Abstract
Fanconi anemia (FA) is an autosomal recessive disorder characterized by developmental defects, bone marrow failure, and cancer susceptibility. Cells derived from FA patients are sensitive to crosslinking agents and have a prolonged G2 phase, suggesting a cell cycle abnormality. Although transfection of type-C FA cells with the FAC cDNA corrects these cellular abnormalities, the molecular function of the FAC polypeptide remains unknown. In the current study we show that expression of the FAC polypeptide is regulated during cell cycle progression. In synchronized HeLa cells, FAC protein expression increased during S phase, was maximal at the G2 /M transition, and declined during M phase. In addition, the FAC protein coimmunoprecipitated with the cyclin-dependent kinase, cdc2. We next tested various mutant forms of the FAC polypeptide for binding to cdc2. A patient-derived mutant FAC polypeptide, containing a point mutation at L554P, failed to bind to cdc2. The FAC/cdc2 binding interaction therefore correlated with the functional activity of the FAC protein. Moreover, binding of FAC to cdc2 was mediated by the carboxyl-terminal 50 amino acids of FAC in a region of the protein required for FAC function. Taken together, our results suggest that the binding of FAC and cdc2 is required for normal G2 /M progression in mammalian cells. Absence of a functional interaction between FAC and cdc2 in FA cells may underlie the cell cycle abnormality and clinical abnormalities of FA.
FANCONI ANEMIA (FA) is an autosomal recessive disease characterized by congenital abnormalities, aplastic anemia, and cancer susceptibility.1-3 Compared with normal cells, FA cells exhibit genomic instability, increased percentage of cells in the G2 phase of the cell cycle, and increased cellular sensitivity to crosslinking agents.2,4,5 Somatic cell hybrid studies have revealed at least five complementation groups of FA.6-9 The cDNAs for two complementation groups (types A and C) have been cloned,10-13 and the chromosomal locus of one additional FA gene (type D) has been mapped.14 Cells derived from mice with targeted disruptions of the FAC gene also exhibit genomic instability comparable with that observed in FA cell lines.15,16 Although the function of the FAC polypeptide remains unknown, the protein has been reported to be localized to the cytoplasm and is therefore unlikely to play a direct role in DNA repair.17 18
Several studies suggest that FA results from a molecular defect in cell cycle progression. First, FA cells have a prolonged G2 transit time,19 which is enhanced by treatment with chemical crosslinking agents.20 Second, the G2 arrest and reduced proliferation of FA cells can be partially corrected by overexpression of a protein, SPHAR, which is a member of the cyclin family of proteins.21 Third, caffeine abrogates the G2 arrest of FA cells.22,23 Consistent with these results, caffeine is known to constitutively activate cdc224 and to override a normal G2 cell cycle checkpoint, which may be defective in FA cells. Fourth, other diseases characterized by genomic instability, such as Li-Fraumeni25 and ataxia telangiectasia (AT),26 result from abnormalities in cell cycle regulation.
The cell cycle abnormality, as evidenced by excessive percentage of G2 , and genomic instability of FA are independent of the p53 pathway. p53 is a tumor suppressor protein known to play an important role in G1 /S25,27 and in G2 /M transitions.28 Unlike AT cells,26 FA cells show normal p53 induction in response to MMC and other DNA damaging agents.29 30 Moreover, once p53 is induced in FA cells p53 dependent apoptosis is normal. Also, following treatment with MMC, FA cells and normal cells exhibit no differences in cell cycle progression from G1 phase to S phase. Taken together, these results suggest that p53 induction and G1 /S transition is normal in FA cells.
The regulation of cell cycle progression from G2 to M phase in mammalian cells has become better described. cdc2 is a cyclin-dependent kinase that assembles with cyclin A and cyclin B and directs G2 progression.31,32 Activated cdc2 phosphorylates multiple substrates, including p53, histone H1, and laminin, and regulates G2 progression. cdc2 is regulated posttranslationally in a series of phosphorylation and dephosphorylation events.33-35 The phosphorylation of cdc2 by the kinase wee1 results in decreased cdc2 kinase activity. The dephosphorylation of cdc2 by the phosphatase cdc25 results in increased cdc kinase activity and progression of the cell cycle from G2 to M phase. Interestingly, the control of activation of cdc2 by wee1 and cdc25 is sensitive to DNA damage.36-41 Cellular treatment with cisplatin, for example, results in increased tyrosine phosphorylation of cdc2 and cell cycle arrest at G2 /M.
