to 5 μg/mL in TBS supplemented with 100 μg/mL BSA (TBSA) and serial 1:2 dilutions of this stock solution were made in TBSA. Sixty microliters of the stock solution and each serial dilution were tested for factor XI activity as described above. Results were compared with standard curves prepared with pooled normal human or murine plasma. The factor XI concentration of undiluted pooled normal human plasma was considered to represent 100% activity (1 U factor XI/mL). Preparations were also tested for factor IX activity by substituting factor IX–deficient plasma for factor XI–deficient plasma in this assay. Recombinant factor XI preparations did not show factor IX activity.
Preparation and activity of recombinant factor XIa.Purified recombinant murine or human factor XI (100 μg/mL) was supplemented with human factor XIIa (2 μg/mL) and incubated at 37°C. Activation was confirmed by demonstrating complete conversion of the single chain zymogen to the two-chain active form on Coomassie Blue–stained SDS-polyacrylamide gels run under reducing conditions. Factor XIIa was neutralized by the addition of corn trypsin inhibitor (CTI) as previously described.26 Kinetic parameters for the cleavage of S-2366 by factor XIa were determined as previously reported.24 Briefly, 20 μL of factor XIa at 5 μg/mL in TBS with 0.1% BSA (TBSA) was mixed with 75 μL of TBSA and 5 μL of CTI and incubated for 20 minutes at room temperature. The mixture was diluted to 900 μL with TBSA and 100 μL of chromogenic substrate S-2366 at varying concentrations (50 to 1,000 μmol/L final concentration) was added. Cleavage of S-2366 was followed by measuring the change in absorbance at 405 nm with a Beckman DU-640 spectrophotometer. Michaelis-Menten constants (Km and Vmax) for the cleavage of the chromogenic substrates were determined by standard methods. The value for Vmax was converted to nanomolar para-nitroanaline (pNA) generated/sec using an extinction coefficient for pNA of 9,800 optical density (OD) units (405 nm)/mol pNA. Turn-over number (kcat ) was calculated from the ratio of Vmax to enzyme concentration.
The activation of factor IX by recombinant factor XIa was determined by our previously published method.24 Purified human factor IX (0.05 to 10.0 μmol/L) was incubated with 5 mmol/L CaCl2 and 0.5 nmol/L factor XIa for 60 seconds at 37°C. The reaction was stopped by adding EDTA to a final concentration of 25 mmol/L and chilling to 4°C. The reaction mixture was diluted 1:100 in TBSA and 10 μL of the dilution was mixed with 50 μL of human factor VIII (8 U/mL), 10 mmol/L CaCl2 , rabbit brain cephalin (1:5 dilution of stock) and 10 μL of human thrombin (0.6 U/mL). After incubation at 37°C for 60 seconds to allow the thrombin to activate the factor VIII, 30 μL of human factor X (450 nmol/L) was added and the incubation was continued for an additional 5 minutes. The activation of factor X by factor IXa was stopped by adding EDTA to 25 mmol/L final concentration and placing the reaction on ice. Fifty microliters of the reaction was diluted into 490 μL of TBSA, 60 μL of 5 mmol/L chromogenic substrate S-2765 was added, and the change in absorbance at 405 nm was followed. The results were compared with a control curve constructed with known concentrations of factor IXa to determine the extent of factor IX activation by factor XIa. Michaelis-Menten kinetic parameters were determined using the average of two sets of experiments for each enzyme.
Western blot analysis of activation of factor XI by factor XIIa, thrombin, and factor XIa.Recombinant factor XI was diluted to 5 μg/mL in TBS supplemented with 1 μg/mL dextran sulfate (average molecular weight, 500,000 daltons), in the absence or presence of human factor XIIa (2.5 nmol/L), thrombin (2.5 nmol/L), or factor XIa (either murine or human, 5 nmol/L). All reactions were 400 μL in volume and were incubated at 37°C. At various time intervals, 30-μL samples were removed and mixed with 10 μL of reducing SDS-sample buffer (500 mmol/L Tris-HCl, pH 6.8, 40% glycerol, 20% 2-mercaptoethanol, 10% SDS). Samples were run on 10% polyacrylamide-SDS gels, transferred to nitrocellulose membranes using a Bio-Rad mini-protean II electrophoresis apparatus, and then blocked for 2 hours in 5% powdered milk in TBS. The primary detection antibody was a rabbit antihuman factor XI polyclonal IgG, and the secondary antibody a goat-antirabbit IgG conjugated to alkaline phosphatase. Blots were developed with a solution of 100 mmol/L Tris-HCl, pH 9.0, 100 mmol/L NaCl, 5 mmol/L MgSO4 , 100 μg/mL NBT, and 50 μg/mL BCIP.
Northern blot analysis of factor XI, factor XII, and prekallikrein in human and murine tissue.Two microgram samples of murine liver and pancreas polyA RNA were size fractionated on a 1.2% agarose, 0.62 mol/L formaldehyde gel in 20 mmol/L 3-[N-morpholino]propane-sulfonic acid (MOPS), pH 7.0, 8 mmol/L sodium acetate, 1 mmol/L EDTA followed by transfer to a nylon membrane using a Turbo Blot apparatus (Schleicher and Schuell, Keene, NH). Full-length human factor XI, factor XII and prekallikrein cDNAs, and the full-length murine factor XI cDNA were labeled with 32P-dATP using the random hexamer primer technique.22 In addition, the human factor XI cDNA was digested with restriction endonuclease Pst I and Bst EII and size fractionated on a 1.2% agarose gel in TBE buffer. The three cDNA fragments were cut from the gel, purified using a Qiaex II gel extraction kit (Qiagen, Chatworth, CA), and then labeled with 32P as above. Finally, a human β-actin probe, used as a control to insure equal lane loading of RNA, was labeled in a similar manner. Blots were prehybridized in 5× SSPE, 50% formamide, 5× Denhardt's solution, 10% high molecular weight dextran sulfate, 1% SDS with 100 μg/mL boiled salmon sperm DNA for 2 hours at 42°C. Hybridization with 32P-labeled probes was performed in fresh prehybridization solution overnight at 42°C. Blots were washed with two exchanges of 2× SSC, 0.1% SDS at room temperature (30 minutes for each exchange), and then twice with 0.1× SSC, 0.1% SDS at 55 to 60°C (20 minutes per exchange). Autoradiography was done at −70°C with enhancer screens for 24 to 72 hours using XAR-5 film (Kodak, Rochester, NY).