In the current study, we show that expression of the FAC protein is regulated during the cell cycle, with peak levels observed at the G2 /M transition period. Also, FAC forms a physical complex with cdc2. Interestingly, FAC interacts with cdc2 through the carboxyl terminal 50 amino acids of FAC, known to be required for its biological activity. We hypothesize that this physical interaction between FAC and cdc2 may underlie the cell cycle abnormality of FA cells.
MATERIALS AND METHODS
Cell culture.Epstein-Barr virus (EBV) transformed lymphoblasts were maintained in suspension cultures in RPMI 1640 media supplemented with 15% heat-inactivated fetal calf serum (FCS) and grown in a humidified 5% CO2 -containing atmosphere at 37°C.10,17 A type-C FA lymphoblast cell line, HSC536N, was transfected with the wild-type FAC cDNA, as previously described.10 17 The resulting corrected cell line, HSC536N (+FAC), was maintained in the same conditions with the addition of 100 μg/mL hygromycin. HeLa cells were maintained in DMEM media and 10% FCS and grown in plates at 37°C. The NSK cell line, an EBV-immortalized line made from normal adult peripheral blood (PB) lymphocytes, was obtained from American Type Culture Collection (Rockville, MD).
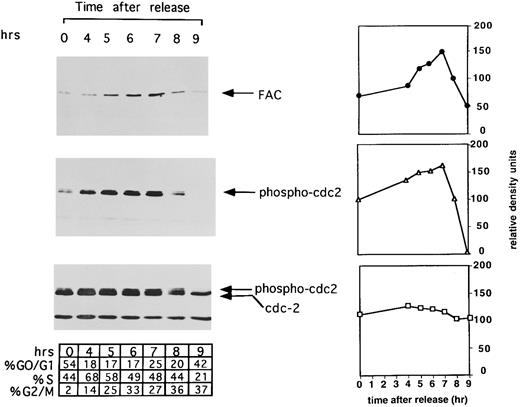
The expression of FAC is regulated during cell cycle progression. HeLa cells were synchronized at G1 /S by double thymidine block and released into regular media. Cell aliquots (1 mL) from the indicated timepoints were washed, suspended in propidium iodide, and assayed by FACS analysis, as described in Materials and Methods. The percentage of cells in each phase of the cell cycle was determined by analyzing FACS data with the computer program CELLFIT (Becton Dickinson). Synchronized cells from each time interval were lysed, whole cell extracts were prepared, and cellular proteins were immunoprecipitated and immunoblotted with an antiserum against FAC (top) or immunoprecipitated with monoclonal against cdc2 followed by immunoblotting with anti–phosphotyrosine-cdc2 (middle) or anti-cdc2 (bottom). Immunoblots were scanned and analyzed by NIH Image. Values along the y-axis are in relative density units and are plotted against time in hours after release from G1 /S synchrony. Each plot is placed along side the corresponding immunoblot with symbols as follows: FAC (•); tyrosine phosphorylated cdc2 (▵); and total cdc2 (□).
The expression of FAC is regulated during cell cycle progression. HeLa cells were synchronized at G1 /S by double thymidine block and released into regular media. Cell aliquots (1 mL) from the indicated timepoints were washed, suspended in propidium iodide, and assayed by FACS analysis, as described in Materials and Methods. The percentage of cells in each phase of the cell cycle was determined by analyzing FACS data with the computer program CELLFIT (Becton Dickinson). Synchronized cells from each time interval were lysed, whole cell extracts were prepared, and cellular proteins were immunoprecipitated and immunoblotted with an antiserum against FAC (top) or immunoprecipitated with monoclonal against cdc2 followed by immunoblotting with anti–phosphotyrosine-cdc2 (middle) or anti-cdc2 (bottom). Immunoblots were scanned and analyzed by NIH Image. Values along the y-axis are in relative density units and are plotted against time in hours after release from G1 /S synchrony. Each plot is placed along side the corresponding immunoblot with symbols as follows: FAC (•); tyrosine phosphorylated cdc2 (▵); and total cdc2 (□).
Cell synchronization.HeLa cells were synchronized as previously described.42 Briefly, cells (5 × 105/mL) were treated sequentially with 2 mmol/L thymidine for 17 hours, thymidine-free media for 10 hours, and an additional 2 mmol/L thymidine for 12 hours to arrest the cell cycle at the G1 /S boundary. The cells were then released into growth media (RPMI 1640 + 15% FCS) and analyzed at various time intervals. We have performed this method multiple times with consistent cell cycle kinetics. Nocodazole (2 μmol/L) was added for 8 hours after release to enrich for cell populations in G2 /M phase. Synchronized cells were analyzed at different timepoints by staining with propidium iodide as previously described.43 Approximately 10,000 cells were analyzed, and flow histograms were generated (Becton Dickinson, Franklin Lakes, NJ).