Reverse transcription/polymerase chain reaction (RT/PCR) detection of murine factor XI transcripts in RNA.RT/PCR was performed on 1 μg each of murine liver and pancreas polyA RNA using a First-Strand cDNA Synthesis Kit (Pharmacia, Piscataway, NJ) according to the manufacturer's recommendations. Murine factor XI primers were: 5′ATCAGCTTTCAAGGAGGT (representing sequence in the third exon of the murine factor XI gene) and 5′ACGACAGACAAAAGCATCTGG (within the seventh exon). Murine p53 (control) primers were: 5′AGAAGTCACAGCACAT-GACGGAGG (upstream primer) and 5′TGTTTTTTCTTTTGCGGGGGAGAGG (downstream primer). PCR products were size fractionated on a 2.0% agarose gel, stained with ethidium bromide, and photographed.
RESULTS
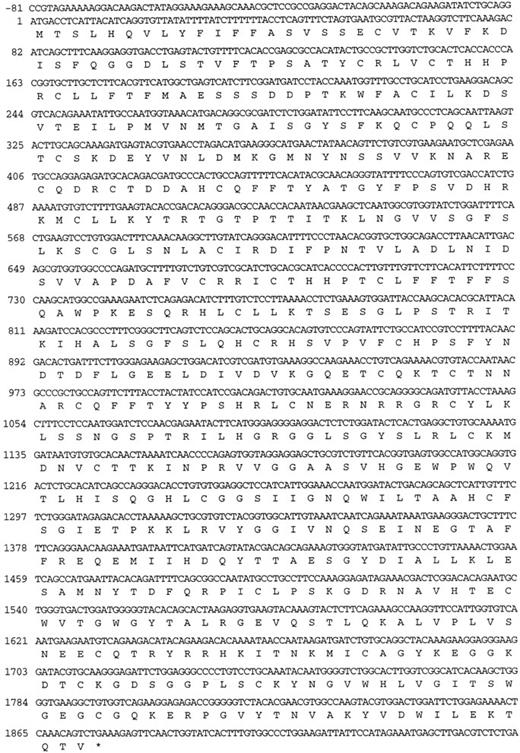
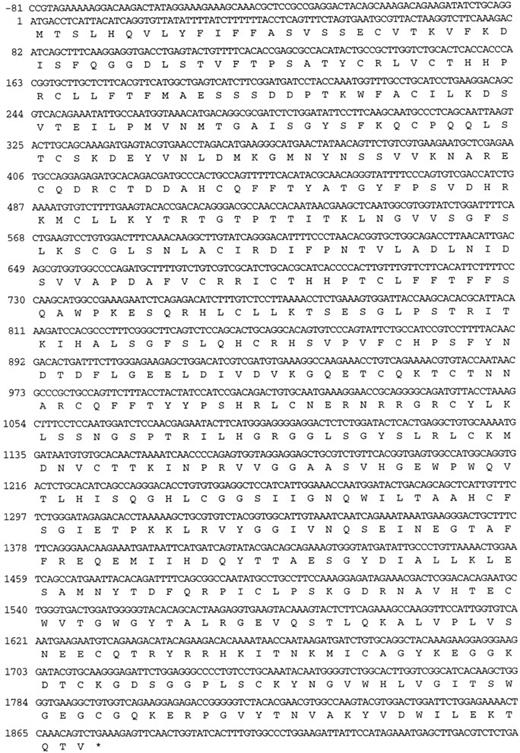
The murine factor XI cDNA.Two clones from the murine liver cDNA library, designated λMFXI-1 and λMFXI-2, were hybridized with the human factor XI cDNA probe and purified for further analysis. The cDNA inserts of both clones were 2.8 kb in size, and sequencing of the 5′ and 3′ ends of the inserts, as well as with an oligonucleotide recognizing a sequence in the human factor XI catalytic (light chain) domain, confirmed that they were identical. λ MFXI-1 contained an 1,872-bp open reading frame coding for a 624 amino acid protein, including an 18 amino acid leader peptide (Fig 1). The amino acid sequence coding for mature murine factor XI is 78% homologous to human factor XI (Fig 2) and 58% homologous to mouse prekallikrein.17,23 By comparison, human factor XI and human prekallikrein are also 58% homologous.17 λMFXI-1 contained approximately 140 bp of 5′ and 800 bp of 3′ untranslated sequence terminating in a polyadenylated tail.
Nucleotide sequence and predicted amino acid sequence of the opened reading frame of the cDNA in λMFXI-1. The nucleotide numbering system starts at the ATG triplet coding for the initiator methionine residue of the leader peptide.
Nucleotide sequence and predicted amino acid sequence of the opened reading frame of the cDNA in λMFXI-1. The nucleotide numbering system starts at the ATG triplet coding for the initiator methionine residue of the leader peptide.
Comparison of the amino acid sequences of human and murine factor XI. The amino acid numbering system used is for human factor XI.17 Amino acids −18 to −1 represent the leader peptide and the N-terminal heavy chain is numbered 1-369. The C-terminal catalytic light chain is numbered 1-238 and begins immediately after the heavy chain sequence. Alignment required the insertion of one gap in the human factor XI sequence after amino acid 325 of the heavy chain and one gap in the murine sequence after amino acid 20 of the light chain. The black arrowhead after Arg369 of the heavy chain designates the factor XIIa and thrombin activation cleavage site. The positions of the serine protease catalytic triad of His, Asp, Ser in the light chain are designated by black circles. The asterisk (*) designates the cystine residue at position 321 of the heavy chain involved in the disulfide bond connecting the two polypeptides of the homodimer. Amino acid positions with identical residues are enclosed in the shaded boxes.
Comparison of the amino acid sequences of human and murine factor XI. The amino acid numbering system used is for human factor XI.17 Amino acids −18 to −1 represent the leader peptide and the N-terminal heavy chain is numbered 1-369. The C-terminal catalytic light chain is numbered 1-238 and begins immediately after the heavy chain sequence. Alignment required the insertion of one gap in the human factor XI sequence after amino acid 325 of the heavy chain and one gap in the murine sequence after amino acid 20 of the light chain. The black arrowhead after Arg369 of the heavy chain designates the factor XIIa and thrombin activation cleavage site. The positions of the serine protease catalytic triad of His, Asp, Ser in the light chain are designated by black circles. The asterisk (*) designates the cystine residue at position 321 of the heavy chain involved in the disulfide bond connecting the two polypeptides of the homodimer. Amino acid positions with identical residues are enclosed in the shaded boxes.
Human plasma factor XI is a 160-kD glycoprotein that is a dimer of two identical 80-kD polypeptides connected by a single disulfide bond.5,17,27 Each polypeptide consists of an N-terminal heavy chain comprised of four tandem repeats called apple domains, and a C-terminal trypsin-like catalytic light chain.17,27 Activation of factor XI by either factor XIIa or thrombin is accomplished by a single proteolytic cleavage after amino acid 369 of the heavy chain (Fig 2).7,8,17 The λ MFXI-1 sequence predicts that each murine factor XI polypeptide contains 34 half-cystine residues compared with 35 residues in the human molecule. The cystine residue at position 11 in the human molecule is replaced with serine in the mouse. In the human molecule, Cys11 forms a disulfide bond with free cystine, and its function is unknown.27 Otherwise, the location of the cystine residues are identical in the two species indicating that murine and human factor XI share a similar tertiary structure. The sequence Asn-Pro-Arg immediately N-terminal to the factor XIIa cleavage site in murine factor XI resembles the Lys-Pro-Arg sequence found at this location in the human molecule and is a typical sequence for a thrombin cleavage site.28 Cys321 is involved in the interchain disulfide bond between the two polypeptides of the factor XI homodimer, and its conservation in the murine cDNA indicates that mouse factor XI may also be dimeric.27
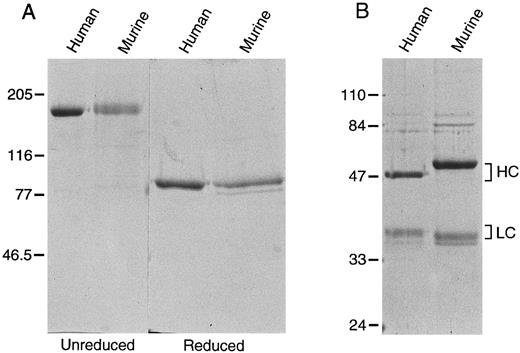
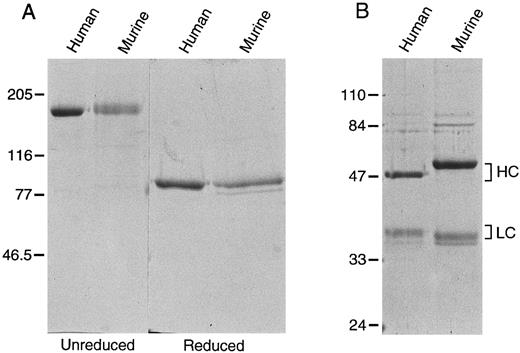
Recombinant factor XI.293 cells, a human fetal kidney fibroblast line that does not constitutively express factor XI, were used to produce recombinant proteins. On SDS-polyacrylamide gel, murine and human recombinant factor XI migrate as a single band of 160 kD under nonreducing conditions, and as single bands at 80 kD when reduced, confirming that murine factor XI is a disulfide-linked dimer (Fig 3A). Using a modified aPTT assay in human factor XI-deficient substrate plasma, the specific activity of the recombinant human protein was determined to be approximately 200 U/mg, virtually identical to plasma derived factor XI.5 24 Murine factor XI has 50% to 70% of the activity (100 to 140 U/mg) of human factor XI when tested in this human plasma system.
Recombinant murine and human (A) factor XI and (B) factor XIa. Recombinant protein expressed in 293 fibroblasts was purified as described in Materials and Methods. Two microgram samples of each protein were size fractionated under reducing and nonreducing conditions on a 10% polyacrylamide SDS-gel, followed by staining with Coomassie brilliant blue. The positions of molecular weight markers in kD are shown at the left of the figure.