Mitomycin C treatment.Asynchronous cells or cells synchronized at G1 /S were incubated with MMC (0.1 μmol/L) for 24 hours. Asynchronous cells were treated for 24 hours and analyzed before the onset of apoptosis.30 Cells were obtained (5 × 105), washed in phosphate-buffered saline (PBS), and prepared for FACS analysis as above.
Metabolic labeling and immunoprecipitation.Metabolic labeling of cells with 35S-methionine and immunoprecipitation (IP) was performed as described previously.44 Whole cell extracts or extracts fractionated from nucleus or cytoplasm were prepared as previously described.17 Extracts (1-mL vol) in TBS (50 mmol/L Tris, 150 mmol/L NaCl, 1% Triton X-100, 0.2% sodium dodecyl sulfate [SDS]) were precleared with preimmune serum and mixed with an affinity purified anti-FAC antiserum (2 μg).17 Protein A–sepharose bound immune complexes were washed three times in TBS containing 1% Triton X-100 and 0.1% SDS and subjected to 10% polyacrylamide SDS gel electrophoresis (PAGE). Following electrophoresis, gels were sequentially washed in concentrated acetic acid, 2,5-diphenyloxazole (PPO), and glycerol. Dried gels were exposed to XAR50 film (Kodak, Rochester, NY) at −70°C.
Absorption of cellular proteins with glutathione-S-transferase fusion proteins.Glutathione-S-transferase fusion proteins containing the amino terminal 100 amino acids of FAC (GST-N), the carboxyl terminal 280 amino acids of FAC (GST-FAC), the carboxyl terminal 280 amino acids of FAC containing the L554P mutation (GST-L554P), and the carboxyl terminal 50 amino acids of FAC (GST-C1) were cloned into the pGEX2T vector (Clontech, Palo Alto, CA), as previously described.17 GST-fusion proteins were prepared by expression in Escherichia coli, cell lysis, incubation with glutathione-sepharose beads, and elution with glutathione. Protein concentrations were determined by the method of Bradford.45 GST-fusion protein (5 μg) was prebound to glutathione sepharose beads (Pharmacia, Piscataway, NJ; 50 μL) in 250 μL of lysis buffer. Washed beads were then added to cell lysates (2 mg protein/mL) prepared from HeLa cells. Lysates were precleared with an overnight incubation with GST bound to glutathione-sepharose beads. The beads were washed three times (0.1% Triton-X 100, 50 mmol/L Tris pH 7.4, 150 mmol/L NaCl) and boiled in loading buffer (100 mmol/L sodium phosphate pH 7.2, 1% SDS, 10% glycerol, 0.001% bromophenol blue) before SDS-PAGE.
Western blotting.Proteins electrophoresed on SDS-PAGE gels were transferred to nitrocellulose in transfer buffer (Tris 25 mmol/L, glycine 200 mmol/L) at 400 mA at 4°C. Ponceau stain (Sigma) was performed to ensure equal loading. Filters were blocked for 1 hour in 5% milk in TBS (50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl) and were incubated in primary antibody in TBS plus Tween20 (TBS-T) overnight. After wash in TBS-T and exposure to horseradish peroxidase-protein A, enzyme-linked chemiluminescence (Amersham, Arlington Heights, IL) was performed. Densitometry was performed on Western blots by scanning into a computer file, followed by analysis using NIH Image.
RESULTS
Expression of the FAC polypeptide is regulated during the cell cycle.Initially, we analyzed the expression of FAC during the cell cycle. HeLa cells were synchronized by double thymidine block,42 released into S phase, and analyzed at different times (Fig 1). FAC protein was immunoprecipitated and immunoblotted with an anti-FAC antiserum (Fig 1, FAC immunoblot). FAC expression increased during S phase (hours 4 to 6), reached a maximum at G2 /M (hour 7), and declined during mitosis (hour 9). To ensure synchrony throughout the cell cycle, cdc2 was immunoprecipitated and immunoblotted from the same cell extracts. Previous studies have shown that cdc2 remains tyrosine phosphorylated until the G2 /M transition when it is dephosphorylated by cdc25.32,33 Cdc2 was dephosphorylated at 9 hours following release from double thymidine block (Fig 1, phospho-cdc2 immunoblot), showing cell synchronization throughout S phase and G2 phase. Unphosphorylated cdc2 protein levels remained constant throughout the cell cycle, whereas phosphorylated forms increased and then decreased (Fig 1, cdc2 immunoblot), consistent with previous studies.32 46 To quantify the protein levels detected by the immunoprecipitation/Western blot experiment, we analyzed the autoradiography by densitometry (NIH Image). The densitometry quantification, expressed in relative density units, confirmed the increase and decrease in the amount of FAC protein and tyrosine phosphorylated cdc2 during the cell cycle, whereas total cdc2 remained relatively stable.
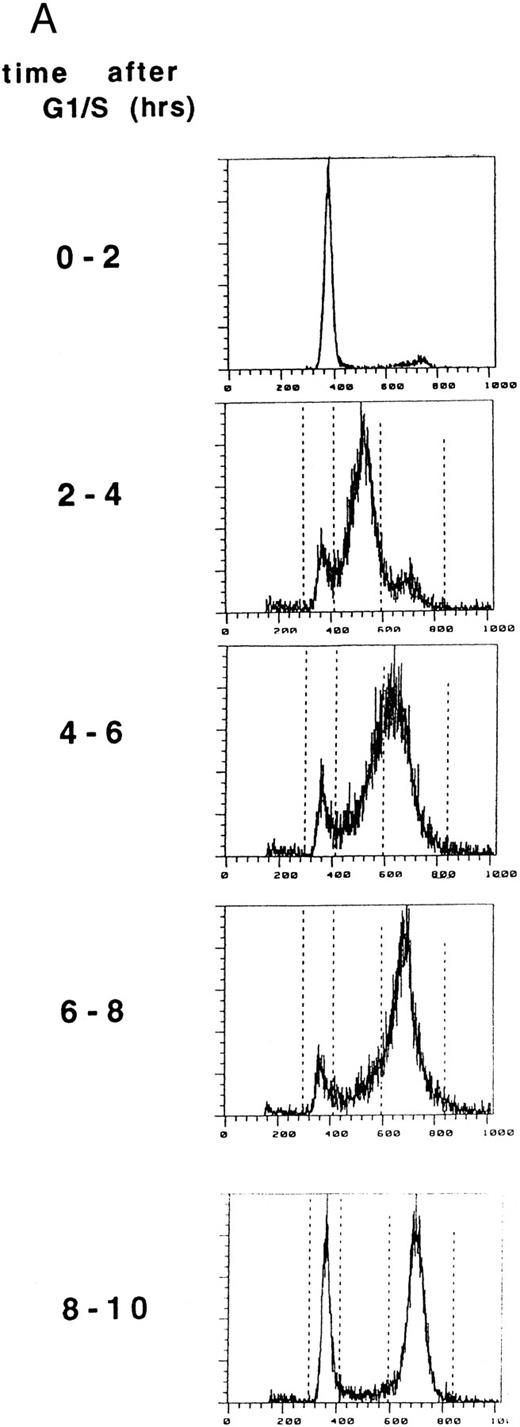
FAC localization does not change during the cell cycle. (A) HeLa cells were synchronized at G1 /S by double thymidine block and released into growth media. Cell synchrony was analyzed by FACS, as described in Materials and Methods. The percentage of cells in each phase of the cell cycle was determined by analyzing FACS data with the computer program CELLFIT (Becton Dickinson).
(B) Cells were metabolically labeled with 35S-methionine during the 2-hour intervals indicated. Proteins from whole cells (T), nuclear (N) extracts, or cytoplasmic (C) extracts were immunoprecipitated with anti-FAC antibody. Alternatively, protein from whole cells was immunoprecipitated with a preimmune serum (P). Immune complexes were resolved by SDS-PAGE. Molecular weight markers are in kilodaltons.
FAC localization does not change during the cell cycle. (A) HeLa cells were synchronized at G1 /S by double thymidine block and released into growth media. Cell synchrony was analyzed by FACS, as described in Materials and Methods. The percentage of cells in each phase of the cell cycle was determined by analyzing FACS data with the computer program CELLFIT (Becton Dickinson).
(B) Cells were metabolically labeled with 35S-methionine during the 2-hour intervals indicated. Proteins from whole cells (T), nuclear (N) extracts, or cytoplasmic (C) extracts were immunoprecipitated with anti-FAC antibody. Alternatively, protein from whole cells was immunoprecipitated with a preimmune serum (P). Immune complexes were resolved by SDS-PAGE. Molecular weight markers are in kilodaltons.