Recombinant murine and human (A) factor XI and (B) factor XIa. Recombinant protein expressed in 293 fibroblasts was purified as described in Materials and Methods. Two microgram samples of each protein were size fractionated under reducing and nonreducing conditions on a 10% polyacrylamide SDS-gel, followed by staining with Coomassie brilliant blue. The positions of molecular weight markers in kD are shown at the left of the figure.
Recombinant factor XIa.Recombinant murine and human factor XIa both appear as two bands when run under reducing conditions on SDS-polyacrylamide gels (Fig 3B). The heavy chain of the murine molecule appears to be larger than its human counterpart. This difference cannot be entirely accounted for by the fact that the murine heavy chain is two amino acids longer than the human protein, and may be due to differences in glycosylation. The murine light chain, which is three amino acids shorter than the human light chain, runs slightly smaller than its human counterpart. S-2366 (L-pyroglutamyl-prolyl-arginine-p-nitroaniline) is a chromogenic substrate commonly used to measure human factor XIa activity.8 24 It is also readily cleaved by rabbit factor XIa (D. Gailani, unpublished observation, November 1991). We compared the kinetic parameters of S-2366 cleavage by recombinant human and murine factor XIa (Table 1). The calculated catalytic efficiency (kcat /Km ) is approximately twofold to threefold higher for human factor XIa, mostly attributable to a higher turn-over number (kcat ) for the human enzyme.
The kinetic parameters for the activation of human factor IX by human and murine factor XIa are shown in Table 1. As there is no commercially available chromogenic substrate for factor IX, a two-stage assay was used in which factor IX is activated by factor XIa in the first step and the resulting factor IXa activates factor X in the presence of factor VIIIa and phospholipid in the second step. Factor Xa generation is then easily determined by chromogenic substrate assay.24 The results of the analysis show that recombinant factor XIa from both species activate human factor IX similarly and are consistent with our previous data for factor IX activation by human factor XIa using this technique.24
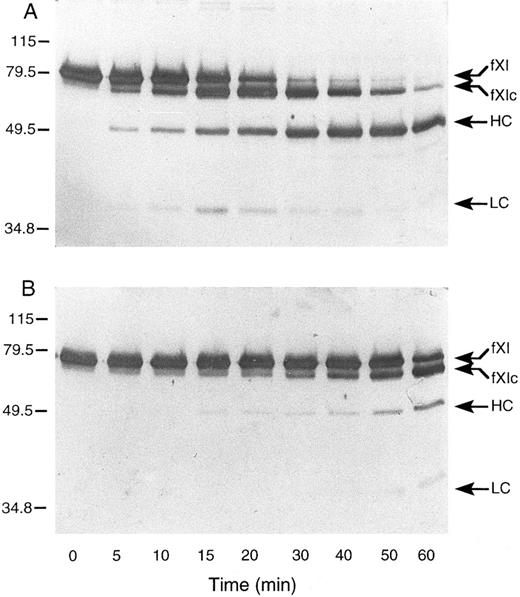
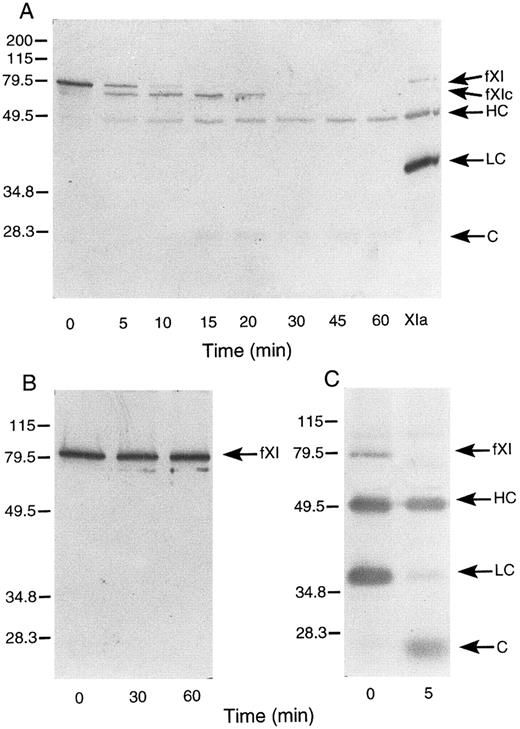
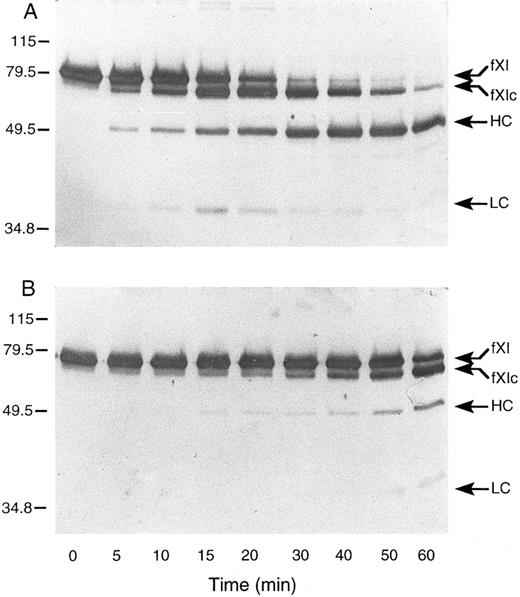
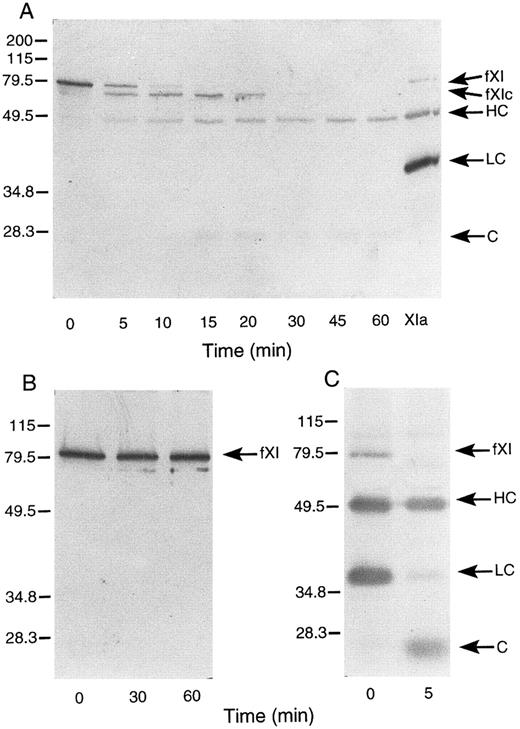
Activation of murine factor XI by factor XIIa, thrombin, and autoactivation.In purified systems, human factor XI is activated slowly by factor XIIa or thrombin in the absence of a negatively charged surface.8 The rate of these reactions are markedly increased by polyanions such as dextran sulfate.7,8 In addition, dextran sulfate promotes the activation of factor XI by factor XIa (autoactivation).7,8 The activation of human factor XI in the presence of dextran sulfate may be followed indirectly by noting the conversion of the 80-kD zymogen to a 45- to 50-kD heavy chain and 35- to 38-kD light chain on western blots of reduced protein.8 As in the case with the human protein, murine factor XI was slowly activated by either human factor XIIa or thrombin in the absence of a negatively charged surface (data not shown). When murine factor XI is incubated with factor XIIa or thrombin in the presence of dextran sulfate, activation occurs much more rapidly, however, an additional band (fXIc, for factor XI cleaved) is noted at approximately 75 kD (Fig 4) that is not seen in human factor XI activation under similar conditions (data not shown). The protease responsible for this cleavage could