We next examined the cellular localization of the FAC polypeptide during the cell cycle (Fig 2). In a separate experiment, HeLa cells were again synchronized at G1 /S, using a double thymidine block (Fig 2A). Following release from the block, the progression of the cells through the cell cycle was monitored by DNA flow histograms every 2 hours. During each 2-hour period shown, cells were metabolically labeled with 35S-methionine, lysed, and fractionated into cytoplasmic and nuclear fractions.17 Labeled proteins were immunoprecipitated with an anti-FAC antiserum. The FAC polypeptide was localized to the cytoplasmic fraction throughout the cell cycle (compare lanes 3, 7, 12, 15, and 19). Although metabolic labeling and IP is not as quantitative as IP followed by Western blot, this experiment suggests that FAC expression was maximal during the period 6 to 8 hours after release from G1 /S arrest (lane 14), consistent with results in Fig 1. A 34-kD protein coimmunoprecipitated with FAC from total cell lysates prepared from cells labeled 4 to 6 hours (lane 10) and 6 to 8 hours (lane 14) after release.
cdc2 binds to the FAC polypeptide.We next used a coimmunoprecipitation procedure to identify proteins that bind to the FAC polypeptide (Fig 3). Given the abnormal cell cycle phenotype of FA cells and the presence of a 34-kD protein (p34) in Fig 2, we tested if p34 was immunoreactive with a specific monoclonal antibody against a cyclin-dependent kinase, cdc2. An immunoprecipitation with FAC antiserum followed by immunoblotting with a monoclonal antibody to cdc2 (Santa Cruz, Santa Cruz, CA) identified p34 as a cdc2 (lane 2).
The FAC polypeptide binds to the cyclin-dependent kinase, cdc2. Proteins from HSC536N (+FAC) were immunoprecipitated with a preimmune (lane 1) or with anti-FAC affinity purified antibody (lane 2). SDS-PAGE gel was blotted onto nitrocellulose and probed with anti-cdc2 monoclonal antibody or anti-FAC antibody. Molecular weight markers are in kilodaltons.
The FAC polypeptide binds to the cyclin-dependent kinase, cdc2. Proteins from HSC536N (+FAC) were immunoprecipitated with a preimmune (lane 1) or with anti-FAC affinity purified antibody (lane 2). SDS-PAGE gel was blotted onto nitrocellulose and probed with anti-cdc2 monoclonal antibody or anti-FAC antibody. Molecular weight markers are in kilodaltons.
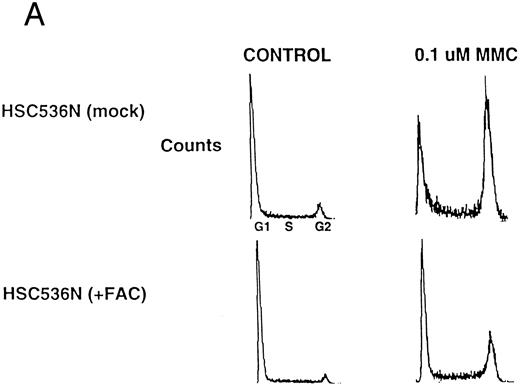
cdc2 binding correlates with the functional activity of FAC.Previous studies have shown that expression of wild-type FAC polypeptide in FA(C) cells confers MMC resistance and corrects the G2 abnormality of FA(C) lymphoblasts.10 43 An FA(C) cell line, HSC536N (mock), expressing a mutant form of the FAC polypeptide, FAC(L554P), accumulated in the G2 phase following treatment with MMC, 0.1 μmol/L, for 24 hours (Fig 4A, upper right). An isogenic cell line, HSC536N (+FAC), transfected with the wild-type FAC cDNA, displayed less accumulation in G2 phase (Fig 4A, lower right). We next tested for the differential ability of wild-type FAC protein and mutant FAC protein to bind to cdc2 (Fig 4B). The mutant FAC protein (FAC L554P) was immunoprecipitated from HSC536N (mock) cells by two independent anti-FAC antisera (Fig 4B, FAC immunoblot, lanes 2 and 4). The mutant FAC protein had the same electrophoretic mobility as the wild-type FAC protein immunoprecipitated from the HSC536N (+FAC) cells (FAC immunoblot, lanes 1 and 3). Interestingly, the wild-type FAC protein coimmunoprecipitated with cdc2 (Fig 4B, cdc2 immunoblot, lanes 1 and 3). Mutant FAC protein (L554P) failed to coimmunoprecipitate with cdc2 (cdc2 immunoblot, lanes 2 and 4).