be the factor XIIa or thrombin used to initiate the reaction or the factor XIa subsequently generated. To evaluate the later possibility, murine factor XI was incubated with dextran sulfate and murine factor XIa (Fig 5A). The result indicates that murine XIa prefers to cleave murine factor XI at a site other than the activation cleavage site, as the fXIc band is generated more rapidly than the heavy chain of factor XIa. The 38-kD band representing the light chain of factor XIa is not seen in this time course, probably because the cleavage that produces fXIc is within the light chain. An approximately 28-kD band (C, for cleavage) is faintly seen and may represent a portion of the cleaved light chain. A similar result was obtained when murine factor XI was incubated with dextran sulfate and human XIa (data not shown) indicating that murine factor XI zymogen differs from human zymogen, which is preferentially cleaved by factor XIa at the activation site between Arg369 and Ile370 in the presence of polyanions.7 8 The preference for cleavage at a site other than the activation site suggests that zymogen murine factor XI would undergo autoactivation on dextran sulfate slowly, if at all. This is confirmed by the experiment shown in Fig 5B. Figure 5C shows murine factor XIa, before and after incubation with dextran sulfate and supports the premise that the active enzyme makes a proteolytic cleavage within the light chain.
Western blots of recombinant murine factor XI activated by (A) thrombin and (B) factor XIIa in the presence of dextran sulfate. Murine factor XI (5 μg/mL) was incubated with 1 μg/mL dextran sulfate and either human thrombin or factor XIIa (2.5 nmol/L) at 37°C. At various time points, samples were removed and mixed with SDS-reducing sample buffer. Western blots were prepared as described in Materials and Methods. The positions of molecular weight markers in kD are shown at the left of the figure. Abbreviations: fXI, factor XI zymogen; fXIc, factor XI cleaved; HC, factor XIa heavy chain; and LC, factor XIa light chain.
Western blots of recombinant murine factor XI activated by (A) thrombin and (B) factor XIIa in the presence of dextran sulfate. Murine factor XI (5 μg/mL) was incubated with 1 μg/mL dextran sulfate and either human thrombin or factor XIIa (2.5 nmol/L) at 37°C. At various time points, samples were removed and mixed with SDS-reducing sample buffer. Western blots were prepared as described in Materials and Methods. The positions of molecular weight markers in kD are shown at the left of the figure. Abbreviations: fXI, factor XI zymogen; fXIc, factor XI cleaved; HC, factor XIa heavy chain; and LC, factor XIa light chain.
Western blots of murine factor XI. (A) Murine factor XI activation by murine factor XIa in the presence of dextran sulfate. Murine factor XI (5 μg/mL) was incubated with 1 μg/mL dextran sulfate and murine factor XIa (5 nmol/L) at 37°C. (B) Murine factor XI autoactivation. Murine factor XI (5 μg/mL) was incubated with 1 μg/mL dextran sulfate at 37°C. (C) Murine factor XIa (5 μg/mL) was incubated with 1 μg/mL dextran sulfate at 37°C. Western blots were prepared for all experiments as described in Materials and Methods. The positions of molecular weight markers in kD are shown at the left of the figure. Abbreviations: fXI, factor XI zymogen; fXIc, factor XI cleaved; HC, factor XIa heavy chain; LC, factor XIa light chain; and C, cleavage product-possibly of the light chain. The sample in lane XIa is an activated murine factor XI standard prepared by incubating factor XI with factor XIIa in the absence of dextran sulfate.