The FAC/cdc2 interaction correlates with the functional activity of FAC. (A) HSC536N (mock) cells and HSC536N (+FAC) cells were harvested following MMC treatment, stained with propidium iodide, and analyzed by FACS, as described in Materials and Methods. (B) Whole cell extracts from HSC536N (mock) cells (lanes 2 and 4) or HSC536N (+FAC) cells (lanes 1 and 3) were immunoprecipitated with affinity purified anti-FAC antisera prepared from two different New Zealand white rabbits. Immunoprecipitated proteins were electrophoresed by SDS-PAGE, blotted to nitrocellulose, and probed with anti-cdc2 antiserum or anti-FAC antiserum. Molecular weight markers are in kilodaltons.
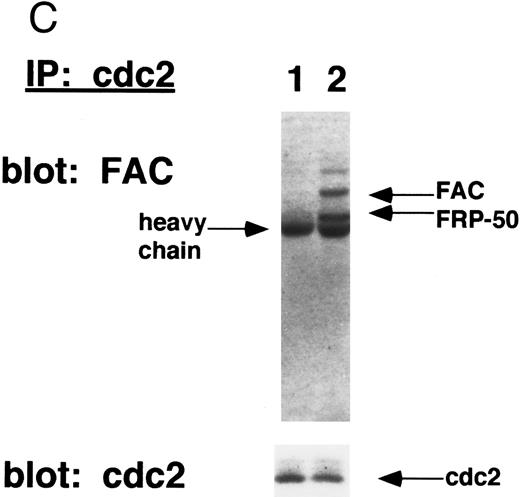
(C) Whole cell extracts from HSC536N (mock) cells (lane 1) or HSC536N (+FAC) cells (lane 2) were immunoprecipitated with anti-cdc2 monoclonal antibody. Immunoprecipitated proteins were electrophoresed by SDS-PAGE, blotted to nitrocellulose, and probed with affinity purified anti-FAC antisera. Molecular weight markers in kilodaltons.
The FAC/cdc2 interaction correlates with the functional activity of FAC. (A) HSC536N (mock) cells and HSC536N (+FAC) cells were harvested following MMC treatment, stained with propidium iodide, and analyzed by FACS, as described in Materials and Methods. (B) Whole cell extracts from HSC536N (mock) cells (lanes 2 and 4) or HSC536N (+FAC) cells (lanes 1 and 3) were immunoprecipitated with affinity purified anti-FAC antisera prepared from two different New Zealand white rabbits. Immunoprecipitated proteins were electrophoresed by SDS-PAGE, blotted to nitrocellulose, and probed with anti-cdc2 antiserum or anti-FAC antiserum. Molecular weight markers are in kilodaltons.
(C) Whole cell extracts from HSC536N (mock) cells (lane 1) or HSC536N (+FAC) cells (lane 2) were immunoprecipitated with anti-cdc2 monoclonal antibody. Immunoprecipitated proteins were electrophoresed by SDS-PAGE, blotted to nitrocellulose, and probed with affinity purified anti-FAC antisera. Molecular weight markers in kilodaltons.
To further confirm the binding of cdc2 with wild-type FAC, we next performed a reciprocal IP (Fig 4C). cdc2 was immunoprecipitated from either HSC536N (mock) or HSC536N (+FAC) cells. Again, wild-type FAC and a naturally occurring truncated variant of FAC, Fanconi-related protein 50 (FRP-50),17 coimmunoprecipitated with cdc2 (FAC immunoblot, lane 2). Mutant FAC failed to bind cdc2 (lane 1).
These data suggest that mutant FAC fails to bind directly to cdc2 or fails to colocalize to the proper cellular compartment in which cdc2 binding occurs. The cdc2 binding activity of the FAC protein therefore correlates with its ability to correct the G2 cell cycle abnormality.