Western blots of murine factor XI. (A) Murine factor XI activation by murine factor XIa in the presence of dextran sulfate. Murine factor XI (5 μg/mL) was incubated with 1 μg/mL dextran sulfate and murine factor XIa (5 nmol/L) at 37°C. (B) Murine factor XI autoactivation. Murine factor XI (5 μg/mL) was incubated with 1 μg/mL dextran sulfate at 37°C. (C) Murine factor XIa (5 μg/mL) was incubated with 1 μg/mL dextran sulfate at 37°C. Western blots were prepared for all experiments as described in Materials and Methods. The positions of molecular weight markers in kD are shown at the left of the figure. Abbreviations: fXI, factor XI zymogen; fXIc, factor XI cleaved; HC, factor XIa heavy chain; LC, factor XIa light chain; and C, cleavage product-possibly of the light chain. The sample in lane XIa is an activated murine factor XI standard prepared by incubating factor XI with factor XIIa in the absence of dextran sulfate.
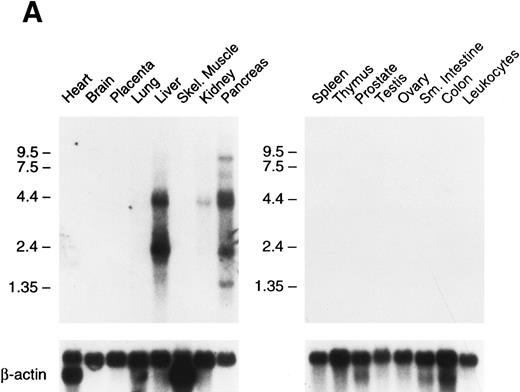
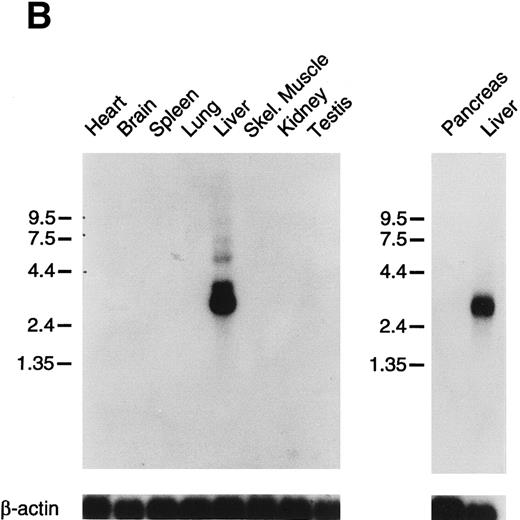
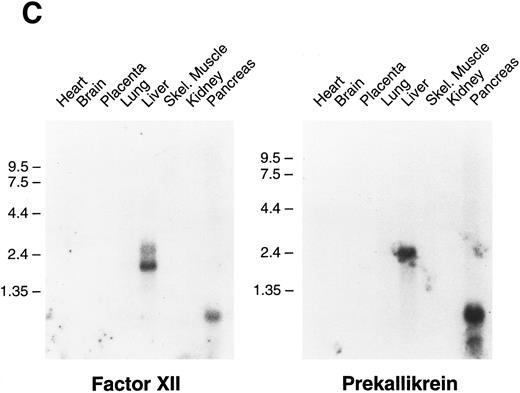
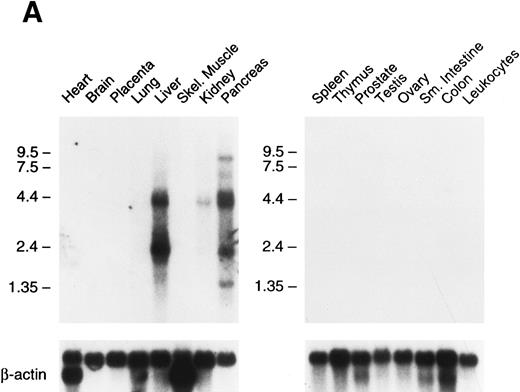
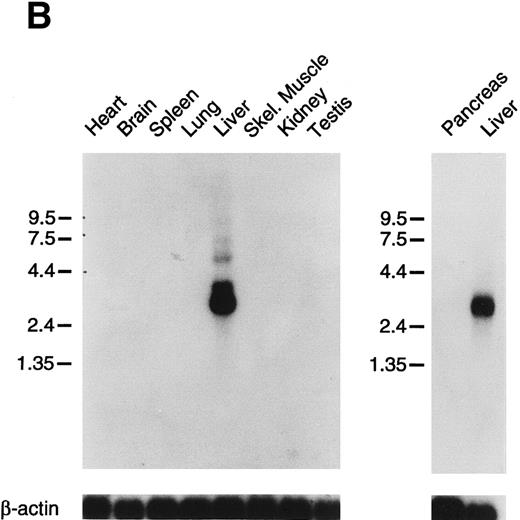
Tissue expression of factor XI messenger RNA.To identify tissues in which factor XI message is expressed, multitissue northern blots containing polyA RNA were initially hybridized with 32P-labeled full-length factor XI cDNA probes (Fig 6A and B). Factor XI message is present, as expected, in the liver of both species. Two messages are present in human liver at approximately 2.4 and 4.0 kb, while the mouse message is primarily a single species of 2.8 kb. Surprisingly, 2.4 and 4.0 kb messages were also detected in human pancreas and kidney (Fig 6A). The 2.4 kb message in kidney is not seen well on the blot shown, but was detected on some blots in several repeat experiments. Several higher molecular weight bands are seen in the pancreas lane and probably represent incompletely spliced factor XI mRNA. Identical high molecular weight bands were also detected in some experiments in RNA from liver. To examine the possibility that signals seen in human pancreas and kidney represent RNA species related to, but distinct from factor XI, the human factor XI cDNA was divided into three pieces and each was used as a probe for the northern blot. Identical results to those seen in Fig 6A were obtained with probes representing: (1) the signal peptide and first three apple domains, (2) the fourth apple domain and the first 15 amino acids of the light chain, and (3) the remainder of the catalytic light chain (data not shown). This indicates that the signals in human pancreas and kidney are, indeed, factor XI mRNA. The human Northern blots were subsequently probed with full-length cDNA probes for the human contact activation proteases, factor XII, and prekallikrein (Fig 6C). Full-length message for these proteins was detected only in RNA from liver. The signal at approximately 0.8 to 1.0 kb seen for both proteins in the pancreas lane is too small to encode either factor XII or prekallikrein and likely represents a related protease species, such as trypsinogen.
Northern blots of human and murine tissues for factor XI and contact activation protease expression. (A) Human tissue Northern blots hybridized with a full-length human factor XI cDNA probe, (B) murine tissue Northern blots hybridized with a full-length murine factor XI cDNA probe, and (C) human tissue Northern blots hybridized with either a full-length human factor XII or human prekallikrein cDNA probe. Blots were subsequently stripped and hybridized with a human β-actin cDNA probe to show equal lane loading of RNA. The position of molecular weight markers in kilobases is shown at the left of the figures.
Northern blots of human and murine tissues for factor XI and contact activation protease expression. (A) Human tissue Northern blots hybridized with a full-length human factor XI cDNA probe, (B) murine tissue Northern blots hybridized with a full-length murine factor XI cDNA probe, and (C) human tissue Northern blots hybridized with either a full-length human factor XII or human prekallikrein cDNA probe. Blots were subsequently stripped and hybridized with a human β-actin cDNA probe to show equal lane loading of RNA. The position of molecular weight markers in kilobases is shown at the left of the figures.
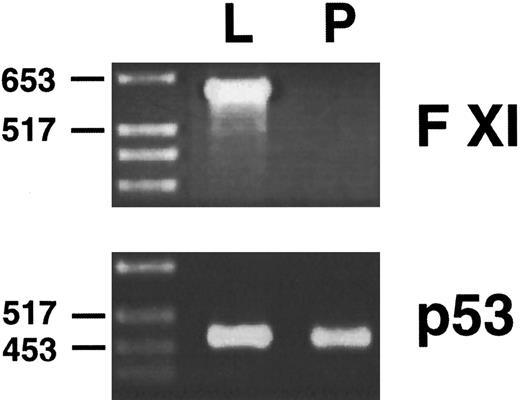
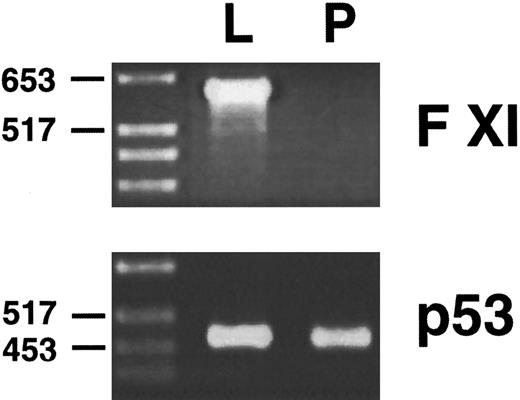
Northern blots are not particularly sensitive assays, and it is possible that a tissue such as murine pancreas could be expressing factor XI mRNA at levels below the detection threshold of the Northern blot. To examine this possibility, polyA RNA from murine liver and pancreas were reverse transcribed, and PCR was performed on the reverse transcribed material with oligonucletoides that specifically copy a 600-bp fragment from the murine factor XI cDNA. Oligonucleotides specific for the murine p53 cDNA were used as controls to demonstrate that reverse transcription was successful. This technique is several orders of magnitude more sensitive than northern blot hybridization. The results of this analysis (Fig 7) show that factor XI message, while easily detected in murine liver, is not present in murine pancreas.
Reverse transcription/PCR analysis of murine liver and pancreas for factor XI expression. PolyA RNA from liver (L) and pancreas (P) were reverse transcribed as described in Materials and Methods, followed by PCR with oligonucleotides specific for the murine factor XI or murine p53 cDNAs. PCR products were size fractionated on a 2.0% agarose gel followed by staining with ethidium bromide. The position of molecular weight markers in DNA kilobases is shown at the left of the figure.
Reverse transcription/PCR analysis of murine liver and pancreas for factor XI expression. PolyA RNA from liver (L) and pancreas (P) were reverse transcribed as described in Materials and Methods, followed by PCR with oligonucleotides specific for the murine factor XI or murine p53 cDNAs. PCR products were size fractionated on a 2.0% agarose gel followed by staining with ethidium bromide. The position of molecular weight markers in DNA kilobases is shown at the left of the figure.
DISCUSSION
We have successfully isolated and expressed a complimentary DNA for murine factor XI and compared the properties of this protein with human factor XI. While factor XI has been purified from the plasma of several species,5,29-31 the nucleotide and amino acid sequences are known only for the human form.17 Human factor XI consists of a C-terminal trypsin-like catalytic light chain and an N-terminal noncatalytic heavy chain comprised of four repeated 90-91 amino acid subunits called apple domains.5,6,17 Only the highly related protease plasma prekallikrein has been shown to contain similar domains.18 The murine factor XI cDNA predicts a high degree of sequence homology (78%) with human factor XI, and the two proteins appear to contain identical disulfide bonds and domain structures. A notable difference is the absence of the cystine residue at amino acid position eleven in murine factor XI. Cys11 in human factor XI forms a disulfide bond with a molecule of free cystine, and its function, if any, is not clear.27 Meijers et al32 have reported that this residue may be changed in recombinant factor XI to serine by site directed mutagenesis without altering the properties of the enzyme in an aPTT assay.