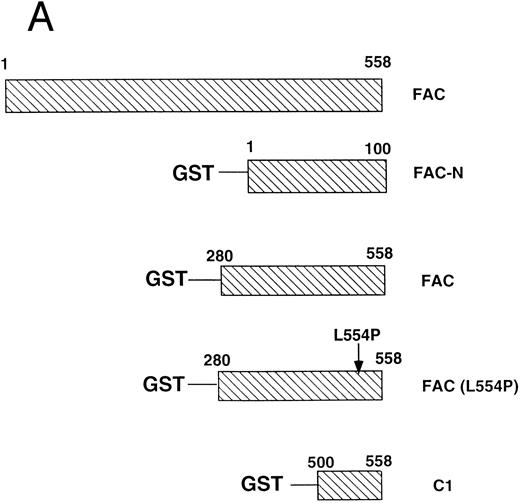
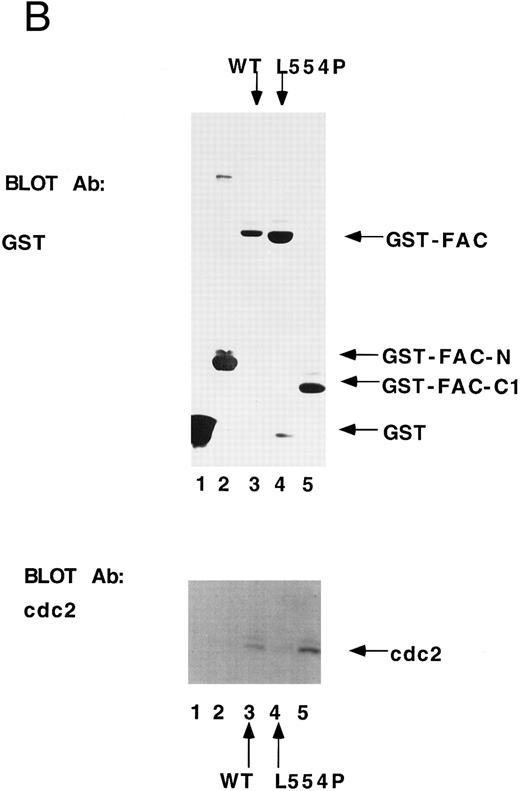
cdc2 specifically binds to the carboxyl terminus of FAC.Previous studies have shown that the carboxyl terminus of the FAC protein is required for its biological activity.10,43 47-49 To assess a direct binding interaction between FAC and cdc2, we prepared GST fusion proteins containing variable regions of the FAC polypeptide (Fig 5A). The GST-FAC fusion proteins were prebound to glutathione sepharose and incubated with cellular proteins prepared from HeLa cells first synchronized by double thymidine block at G1 /S and then released for 8 hours in the presence of nocodazole to enrich for cells at G2 /M. All fusion proteins were added at comparable levels, as judged by anti-GST immunoblot (Fig 5B, GST immunoblot). Fusion proteins containing the wild-type carboxyl terminus of FAC bound to cdc2 (Fig 5B, cdc2 immunoblot, lanes 3 and 5), whereas fusion proteins derived from the N-terminus of FAC (lane 2) or containing the carboxyl terminus with the L554P missense mutation (lane 4) failed to bind to cdc2. Taken together, these results suggest that the binding of FAC and cdc2 is mediated by the carboxyl terminal 50 amino acids of FAC.
cdc2 specifically binds to the carboxyl terminus of FAC. (A) GST fusion proteins used for in vitro mixing experiments are shown schematically. (B) Whole cell extract (unlabeled) was made from HeLa cells first synchronized at G1 /S by double thymidine block and then released for 8 hours in nocodazole.41 GST (lane 1), GST-N (lane 2), GST-FAC (wild-type; lane 3), GST-FAC (L554P, lane 4), or GST-C1 (lane 5), prebound to glutathione-sepharose beads, was mixed with the extract. Bound cellular proteins were electrophoresed by SDS-PAGE and transferred to nitrocellulose. The filter was probed with either anti-GST monoclonal antibody or anti-cdc2 monoclonal antibody.
cdc2 specifically binds to the carboxyl terminus of FAC. (A) GST fusion proteins used for in vitro mixing experiments are shown schematically. (B) Whole cell extract (unlabeled) was made from HeLa cells first synchronized at G1 /S by double thymidine block and then released for 8 hours in nocodazole.41 GST (lane 1), GST-N (lane 2), GST-FAC (wild-type; lane 3), GST-FAC (L554P, lane 4), or GST-C1 (lane 5), prebound to glutathione-sepharose beads, was mixed with the extract. Bound cellular proteins were electrophoresed by SDS-PAGE and transferred to nitrocellulose. The filter was probed with either anti-GST monoclonal antibody or anti-cdc2 monoclonal antibody.