Human factor XI is unique among the proteases of the coagulation cascade in that it circulates as a disulfide bond-linked homodimer with two catalytic sites.5,17,32 The cystine residue involved in the interchain bond in the human cDNA (Cys321 ) is conserved in the murine cDNA and, indeed, murine factor XI is expressed as a dimer. Factor XI purified from bovine and porcine plasma are also dimeric, however, rabbit factor XI lacks the interchain disulfide bond.29-31 The significance of this is not clear and rabbit factor XI may circulate as a noncovalent dimer.31 In fact, mutation of Cys321 in human factor XI to serine does not affect dimer formation, as other interactions in the fourth apple domain effectively hold the dimer together.32 It is not known if factor XI must exist as a dimer to be functional in vivo. Recently, we prepared a factor XI mutant in which the entire fourth apple domain was replaced with the corresponding domain from prekallikrein (FXI/PKA4).24 This effectively removes the sequences required for dimer formation and results in a monomeric protein. FXI/PKA4 is activated by factor XIIa, and activated FXI/PKA4 activates factor IX with similar kinetic parameters to those of wild type factor XI.24
Human factor XI is activated by factor XIIa, thrombin, and activated factor XI (autoactivation) on a negatively charged surface.5,7,8,14 The amino acid sequence immediately preceding the activation cleavage site is of interest. Thrombin cleaves many of its substrates immediately after the sequence X-Pro-Arg (P3-P1 positions, respectively), and this sequence is present in both human and murine factor XI.17 By comparison, the sequence preceding the factor XIIa cleavage site in murine and human prekallikrein are Asn-Ala-Arg and Ser-Thr-Arg, respectively.18,23 These are not typical thrombin cleavage sites, and human prekallikrein is not activated by thrombin.33 We have prepared a recombinant factor XI molecule in which the factor XIIa cleavage site has been changed to that of prekallikrein (manuscript in preparation). This molecule is activated normally by factor XIIa, but is not activated by thrombin confirming the importance of this sequence to thrombin mediated cleavage. Like human factor XI, murine factor XI is activated by factor XIIa and thrombin. In contrast, the murine protein undergoes autoactivation poorly on dextran sulfate because murine factor XIa prefers to cleave its zymogen at a location other than the activation cleavage site. The physiologic importance of factor XI autoactivation is not clear. Factor XII and factor VII bound to tissue factor have been reported to undergo autoactivation, and one could postulate that these reactions may be physiologic triggers for contact activation and the extrinsic pathway, respectively.34 35 Alternatively, autoactivation could serve to amplify an enzymatic reaction after initial activation from another protease.
The major source of plasma factor XI is thought to be the liver. Factor XI levels decrease in liver disease, and a patient acquired mild factor XI deficiency after orthotopic liver transplantation from a factor XI–deficient donor.36 Factor XI activity has been reported to be associated with a 200-kD protein on platelet membranes, however, the activity has not been completely characterized, and it has not been definitively determined that this protein is a product of the factor XI gene.37 The identification of mRNA for human factor XI in organs other than the liver, therefore, is somewhat surprising. Furthermore, the absence of detectable expression in murine kidney and pancreas raises the possibility that factor XI may be required for somewhat different functions in the two species. The presence of mRNA in renal tissue is particularly intriguing as bleeding from the urinary tract, particularly after surgical procedures, is common in severe factor XI deficiency in humans.2,13 Factor XI has been postulated to be required for fibrin clot stability in the face of fibrinolysis, and the tissues of the urinary tract contain significant amounts of urokinase.11-14 Local expression of factor XI may, therefore, be required for maintaining fibrin integrity in the face of brisk fibrinolysis. The expression of factor XI mRNA in human pancreas is more difficult to explain. The result does not appear to be due to the nonspecific expression of a trypsin-like protease because mRNA for prekallikrein, a protease with a high degree of homology to factor XI, is not seen in pancreas. It is conceivable that factor XI has specific activities within the pancreas or gut, as trypsin has been shown to effectively activate human factor XI in a purified protein system.38
It is important to note that the presence of mRNA in a tissue may represent illegitimate transcription and is not proof of protein expression. We attempted to demonstrate factor XI protein in human liver tissue by immunohistochemistry using monoclonal antibodies24 that easily detect factor XI in human plasma by Western blot and enzyme-linked immunosorbent assay (ELISA) (data not shown). Unfortunately, these studies were not successful, possibly because the amount of factor XI in hepatic tissue is small. In the absence of a clear demonstration of factor XI protein, therefore, our northern blot results must be considered preliminary evidence for extra-hepatic expression of factor XI, rather than definitive proof. Even if organs other than the liver are expressing factor XI, it is unlikely that they contribute substantially to plasma levels of factor XI.
Our data show that murine and human factor XI are structurally homologous and have similar enzymatic properties, particularly in regard to the activation of factor IX. The autoactivation studies in the purified protein systems, however, suggest that there are subtle differences in enzymatic properties. Furthermore, there are differences in tissue specific expression of factor XI mRNA between humans and mice. These findings raise the possibility that factor XI functions in hemostasis differently in the two species, or that the protein may be involved in functions unrelated to hemostasis.
Supported by Grant No. HL02917 to D.G. from the National Heart, Lung and Blood Institute (Bethesda, MD). Y.S. is an Ortho Biotech Hematology Fellow.
Address reprint requests to David Gailani, MD, The Department of Pathology and Division of Hematology, Vanderbilt University School of Medicine, 538 Medical Research Bldg II, 2220 Pierce Ave, Nashville, TN 37232-6350.