DISCUSSION
Previous studies have suggested that FA cells have a defect in cell cycle progression, confined to the G2 /M transition phase.2,4,5 In the current manuscript, we show that expression of FAC protein is regulated during the cell cycle, with peak expression at the G2 /M transition point. Also, a 34-kD coprecipitating protein is observed, consistent with a previous report that FAC binds to a protein of 34 kD.50 We have shown that FAC binds to cdc2, which may well be one of the previously reported FAC-binding proteins. The binding interaction between FAC and cdc2 is mediated by the carboxyl terminus of FAC, a region important for functional activity of FAC in the cell. A FA patient-derived nonfunctional FAC polypeptide, with a L554P mutation in the carboxyl terminus, failed to bind to cdc2.
There are several cellular factors that may regulate the interaction between FAC and cdc2. Because FAC mRNA levels change minimally during cell cycle progression (data not shown), FAC protein levels appear to be regulated at a posttranscription level. Like other cdc2 binding proteins, such as cyclin B, FAC accumulates during S phase and is degraded during M phase. This similar pattern of degradation of cyclin B and FAC in mitosis may result from a common degradation mechanism, such as the anaphase promoting complex.51 Our data suggest that the FAC/cdc2 binding interaction is not regulated at the level of protein localization. FAC remains in the cytoplasm throughout the cell cycle. In addition, the binding interaction does not appear to be regulated by phosphorylation. There is no evidence for phosphorylation of FAC (Yamashita, unpublished observation, July 1994), and FAC does not contain consensus cdc2 phosphorylation sites. In contrast to FAC, unphosphorylated cdc2 levels remain constant during the cell cycle (Fig 1C), consistent with previous studies.32,46 Also, cdc2 is found in both the cytoplasm and the nucleus.52 Interestingly, the phosphorylation state and kinase activity of cdc2 are regulated during cell cycle progression. cdc2 is tyrosine phosphorylated on Y15 during S phase and dephosphorylated by cdc25 during the G2 /M transition.32-34,53 54 Taken together, our data suggest that the FAC/cdc2 interaction may be regulated by the amount of FAC in the cell or by the state of phosphorylation or activity of cdc2. Whether or not additional cellular proteins, such as cyclin B, bind to the FAC/cdc2 complex remains unknown.
The significance of the FAC/cdc2 binding interaction also remains unclear. Several potential cellular events resulting from the interaction are possible. In one model, FAC might be downstream of the cdc2 kinase. Once activated, cdc2 is known to phosphorylate several protein substrates, which mediate mitotic cellular events, including nuclear membrane disassembly and chromosome condensation.32 Consistent with this model, FAC may be a component of a mitotic DNA repair pathway downstream of active cdc2, similar to pathways observed in yeast.55-57 In an alternative model, FAC may bind and functionally regulate the activity of cdc2 by acting in concert with a cdk inhibitor that could couple a transient G2 /M pause with DNA repair. cdc2 exhibits persistent tyrosine phosphorylation after DNA damage, resulting in decreased cdc2/cyclin B kinase activity and cell cycle arrest.37,58 59 The interaction of cdc2 and FAC provides the opportunity for modulating this DNA damage-inducible checkpoint. In either case, a signal transduction cascade triggered by DNA damage might result, requiring interaction of cell cycle and DNA repair machinery at a G2 /M checkpoint.
The functional interaction between cdc2 and FAC also suggests that mutations in cdc2 could result in cancer susceptibility and an FA phenotype. The cdc2 gene (chromosome 10) does not map to known FA gene loci, but cdc2-like kinases and cdk inhibitors have been linked to tumor susceptibility.31,60 61 Accordingly, our data would suggest that the FAC pathway is inactive in cdc2 mutant cell lines.
Although the significance of the FAC/cdc2 protein interaction remains unclear, the interaction is consistent with the cell cycle abnormality of increased G2 percentage previously observed in FA cells.2,19 62 FAC levels and FAC/cdc2 interaction are maximal at the G2 /M transition. Accordingly, this is the point in the cell cycle at which FAC cells (lacking functional FAC protein) accumulate following crosslinking damage. Cell lines derived from FA patients with other types also accumulate abnormally at the G2 /M transition (Kupfer, unpublished observations, May 1996). As other FA genes are cloned, it will be interesting to investigate binding interactions between these proteins and the FAC/cdc2 complex.
ACKNOWLEDGMENT
We thank David Pellman, Bernard Mathey-Prevot, Chiang Li, and members of the D'Andrea laboratory for helpful discussions.
Supported in part by National Institutes of Health Grant No. R01-HL5725. G.M.K. is supported by Physician-Scientist Award No. K08-H103420, A.D.D. is a Scholar of the Leukemia Society of America (LSA), and D.N. is a Fellow of the LSA.
Address reprint requests to Gary M. Kupfer, MD, Division of Pediatric Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal