Abstract
Low levels of telomerase activity have recently been detected in human primitive hematopoietic cells, however, blood cells exhibit telomere shortening on cell proliferation. This challenging observation led us to study telomerase regulation and telomere length in human hematopoietic progenitor cells from fetal liver (FL), cord blood (CB), peripheral blood (PB), and bone marrow (BM). We found telomerase activity in CD34+/CD38+ cells exceeding levels in CD34+/CD38−, CD34−, and mononuclear cells (P < .05). Baseline telomerase activity was highest in BM (n = 5) CD34+ cells, followed by PB (n = 20), CB (n = 11), and FL (n = 1). Within 48 hours to 72 hours of in vitro culture of CD34+ cells in the presence of cytokines (KL, interleukin-3 [IL-3], IL-6, erythropoietin, granulocyte colony-stimulating factor), telomerase activity was upregulated, peaked after 1 week of culture, and decreased to baseline levels or below detection after 3 to 4 weeks. Stimulation of CD34+ cells with single cytokines resulted in no or minor telomerase upregulation, whereas cytokine combinations resulted in a significant telomerase increase (P < .001). There was a correlation between telomerase activity, cell cycle status by BrdU incorporation, and induction of phosphorylated retinoblastoma protein, CDC2, CDK2, cyclin D1, and cyclin A, but not cyclin E and B1 after 72 hours with multiple (but not single) cytokines. In nonexpanding CD34+ cells, telomerase was low or undetectable. Secondary CD34+ cells showed a reduced ability to upregulate telomerase activity. Antiproliferative cytokines such as transforming growth factor-β1 and high concentrations of all-trans–retinoic acid in cytokine-supported CD34+ cultures downmodulated telomerase activity. Average telomere lengths were 10.4 kbp, 7.4 kbp, and 7.6 kbp in CB, PB, and BM CD34+ cells, respectively. In ex vivo expansion cultures, an average telomeric DNA loss of 1 to 2 kbp over 4 weeks was observed. However, the rate of base pair loss per population doubling was significantly lower during the first 2 weeks, when telomerase was upregulated, than during weeks 3 and 4 of culture. In summary, telomerase is upregulated in response to cytokine-induced proliferation and cell cycle activation in primitive hematopoietic cells. Telomerase is downregulated between weeks 3 and 4 of ex vivo expansion culture linked with decreased proliferation and greater expansion of more mature cell subsets. Our data suggest that telomerase activity in hematopoietic cells reduces, but does not prevent, telomere shortening on proliferation.
HUMAN TELOMERES are regions of tandem TTAGGG repeats at chromosomal ends that protect chromosomes from degradation, fusion, and recombination.1 Because of the end replication problem, a loss of 50 to 100 bp occurs with each cell replication leaving nonreplicated DNA at the 3′ end of the DNA template in the absence of compensatory mechanisms.2,3 In most normal somatic cells such as fibroblasts, blood leukocytes, and endothelial cells, telomere shortening has been observed.4-6 When telomeres reach a critical degree of shortening, cells lose their proliferative potential, suggesting that telomeres signal cell senescence and can be used to measure population doublings and the replicative history of cells.7-13 In contrast, spontaneously immortalized tumor cell lines and germ cell tissue show stable telomeres and unlimited proliferative potential, which is thought to be due to telomerase activation compensating for telomere base pair loss by adding TTAGGG repeats onto chromosomes.1-6,13-15 Although in most somatic cells telomerase activity is lacking, primitive hematopoietic cells have recently been shown to exhibit low telomerase activity.4-6,16,17 Despite telomerase activity, telomere shortening is observed in blood leukocytes with age and in vitro hematopoietic progenitor cultures.8-10
This observation led us to thoroughly study telomerase and telomere length regulation in hematopoietic cells. CD34+ cells from various sources were analyzed both in unpertubed conditions and in ex vivo expansion cultures. Telomerase activity was detected in CD34+ cells from all sources and increased significantly after ex vivo expansion. As there is an indication that telomerase activity may be related to cell cycle entry in tumor cells,18,19 we analyzed telomerase activity and cell cycle status in primitive hematopoietic cells during ex vivo expansion. Using the rapid and highly sensitive telomeric repeat amplification protocol (TRAP) assay, we found that proliferation of ex vivo expanded CD34+ cells and cell cycle activation in response to cytokine support was closely linked with telomerase upregulation suggesting that telomerase is activated as cells progress from G0 into S-phase. The regulation of cell proliferation is known to be controlled by a series of essential proteins including cyclins and cyclin-dependent kinases (CDKs), which enable cells to proceed through specific phases of the cell cycle: the preparatory or checkpoint phases (G1 and G2), DNA synthesis (S), and mitosis (M).20-22 The activities of CDKs appear to be most critical to the restriction point transitions, and these are positively regulated by their binding to D-type cyclins.20-22 CDKs associate with G1 cyclins and proliferating cell nuclear antigen to form a catalytically active kinase complex that phosphorylates RB protein at the G1/S transition, allowing release of the transcription factor E2F, which activates the expression of genes required in DNA synthesis.23 The CDK2 plays an important role in progression from G1 through S in dividing cells, as it is associated with cyclin D1 in late G1/early S phase, and with cyclin A in the S-phase.23-25
Despite telomerase activity, we found an average telomeric DNA loss of 1 to 2 kbp within 4 weeks of ex vivo expansion culture suggesting that hematopoietic cells lose telomeric DNA on each cell division. Therefore, telomerase activity may not be sufficient to completely prevent telomere shortening. The estimated rate of base pair loss per population doubling (PD) was, however, significantly lower during the first 2 weeks, when telomerase was upregulated, than during weeks 3 and 4 of culture. Our data supports the prevailing hypothesis that telomerase activity in primitive hematopoietic cells reduces rather than completely eliminates telomere loss on proliferation, and may thus extend the proliferative life span of hematopoietic cells.
MATERIALS AND METHODS
Cell source and separation of CD34+ cells and subsets.Fetal liver (FL) and cord blood (CB) to be discarded were collected. Discarded CB was exempt from the consent process according to the Institutional Review Board of Research Associates of the New York University Medical Center. Bone marrow (BM) was obtained from healthy donors and peripheral blood (PB) from patients undergoing leukapheresis as part of high-dose chemotherapy protocols with peripheral progenitor support. Leukapheresis products (1 mL) were collected from untreated ovarian cancer (n = 13) or stage IV breast cancer (n = 7) patients. The mobilization regimens consisted of cyclophosphamide 3 g/m2 intravenously followed by granulocyte colony-stimulating factor (G-CSF ) for 14 days. Informed written consent for FL, BM, and PB was obtained previously. Heparinized mononuclear cells (MNC) from all hematopoietic sources were separated by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density-gradient centrifugation (2,000 rpm for 30 minutes), suspended at 1 × 108/mL and incubated for 30 minutes with 50 μg/mL of a mouse monoclonal IgG1 anti-CD34+ antibody (developed in this laboratory and available as clone 11.1.6, Oncogene Science, Uniondale, NY).26 CD34+ cells were selected using the CellPro Ceprate System (kindly provided by CellPro, Bothell, WA) with immunomagnetic beads (Dynal A.S., Oslo, Norway) or colloidal superparamagnetic MultiSort MicroBeads (kindly provided by Miltenyi Biotec, Auburn, CA) according to the manufacturer's recommendation. For separation with the CellPro system, MNCs were washed twice with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), resuspended in 1% BSA to a concentration of 1 to 2 × 108 cells/mL, and incubated for 25 minutes with a biotinylated IgM, mouse antihuman antibody to the CD34 antigen at room temperature. The cells were washed with 1% BSA to remove excess antibody, resuspended in 5% BSA at a concentration of 1 to 2 × 108/mL, and were passed through an avidin column. Using immunomagnetic beads, MNCs were washed twice with 0.1% BSA in PBS and resuspended to 1 × 108 cells/mL. The antimouse IgG1 (Fc) antibody was added to the cells at a concentration of 50 μg/mL for 30 minutes at 4°C. Cells were washed twice with 0.1% BSA in PBS, and immunomagnetic beads were added for 30 minutes at 4°C (4.5 mg/mL, Dynal Inc, Great Neck, NY) at a bead/cell ratio of 16:1. Cells were visualized microscopically for bead rosetting. Rosettes were incubated overnight to allow cell detachment and recovered using a magnet separator. Using the magnetic separation with the Miltenyi System, MNCs were resuspended in 300 μL buffer (buffer: PBS pH 7.2, supplemented with 0.5% BSA and 2 mmol/L EDTA) at a concentration of 1 × 108 cells/mL and incubated with 50 μg/mL of the antimouse IgG1 monoclonal CD34+ antibody and 100 μL CD34+ MultiSort Micro beads for 30 minutes at 6°C. The cell suspension was loaded onto the column, and the negative cells were allowed to pass through. The column was washed, removed from the separator, placed onto a collection tube, and the CD34+ cells were flushed out. To achieve a purity of greater than 90%, the positive fraction was loaded onto a new column and the separation repeated as described above. Cells collected in the CD34+ and CD34− fraction were counted and the percentage of cells in the CD34+ fraction was determined. CD34+ cells collected were subject to progenitor assay (colony assay), suspension culture assay (Delta assay) and frozen at −80°C for TRAP and telomere restriction fragment (TRF) analysis.
CD34+/CD38+ separation.Using the Miltenyi system, CD34+ cells were positively selected as described above. The magnetically preenriched CD34+ cells (>80% CD34+) were incubated with 20 μL release reagent (MACS MultiSort Release Reagent) for 10 minutes at 6°C and loaded onto a column to remove any remaining magnetically labeled cells. The release reaction was stopped using 30 μL of Stop Reagent (MACS MultiSort Stop Reagent) for each 1 × 107 cells/mL and labeled with CD38− fluorescein isothiocyanate (FITC)-antibody (Immunotech Inc, Westbrook, ME) at a concentration of 10 μL for 1 × 106 cells/mL. Anti-FITC MicroBeads (10 μL for 1 × 107 cells/mL) were added to magnetically labeled cells for the second marker, incubated for 15 minutes at 6°C, and separated as described before. The CD34+/CD38− cells were enriched by depletion of CD38+ cells from preselected CD34+ cells. The purity of both CD34+/CD38+ and CD34+/CD38− fractions was determined by flow cytometry.
Colony assay.CD34+ cells (1 × 103/mL) and ex vivo expanded CD34+ cells (2 × 103 to 4 × 104/mL) were cultured in triplicate in Iscove's modified Dulbecco's medium (IMDM) containing 0.36% agarose (FMC Bioproducts, Rockland, ME), 20% fetal calf serum (FCS), and cytokines.26 Following 14 days of incubation, colony-forming unit–granulocyte-macrophage (CFU-GM) were scored using an inverted microscope.
Cytokines.For various assays and suspension cultures, combinations of the following cytokines were used. Recombinant human G-CSF (rh-G-CSF, 1,000 U/mL; Amgen, Thousand Oaks, CA), rh erythropoietin (EPO, 6 U/mL; Amgen), rh interleukin (IL)-1β (100 U/mL; Syntex, Palo Alto, CA), human c-kit–ligand (KL, 20 ng/mL; Immunex, Seattle, WA), rh IL-6 mutein (20 ng/mL; Imclone, New York, NY), rh IL-3 (50 ng/mL; Sandoz, Basel, Switzerland), transforming growth factor-β (TGF-β1, 10, 100 ng/mL; Genentech, San Francisco, CA), Flk-2 ligand (Flk-L, 20 ng/mL; Imclone), and soluble IL-6 receptor (sIL-6R, 1,250 ng/mL; R & D Systems, Minneapolis, MN).
Suspension culture (Delta) assay.CD34+ cells were cultured at 4 × 104 cells/mL in IMDM plus 20% FCS supplemented with gentamycin and monothioglycerol in the presence of a five factor cytokine cocktail of KL, IL-3, IL-6, Epo, and G-CSF (K36EG) unless stated otherwise. Experiments were performed in 6-well plates (Becton Dickinson, Bedford, MA). Cultures were incubated at 37°C, 100% humidity, and 5% CO2 in air.26-28 At various time intervals, the cells in culture were harvested. Viability was assessed using the trypan blue exclusion assay. Cells were frozen for TRAP assay, TRF measurements, and cell cycle analysis. After 7 days of Delta culture, cells were recovered, plated at 2 × 103 cells/mL in agarose for CFU assay, and repassaged at the starting concentration of 4 × 104 cells/mL. Weekly passages were continued for 4 to 5 weeks until no further cellular expansion was observed. Results are expressed as cumulative cell numbers, progenitor numbers and fold expansion (total CFU-GM per culture at week x multiplied by the fold increase in total cells divided by total CFU-GM per 4 × 104 input CD34+ cells).
Secondary CD34+ selection.At weekly intervals, cells were harvested from Delta culture and a second CD34+ selection was performed on days 7, 14, 21, and 28 using immunomagnetic beads. Cells collected in the secondary CD34+ and CD34− fraction were counted and the percentage of secondary CD34+ cells determined. All fractions were frozen for TRAP assay.
Expansion of CD34+ cells in the presence of all-trans–retinoic acid (ATRA).CD34+ cells were exposed to 1 μmol/L ATRA and 0.1% dimethyl sulfoxide (DMSO) (vehicle for ATRA) in Delta culture. Cells were seeded at a density of 4 × 104 cells/mL in the presence of K36EG and were grown with media plus cytokines alone, in media supplemented with DMSO and in media supplemented with ATRA as described previously.29 The ATRA dosage varied from 1 μmol/L to 10 nmol/L. DMSO and ATRA treatment at this dosage had no observed toxic effect on hematopoietic cells. Cells were grown in the dark and harvested at a 7-day interval for analysis. Cellular viability was measured using trypan blue exclusion technique.
TRAP assay.For telomerase activity, cells were incubated with ice-cold lysis buffer (1 × 106 cells/100 μL) for 30 minutes at 4°C, using 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate, 10 mmol/L Tris-HCL (pH 7.5), 1 mmolL MgCl2, 1 mmol/L EGTA, 10% glycerol, 5 mmol/L β-mercaptoethanol, and 1 ng/mL leupeptine], and were centrifuged at 12,000g for 30 minutes at 4°C as described.7,30,31 The supernatant was collected and the protein concentration was determined by the Bradford assay (Bio-Rad Laboratories, Richmond, CA). Aliquots were diluted to 1 μg protein/μL and stored at −80°C. In brief, the TRAP assay was performed as follows.7,29-31 Two micrograms of protein extract was assayed in a 38-μL reaction mixture containing 10× TRAP buffer (0.2 mol/L Tris-HCL [pH 8.3], 0.5 mmol/L MgCl2 , 630 mmol/L KCl, 0.05% Tween 20, 10 mmol/L EGTA, and 1 mg/mL BSA), 2.5 mmol/L dioxynucleoside, and 0.1 mg TS primer at 25°C for 20 minutes. A 10-μL polymerase chain reaction (PCR) mix containing 10× TRAP buffer, 0.1 mg ACX primer, Taq polymerase, and 32[P]dCTP (deoxycytidine triphosphate) was then added to each sample. The products were amplified by 30 cycles at 94°C, 60°C, and 72°C for 30 seconds each and were separated by electrophoresis on a 15% polyacrylamide gel. The gel was dried for 1 hour at 80°C and exposed to an image plate. Using ImageQuant software (Molecular Dynamics, Sunnyvale, CA), we quantified the signal intensity by determining the radioactivity of each repeat ladder corrected for the background and by expressing the total count per reaction (total product generated = TPG) as percentage of activity in a cell line as described previously.6,7,29-31 For consistency, we assayed an extract from a neuroblastoma cell line (SK-N-SH) in parallel on each gel, which shows maximum telomerase activity. The level of specific telomerase activity in SK-N-SH was set to 100%, and the relative specific telomerase activities of each extract was expressed as percentage of the SK-N-SH standard. All results were determined from at least three to six independent TRAP assays and average activity was calculated. The quantitation method is semiquantative, but sufficient for comparative analysis,6 29-31 especially when the results of at least three independent experiments are combined. A sample was scored as telomerase positive when the telomerase-specific, 6-bp DNA ladder was observed. Negative samples were reexposed for 72 hours to exclude weak enzyme activity. Specimens showing no telomerase were reassayed at 0.2 and 0.02 μg of protein, because inhibition of telomerase activity at high protein concentrations has been reported. To exclude Taq polymerase inhibitors that may account for telomerase negativity, negative samples were reassayed using a PCR control TSNT (5′-AATCCGTCGAGCAGAGTTAG[GGTTAG]7 -3′) oligonucleotide and RP plus NT primers under standard TRAP assay conditions (N.W. Kim et al, personal communication, April 1996). In all, the TSNT band present and lack of telomerase activity was confirmed.
Alkaline phosphatase activity.The stability of alkaline phosphatase appears to be similar to the stability of telomerase.30 Therefore, alkaline phosphatase activity was analyzed to assess the possibility of protein degradation as a cause of negative telomerase activity. A total of 30 μg protein extract was assayed in a 200-μL reaction mixture (0.75 mmol/L 4-methyl–umbelliferyl phosphate, 112 mmol/L 2-amino-2 methyl-1 propanol buffer [pH 10.4], and 3.75 mmol/L MgSO4, 1 mg/mL polyvinyl pyrrolidone), was incubated at 37°C for 1 hour and the reaction stopped by adding 20 μL of 1 mol/L NaOH. The fluorescence of the product was read on a fluorescence plate reader (CytoFluor 2350; Millipore, Bedford, MA). Alkaline phosphatase activity was present in all specimens and displayed comparable fluorescence activity in telomerase positive and negative hematopoietic cell samples.
TRF assay.Telomere length was determined by TRF Southern blot analysis as previously described.8-13 Genomic DNA was digested with 400 μL DNA extraction buffer (100 mmol/L NaCl, 40 mmol/L Tris [pH 8.0], 20 mmol/L EDTA [pH 8.0], 0.5% sodium dodecyl sulfate [SDS], and proteinase K (0.1 mg/mL). Extraction was performed using phenol/chloroform. A total of 5 to 10 μg extracted DNA was digested with 10 U of MSP I and RSA I (Boehringer Mannheim, Indianapolis, IN) for 12 hours to 24 hours at 37°C. Integrity of the DNA before digestion and completeness of digestion was monitored by gel electrophoresis. Electrophoresis of digested genomic DNA was performed in 0.5% agarose gels in 45 mmol/L TBE buffer (pH 8.0) for a total of 660 to 700 V/h. After electrophoresis, gels were depurinated in 0.2 N HCL, denatured in 0.5 mol/L NAOH/1.5 mol/L NaCl, neutralized in 0.5 mol/L Tris/1.5 mol/L NaCl, transferred to a nylon membrane (Schleicher & Schuell, Keene, NH) using 20× sodium saline citrate (SSC) and dried for 1 hour at 70°C. The telomeric probe (TTAGGG)3 (Genset, San Francisco, CA) was 5′-end labeled with [γ-32P]-adenosine triphosphate (ATP) using T4 polynucleotide kinase (Boehringer Mannheim). Prehybridization and hybridization were performed at 50°C using 5× Denhardt's solution, 5× SSC/0.1 mol/L Na2HPO4 /0.01 mol/L Na4P2O7 , and 30 μg of salmon sperm DNA per mL/0.1 mmol/L ATP. Membranes were washed in 0.5× SSC/0.1% SDS solution. Telomeric smears were visualized by exposing the membranes to an image plate. The mean lengths of TRFs were analyzed with a Phosphorimager (Molecular Dynamics). Mean TRF lengths were defined as Σ(ODi /Li )/Σ(ODi ) where ODi is the densitometer output and Li is the length of DNA in positioni .9,10,12,13,32 Sums were calculated over the range of 2 to 23 kbp. Telomere peak values were measured by estimating the band size corresponding to the point with the highest absorbence.32
S-phase analysis.After CD34+ selection, cells were stimulated with KL, IL-1, and IL-3 alone, as well as cytokine combinations of IL-1 + IL-3, KL + IL-1, KL + IL-3, and IL-1 + KL + IL-3 for up to 72 hours using the same conditions described for ex vivo expansion cultures. Cells were analyzed for S-phase distribution using a cell proliferation kit (Amersham, Little Chalfont, UK). Cells were incubated at 37°C in 1:500 diluted labeling reagents containing 5-bromo-2′-deoxyuridine (BrdU) for 120 minutes.33 Cytospins were fixed in acetic ethanol and incubated with a murine-BrdU antibody for 120 minutes at 20°C. A secondary peroxidase antimouse antibody was added for 30 minutes, and positive cells were visualized with hydrogen peroxide and 3,3′-diaminobenzidine tetrahydrochloride. A minimum of 500 cells was scored to determine the percentage of cells in S-phase.
Western immunoblotting.CD34+ cells that had been cultured for 8 hours to 72 hours in 1 mL of IMDM + 20%FCS and various cytokine combinations were washed twice with cold PBS on ice, lysed in buffer (50 mmol/L Tris-HCl, pH 8.0; 120 mmol/L NaCl; 0.5% Nonidet P-40; 100 mmol/L NaF; 200 μmol/L NaVO5; 1 mmol/L phenylmethylsulfonyl fluoride; 10 μg/mL leupeptine), incubated on ice for 15 minutes, and centrifuged at 12,000g for 15 minutes at 4°C as described.34 35 The supernatant was taken off and the protein concentration determined (Bio-Rad kit; Bio-Rad, Hercules, CA). Cell lysates containing equal amounts of total protein were resolved on a 7% (for pRB) or 10% (for cyclins and CDKs) SDS-polyacrylamide gel electrophoresis followed by transfer onto nitrocellulose. Membranes were blocked in 2% BSA in PBS followed by probing with the desired Ab, and detection of immune complexes using 125I-protein A. Quantitation of specific protein bands was performed using densitometric scanning.
Calculation of number of cell divisions.An evaluation of the number of PD in hematopoietic cells was performed, based on differences in mean TRF length at baseline (Delta 0) and after 4 weeks in culture (Delta 4), assuming a base pair loss of 50/PD: TRFDelta 0 (kbp) − TRFDelta 4 (kbp)/0.05 = number of PD.
The number of PD was also determined from cell numbers at each weekly Delta passage compared with input cell numbers. The base pair loss/PD based on the population doublings in Delta culture was performed using the following formula: TRFDelta 0 (kbp)TRFDelta 4 (kbp): total number of PD4weeks = bp loss/PD.
Statistics.Comparisons among groups were made with standard statistical tests. Results are expressed as median ± range except when stated otherwise. Statistical significance of the data obtained was analyzed by the Wilcoxon rank sum test and the Student's t-test (Statworks; Cricket Software, Philadelphia, PA). A P value of less than .05 was considered statistically significant.
RESULTS
Cell expansion and colony numbers in FL, CB, PB, and BM.Ex vivo expansion of cells and total colony numbers were highest in CB (n = 11) and FL (n = 1) reaching a maximum for all hematopoietic cell sources on day 21 (Table 1). The effects of different cytokines and combinations on day 3, 5, and 7 using CD34+ cells from PB are shown in Table 2. CD34+ cells expanded moderately (onefold to twofold) with single cytokines (KL, IL-1, IL-3) or cytokine combinations of KL + Flk-L (K + F ) and expanded 2-fold to 3.5-fold with KL + IL-6, KL + IL-6 + IL-6R, IL-1 + IL-3, KL + IL-1, and KL + IL-3. Cell expansion increased further with cytokine combination of KL, IL-3 and IL-6 (K36) (5-fold), with Flk-L, IL-3, IL-6, Epo, and G-CSF (F36EG), with KL, IL-3, IL-6, Epo, and G-CSF (K36EG) or with KL, Flk-L, IL-3, IL-6, Epo, and G-CSF (KF36EG) (12-fold to 35-fold). Addition of TGF-β1 in concentrations of 10 and 100 ng/mL to K36EG inhibited cell expansion considerably (Table 2).
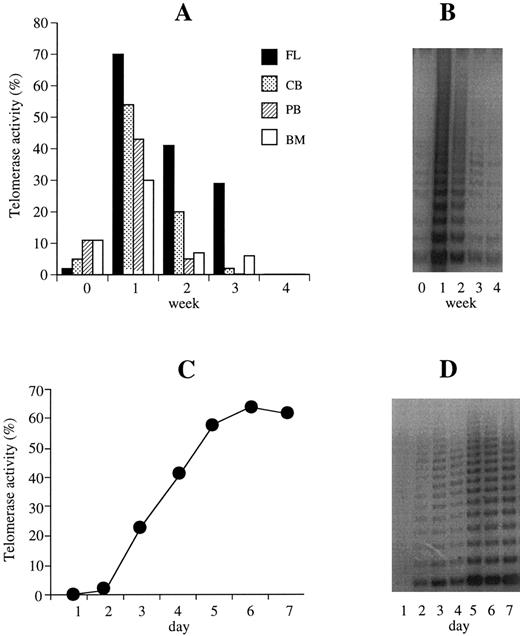
Basal telomerase activity in hematopoietic cells, telomerase activity in ex vivo expansion cultures and time course regulation.Basal telomerase activity was detected in CD34+ cells from FL (n = 1), CB (n = 11), PB (n = 20), and BM (n = 5) samples (median, 9%; range, 1% to 22%) that exceeded levels in CD34− (median, 1%; range, 0% to 13%) and MNCs (median, 0.05%; range, 0% to 4%) (P < .05). After 1 week of liquid culture in the presence of K36EG, telomerase was significantly upregulated in CD34+ cells from all sources (median activity: 46%) compared with baseline levels (median, 9%) (P < .001) (Fig 1A and B). There was no statistically significant difference in basal and in week 1 telomerase levels from different hematopoietic products. After 2 weeks of expansion, telomerase activity declined (median, 11%) decreasing to baseline or below the limits of detection after 3 to 4 weeks of Delta culture (median, 2%) (Fig 1A and B). In the CD34− population, which contains more mature cells, telomerase was also upregulated after 1 week of Delta expansion (median basal activity, 1%, increasing to 11% at week 1; P < .001), but significantly less than in CD34+ cells (P < .001). Median telomerase activity in week 1 CD34− cells increased twofold to threefold (n = 6) compared with basal levels, but 20-fold in CD34+ cells. Median CD34− cell expansion after 1 week of Delta culture was 1.5-fold to twofold compared with 20-fold to 30-fold using CD34+ cells (P < .001). To determine the time course of telomerase upregulation, CD34+ cells from PB were harvested, counted, and assayed for telomerase activity at 4 hours, 6 hours, 12 hours, and 24 hours and day 2 to day 7 in culture (n = 7). Within the first 48 hours of ex vivo expansion, telomerase was not upregulated, after 48 hours to 72 hours, however, telomerase was detectable reaching maximum levels between day 5 and 7 (Fig 1C and D). Without cytokine support, ex vivo expanded CD34+ cells grown in 20% IMDM showed no telomerase activity after 72 hours, day 5 or day 7 of Delta culture. In one PB sample (pretreated patient), CD34+ cells also did not expand, despite K36EG cytokine stimulation, and telomerase was not upregulated.
Telomerase activity of CD34+ cells in liquid culture. (A) Telomerase activity was calculated from the TRAP signal of CD34+ cells from all sources (PB, n = 20; CB, n = 11; BM, n = 5; FL, n = 1) at baseline and weeks 1 to 4 of ex vivo expansion culture. Telomerase is expressed as a percentage of the activity detected in the neuroblastoma (SK-N-SH) cell line control. Telomerase was significantly upregulated in week 1 of ex vivo expanded cytokine-supported (KL, IL-3, IL-6, Epo + G-CSF = K36EG) CD34+ cells from PB, CB, BM, and FL compared with baseline levels (P < .001). After 2 weeks of expansion, telomerase activity declined decreasing to baseline or below detection after 3 to 4 weeks. (B) Representative TRAP of the regulation of telomerase activity (this example corresponds to a PB sample) of CD34+ cells at baseline and weeks 1 through 4 in ex vivo expansion culture. (C) Daily time course experiments in PB CD34+ cells in culture (n = 7) showed that after 48 to 72 hours of ex vivo expansion, telomerase was detectable reaching maximum levels between days 5 and 7. (D) Representative TRAP showing telomerase upregulation within days 2 and 3 and highest activity at days 5 and 7 in cytokine-supported (K36EG) CD34+ cells in ex vivo expansion culture.
Telomerase activity of CD34+ cells in liquid culture. (A) Telomerase activity was calculated from the TRAP signal of CD34+ cells from all sources (PB, n = 20; CB, n = 11; BM, n = 5; FL, n = 1) at baseline and weeks 1 to 4 of ex vivo expansion culture. Telomerase is expressed as a percentage of the activity detected in the neuroblastoma (SK-N-SH) cell line control. Telomerase was significantly upregulated in week 1 of ex vivo expanded cytokine-supported (KL, IL-3, IL-6, Epo + G-CSF = K36EG) CD34+ cells from PB, CB, BM, and FL compared with baseline levels (P < .001). After 2 weeks of expansion, telomerase activity declined decreasing to baseline or below detection after 3 to 4 weeks. (B) Representative TRAP of the regulation of telomerase activity (this example corresponds to a PB sample) of CD34+ cells at baseline and weeks 1 through 4 in ex vivo expansion culture. (C) Daily time course experiments in PB CD34+ cells in culture (n = 7) showed that after 48 to 72 hours of ex vivo expansion, telomerase was detectable reaching maximum levels between days 5 and 7. (D) Representative TRAP showing telomerase upregulation within days 2 and 3 and highest activity at days 5 and 7 in cytokine-supported (K36EG) CD34+ cells in ex vivo expansion culture.
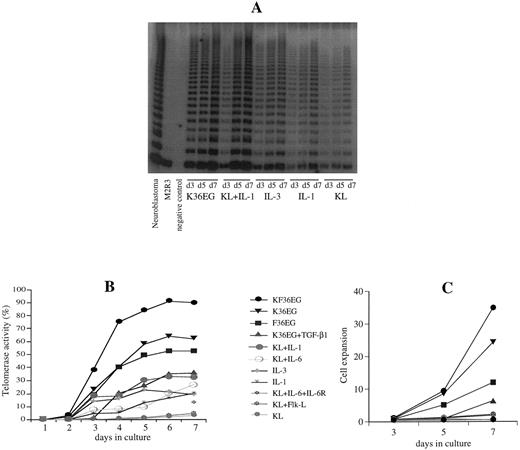
Telomerase upregulation in different cytokine combination supported cells in liquid culture.To determine the effect of various cytokines on telomerase upregulation within 1 week of ex vivo expansion, we assayed telomerase after incubation with KL, IL-1, IL-3, or IL-6 alone, and combinations of IL-1 + IL-3, KL + Flk-L, KL + IL-1, KL + IL-3, KL + IL-6, KL + IL-6 + sIL-6R, K36EG, F36EG, KF36EG and K36EG + TGF-β. Stimulation of CD34+ cells from CB and PB with single cytokines such as KL, IL-1, IL-3, and IL-6 or combinations of IL-1 + IL-3, KL + Flk-L or KL + IL-6 showed no or little telomerase upregulation (Fig 2A and B). Using cytokine combinations of KL + IL-1, KL + IL-3 or multiple cytokines (1K3, K36, 3KG, K36GE, F36GE, KF36GE) that initiate cell proliferation (Fig 2C) and mitotic stimulation resulted in a considerable telomerase increase in Delta culture (Fig 2A and B). Highest telomerase upregulation was seen using KF36EG (Fig 2B). TGF-β1 in combination with stimulatory cytokines (K36EG) downregulated telomerase activity by 50% using 10 ng/mL and by 75% at 100 ng/mL as compared with the five factor cytokine combinations (K36EG) alone.
Telomerase upregulation in different cytokine combination supported cells in liquid culture. (A) Representative TRAP of telomerase regulation in CD34+ cells in response to single cytokines and cytokine combinations on days 3, 5, and 7 of ex vivo expansion culture. Telomerase activity was maximally increased in cultures supported with K36EG or KL + IL-1 as compared with considerably lower activity using single cytokines. (B) Summary of telomerase activity (given in percentage of the neuroblastoma cell line control) of CD34+ cells showing upregulation of telomerase in response to various single and multiple cytokine combinations. Stimulation of CD34+ cells with single cytokines such as KL, IL-1, and IL-3 or combinations of KL + Flk-L or KL + IL-6 + sIL-6R displayed no or little telomerase upregulation. Using cytokine combinations of KL + IL-1 or multiple cytokines (K36GE, F36GE, KF36GE) that initiate cell proliferation and mitotic stimulation resulted in a considerable telomerase increase in Delta culture. Highest telomerase upregulation was seen using KF36EG. TGF-β1 in combination with stimulatory cytokines (K36EG) downregulated telomerase activity compared with the five factor cytokine combinations (K36EG) alone. (C) Cell expansion of CD34+ cells in ex vivo expansion culture using single and multiple cytokine combinations. Cell expansion was highest in multiple cytokine combinations. TGF-β1 in combination with stimulatory cytokines (K36EG) downregulated cell expansion. Combinations of KL + IL-1, KL + IL-6, KL + IL-6 + IL-6R, KL + Flk-L, or single cytokines induced only a minor cell expansion.
Telomerase upregulation in different cytokine combination supported cells in liquid culture. (A) Representative TRAP of telomerase regulation in CD34+ cells in response to single cytokines and cytokine combinations on days 3, 5, and 7 of ex vivo expansion culture. Telomerase activity was maximally increased in cultures supported with K36EG or KL + IL-1 as compared with considerably lower activity using single cytokines. (B) Summary of telomerase activity (given in percentage of the neuroblastoma cell line control) of CD34+ cells showing upregulation of telomerase in response to various single and multiple cytokine combinations. Stimulation of CD34+ cells with single cytokines such as KL, IL-1, and IL-3 or combinations of KL + Flk-L or KL + IL-6 + sIL-6R displayed no or little telomerase upregulation. Using cytokine combinations of KL + IL-1 or multiple cytokines (K36GE, F36GE, KF36GE) that initiate cell proliferation and mitotic stimulation resulted in a considerable telomerase increase in Delta culture. Highest telomerase upregulation was seen using KF36EG. TGF-β1 in combination with stimulatory cytokines (K36EG) downregulated telomerase activity compared with the five factor cytokine combinations (K36EG) alone. (C) Cell expansion of CD34+ cells in ex vivo expansion culture using single and multiple cytokine combinations. Cell expansion was highest in multiple cytokine combinations. TGF-β1 in combination with stimulatory cytokines (K36EG) downregulated cell expansion. Combinations of KL + IL-1, KL + IL-6, KL + IL-6 + IL-6R, KL + Flk-L, or single cytokines induced only a minor cell expansion.
Telomerase in CD34+/CD38+ and CD34+/CD38− cells.CD34+/CD38+ and CD34+/CD38− from PB were used to determine telomerase activity at baseline and after ex vivo expansion. The purity of CD34+/CD38+ was 98.6% and 80% for the CD34+/CD38− fraction as determined by flow cytometry. Ex vivo expansion was performed with both fractions using the following cytokine combinations: K36EG, F36EG, KF36EG, KL + IL-6 and KL + IL-6 + sIL-6R (Fig 3A). Baseline telomerase was present in the CD34+/CD38+ fraction and absent in the CD34+/CD38− population (Fig 3B). The fold increase of cells and colony numbers after 7 days of culture in the CD34+/CD38+ fraction exceeded those in CD34+/CD38− cells. KL + IL-6 and KL + IL-6 + IL-6R only induced cell expansions of 0.7 and 2.1 in CD34+/CD38+ cells and of 0.6 and 1.6 in CD34+/CD38− cells, whereas with K36EG, F36EG, and KF36EG progenitors increased 65.6-fold, 43.8-fold, and 90.6-fold in cultures initiated with CD34+/CD38+ cells and 56.7-fold, 23.3-fold, and 81.6-fold with CD34+/CD38− cells. On day 3, 5, and 7 of expansion, telomerase was upregulated in both fractions, however, to a higher extent in CD34+/CD38+ cells compared with the more quiescent CD34+/CD38− population (Fig 3A and B). Cells stimulated with multiple cytokines (K36EG, F36EG, KF36EG) as opposed to KL + IL-6 or KL + IL-6 + sIL-6R resulted in an increased telomerase expression (Fig 3A and B).
Telomerase in CD34+/CD38+ and CD34+/CD38− cells. (A) Telomerase activity in CD34+/CD38+ and CD34+/CD38− cells on days 3, 5, and 7 of ex vivo expansion culture. Telomerase was upregulated to a higher extent in CD34+/CD38+ cells compared with the more quiescent CD34+/CD38− population. Both fractions when stimulated with multiple cytokines (K36EG, F36EG, KF36EG) as opposed to KL + IL-6 or KL + IL-6 + sIL-6R resulted in an increased telomerase expression. (B) Telomerase activity at baseline with telomerase confined to CD34+/CD38+ cells and absent in CD34+/CD38− fraction is diplayed. After 1 week of ex vivo expansion, telomerase upregulation using combinations of KL + IL-6 was increased in CD34+/CD38+ cells compared with CD34+/CD38− cells. This was even more pronounced when cytokine combinations of KF36EG were used. Lane 1: CD34+/CD38− fraction at baseline (day 0) [no telomerase activity]; lane 2: CD34+/CD38+ fraction at baseline (day 0) [telomerase activity]; lane 3: CD34+/CD38− fraction, 7 days in ex vivo expansion (day 7) with KL + IL-6; lane 4: CD34+/CD38+, 7 days in ex vivo expansion (day 7) with KL + IL-6; lanes 5 to 7: CD34+/CD38− fraction on day 3 (lane 5), day 5 (lane 6), and day 7 (lane 7) of ex vivo exansion with KF36EG; lanes 8 to 10: CD34+/CD38+ fraction on day 3 (lane 8), 5 (lane 9), and 7 (lane 10) with KF36EG.
Telomerase in CD34+/CD38+ and CD34+/CD38− cells. (A) Telomerase activity in CD34+/CD38+ and CD34+/CD38− cells on days 3, 5, and 7 of ex vivo expansion culture. Telomerase was upregulated to a higher extent in CD34+/CD38+ cells compared with the more quiescent CD34+/CD38− population. Both fractions when stimulated with multiple cytokines (K36EG, F36EG, KF36EG) as opposed to KL + IL-6 or KL + IL-6 + sIL-6R resulted in an increased telomerase expression. (B) Telomerase activity at baseline with telomerase confined to CD34+/CD38+ cells and absent in CD34+/CD38− fraction is diplayed. After 1 week of ex vivo expansion, telomerase upregulation using combinations of KL + IL-6 was increased in CD34+/CD38+ cells compared with CD34+/CD38− cells. This was even more pronounced when cytokine combinations of KF36EG were used. Lane 1: CD34+/CD38− fraction at baseline (day 0) [no telomerase activity]; lane 2: CD34+/CD38+ fraction at baseline (day 0) [telomerase activity]; lane 3: CD34+/CD38− fraction, 7 days in ex vivo expansion (day 7) with KL + IL-6; lane 4: CD34+/CD38+, 7 days in ex vivo expansion (day 7) with KL + IL-6; lanes 5 to 7: CD34+/CD38− fraction on day 3 (lane 5), day 5 (lane 6), and day 7 (lane 7) of ex vivo exansion with KF36EG; lanes 8 to 10: CD34+/CD38+ fraction on day 3 (lane 8), 5 (lane 9), and 7 (lane 10) with KF36EG.
Telomerase activity in CD34+ cells obtained after different separation systems and in secondary CD34+ and CD34− cells.CD34+ cells from CB, PB, and BM were separated using the Ceprate column (CellPro) system, the Minimax (Miltenyi) system, or immunomagnetic Dynal beads. All separation systems provided efficient recovery of CD34+ cells. After different separation procedures, telomerase activity in CD34+ cells was comparable at baseline and in Delta expansion culture, if CD34+ cells expanded similarly.
To determine telomerase in primary CD34+ cells versus secondary CD34+ and CD34− cells, progenitor cells from CB (n = 4), PB (n = 5), and BM (n = 2) were secondary separated using immunomagnetic beads on days 7, 14, 21, and 28 of culture. Median recovery of secondary CD34+ cells were 6.1% in CB, and 2% both in PB and BM cells after 1 week, 1.3% and 0.5% after 2 weeks, and 0.3% and 0.4% after 3 weeks of ex vivo expansion culture, respectively. After 1 week of culture, telomerase activity was differently upregulated in primary CD34+, secondary CD34+, and CD34− cells (Fig 4A). Whereas, in primary CD34+ cells, telomerase activity increased 20-fold (baseline → week 1), secondary CD34+ cells isolated at week 1 of culture displayed a 1.4-fold telomerase increase compared with primary CD34+ cells, but a sharp decline after 1 week of expansion with no further upregulation of telomerase (week 1 → week 2) (Fig 4A). At week 2 to 4 of culture, telomerase activity had decreased both in primary and secondary CD34+ cells. Similarly to the reduced upregulation of telomerase activity in secondary CD34+ cells, cellular expansion was also reduced in these cells (Fig 4B). In CD34− cells, telomerase was 5-fold to 10-fold decreased in week 1 and 2 compared with primary CD34+ cells and undetectable in week 3 and 4 (Fig 4A). Cellular expansion was low compared with primary and secondary CD34+ cells and thus correlated with the decreased telomerase upregulation (Fig 4B).
Secondary CD34+ and CD34− cells. (A) Telomerase activity at baseline and weeks 1 to 4 of ex vivo expansion in primary CD34+ (•), secondary CD34+ (▪), and CD34− () cells is shown ([A] depicts the relative telomerase activity in percentage of the SK-N-SH neuroblastoma cell line control). After 1 week of culture, telomerase activity was upregulated in primary CD34+ and CD34− cells. In primary CD34+ cells, telomerase activity increased 20-fold (median) compared with baseline levels. Secondary CD34+ cells isolated at week 1 of culture displayed a 1.4-fold telomerase increase compared with primary CD34+ cells, but a sharp decline after 1 week of expansion and no upregulation of telomerase (week 1 → week 2). There was no upregulation of telomerase activity in primary or in secondary CD34+ cells in the following weeks (weeks 2 to 4) of culture. In CD34− cells, telomerase was fivefold to 10-fold lower in the first 2 weeks of expansion compared with primary CD34+ cells and undetectable in weeks 3 and 4. (B) Cumulative population doublings in primary CD34+, secondary CD34+, and CD34− cells are shown. Similarly to the reduced upregulation of telomerase activity in secondary CD34+ cells, cellular expansion was also reduced.
Secondary CD34+ and CD34− cells. (A) Telomerase activity at baseline and weeks 1 to 4 of ex vivo expansion in primary CD34+ (•), secondary CD34+ (▪), and CD34− () cells is shown ([A] depicts the relative telomerase activity in percentage of the SK-N-SH neuroblastoma cell line control). After 1 week of culture, telomerase activity was upregulated in primary CD34+ and CD34− cells. In primary CD34+ cells, telomerase activity increased 20-fold (median) compared with baseline levels. Secondary CD34+ cells isolated at week 1 of culture displayed a 1.4-fold telomerase increase compared with primary CD34+ cells, but a sharp decline after 1 week of expansion and no upregulation of telomerase (week 1 → week 2). There was no upregulation of telomerase activity in primary or in secondary CD34+ cells in the following weeks (weeks 2 to 4) of culture. In CD34− cells, telomerase was fivefold to 10-fold lower in the first 2 weeks of expansion compared with primary CD34+ cells and undetectable in weeks 3 and 4. (B) Cumulative population doublings in primary CD34+, secondary CD34+, and CD34− cells are shown. Similarly to the reduced upregulation of telomerase activity in secondary CD34+ cells, cellular expansion was also reduced.
Telomerase activity in ex vivo expanded CD34+ cells using differentiation agents (ATRA).CD34+ cells from PB were expanded in Delta supplemented with K36EG and varying concentrations (10−6 mol/L to 10−8 mol/L) of ATRA (n = 2). Telomerase activity was determined after each week of Delta passage. High concentrations of ATRA (10−6 mol/L) downmodulated telomerase activity. Telomerase activity was similar to control cells (without ATRA treatment) using 10−7 mol/L ATRA and was enhanced using 10−8 mol/L ATRA. Mean cell expansion in Delta 1 was 18-fold in control CD34+ cells, 34.4-fold using ATRA 10−8 mol/L, 15-fold with ATRA 10−7 mol/L, and 10-fold with ATRA 10−6 mol/L.
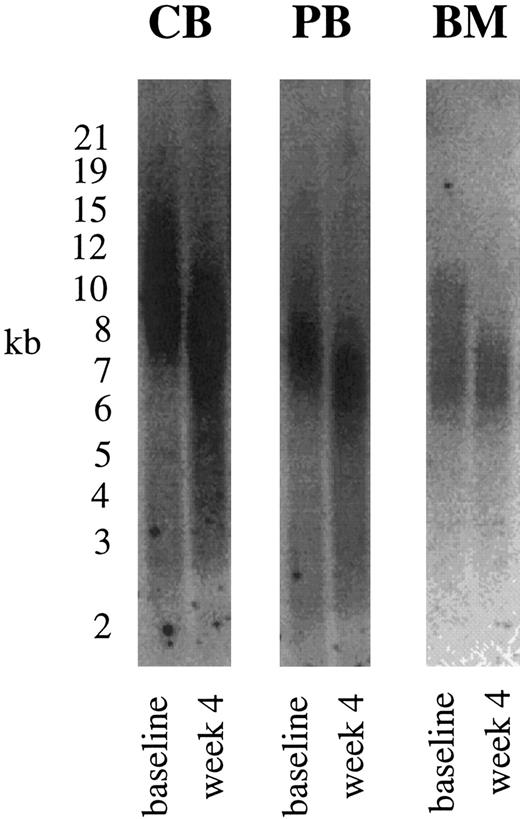
Telomere length in hematopoietic cells.Mean TRF length of CD34+ cells from FL (n = 1) was 11 kbp, 10.4 ± 1.9 kbp from CB (n = 14), 7.4 ± 0.8 kbp from PB (n = 19), and 7.6 ± 0.5 kbp from BM (n = 4). Peak TRFs were similar to mean values with 11.5 kbp, 10.6 ± 1.3 kbp, 7.5 ± 0.7 kbp, and 7.6 ± 0.4 kbp from FL, CB, PB, and BM, respectively. Telomeres from CB CD34+ cells were significantly longer compared with telomere lengths from mostly untreated PB patients and BM from healthy donors (P < .001). Analyzing mean and peak TRF values demonstrated that CD34+ cells over a period of 4 weeks lost on average 1.5 kbp (mean) to 1.7 kbp (peak), respectivly (1.4 kbp in FL [peak, 1.5 kbp], 1.5 ± 0.7 kbp in CB [peak, 1.7 ± 0.5], 1.5 ± 0.4 kbp in PB [peak, 1.6 ± 0.5], 1.3 ± 0.2 kbp in BM [peak, 1.5 ± 0.3]). Telomere loss after 4 weeks of expansion is shown for CB, PB, and BM CD34+ cells in Fig 5. Calculating PD from cell numbers obtained at each week of Delta expansion divided by the input cell number showed a minimum PD of 21.4 over a 4-week period (23 in FL, 22 ± 3 in CB, 21 ± 4 in PB and BM) corresponding to a maximum base pair loss of 73/PD (61 in FL, 71 in CB, 74 in PB and BM cells). Highest PDs were observed in week 1 (15.2 ± 2.3), which decreased in the following weeks (Delta 2: 4.8 ± 1.6, Delta 3: 1.9 ± 1, Delta 4: 1.6 ± 0.5). Numbers of PDs were maximal in primary CD34+ cells, followed by secondary CD34+ and CD34− cells (Fig 4B). Despite telomerase upregulation peaking after 1 week of culture, telomere loss in Delta showed an average telomeric decrease of 0.4 kbp per week.
Telomere length in hematopoietic cells. TRF Southern blot analyses were performed with CD34+ cells at baseline and after ex vivo expansion. Over a 4-week period, CD34+ cells lost on average 1.5 kbp as shown for CB, PB, and BM CD34+ cells.
Telomere length in hematopoietic cells. TRF Southern blot analyses were performed with CD34+ cells at baseline and after ex vivo expansion. Over a 4-week period, CD34+ cells lost on average 1.5 kbp as shown for CB, PB, and BM CD34+ cells.
S-phase analysis.CD34+ cells were prestimulated with various cytokines alone and combinations (KL, IL-1, IL-3, IL-1 + IL-3, KL + IL-1 [K1], KL + IL-3 [K3], IL-1 + KL + IL-3 [1K3]) for up to 72 hours. We determined the percentage of cells in S-phase by incorporation of BrdU. Less than 1.5% of CB CD34+ cells, 8.4% of PB cells, and 30% to 40% of BM CD34+ cells were found in cell cycle at the time of harvest. Within 72 hours of exposure to KL and IL-1 alone, 1% to 2% entered S-phase, 7% with IL-3 alone, and 40% to 45% with combinations of IL-1 + IL-3, K1, K3, or 1K3 resulting in substantial cell and progenitor expansion by 7 days.
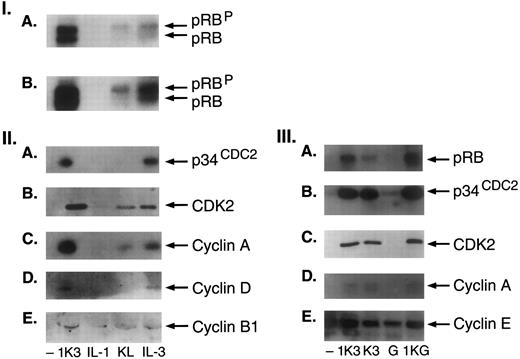
Induction of cell cycle proteins.To investigate the possible role of cell cycle proteins in the proliferative response of progenitor cells and to understand their relationship with telomerase upregulation in hematopoiesis, we examined cell cycle regulatory proteins in response to individual cytokines as KL, IL-1, and IL-3 or combinations such as 1K3. Treatment of CD34+ cells with 1K3 induced the expression of RB protein (pRB), both phosphorylated (pRBP) and underphosphorylated (pRB) forms compared with controls (pRBP:pRB: 60:40) (8-fold to 25-fold, n = 8) (Fig 6I). KL alone stimulated the expression of phosphorylated RB protein, and IL-3 the expression of both pRBP and pRB (Fig 6I). Because phosphorylation of pRB protein is associated with the progression of cells into S phase, regulated by complexes consisting of p34 CDC2 or related kinase and induced synthesis of cyclins, we also examined the effect of 1K3 or single cytokines on the expression of p34CDC2, CDK2, cyclin A, cyclin D, and B1. As shown in Fig 6II, treatment of CD34+ cells with 1K3 induced the expression of p34CDC2, CDK2, and cyclin A and D1. KL induced the expression of cyclin A (twofold) with no effect on p34CDC2 and cyclin D. In contrast, treatment with IL-3 resulted in a significant induction of expression of p34CDC2 (3-fold), CDK2 (4-fold), cyclin A (4.5-fold), and cyclin D1 (2.5-fold) (Fig 6II). The effects of KL and IL-3 on the expression of G1/S cyclins were specific, as there was no significant change in the levels of G2/M cyclin B1. Treatment with single cytokines as IL-1 had no effect on the expression of any cell cycle proteins. Because the effect of 1K3 on the cell cycle could be partially mimicked by KL or IL-3, we next compared the effect of combination of KL and IL-3 (K3) with that of 1K3 on the expression of cell cycle proteins (Fig 6III). Treatment with K3 resulted in the induction of pRB, p34CDC2, CDK2, and cyclin A, comparable to the levels induced with 1K3, suggesting that this combination can mimic the effects of 1K3, although the levels of induced pRB in K3-treated cells were 50% reduced compared with 1K3 (Fig 6III). Replacing G-CSF for IL-3 (1KG) resulted in the induction of RB, of p34CDC2, CDK2, and cyclin A, comparable with levels induced by 1K3 (Fig 6III). As there was no individual effect of IL-1 on pRB expression, it is possible that IL-1 may be required for a synergistic interaction to activate the pathway responsible for pRB expression when combined with KL and IL-3. The observed synergistic effect of 1K3, K3, or 1KG on the expression of pRB and G1 cyclin D1 was relative specific, as there was no change in the expression of another G1/S cyclin E.
Induction of cell cycle proteins. (I) Regulation of expression of G1-S cell cycle regulatory proteins in human CD34+ cells. Equal number of cells were treated without or with 1K3, or IL-1, KL, and IL-3 for 72 hours. Cell extracts were immunoblotted with the indicated antibodies. In panel I, (A and B) are two different exposures of the same autoradiogram. (II) Immunoblotting for p34CDC2 and cyclin B1 was performed from the same blot, and of cyclin D and A from another blot. (III) G-CSF can replace IL-3 to induce the expression of cell cycle proteins in human CD34+ cells. Cells were treated with either 1K3 or combination of IL-3 and KL (K3), G-CSF, and combination of IL-1, KL, and G-CSF (1KG) for 72 hours. Cell extracts were immunoblotted with the indicated antibodies. Immunoblotting for p34CDC2 and cyclin E was performed from the same blot. Results shown are representative of three separate experiments.
Induction of cell cycle proteins. (I) Regulation of expression of G1-S cell cycle regulatory proteins in human CD34+ cells. Equal number of cells were treated without or with 1K3, or IL-1, KL, and IL-3 for 72 hours. Cell extracts were immunoblotted with the indicated antibodies. In panel I, (A and B) are two different exposures of the same autoradiogram. (II) Immunoblotting for p34CDC2 and cyclin B1 was performed from the same blot, and of cyclin D and A from another blot. (III) G-CSF can replace IL-3 to induce the expression of cell cycle proteins in human CD34+ cells. Cells were treated with either 1K3 or combination of IL-3 and KL (K3), G-CSF, and combination of IL-1, KL, and G-CSF (1KG) for 72 hours. Cell extracts were immunoblotted with the indicated antibodies. Immunoblotting for p34CDC2 and cyclin E was performed from the same blot. Results shown are representative of three separate experiments.
DISCUSSION
In the absence of compensatory mechanisms, telomere shortening has been observed with each cell division.2,3 Telomeres are stabilized, however, if telomerase is activated to an appropriate level as found in the majority of immortalized cell lines and tumor specimens.7,14 In concordance with previous studies,8,11,12,16,17 we report telomere shortening of hematopoietic cells on proliferation despite telomerase activity. Our data show that telomerase is expressed at low level in MNCs, CD34−, and CD34+ cells from different hematopoietic sources and is significantly upregulated within 1 week of cytokine-supported ex vivo expansion cultures. The Delta assay where cultures are returned to low cell densities at weekly intervals was chosen, as it better reflects cumulative cell and progenitor expansion hence population doublings than in other systems.27,28 Our consistent detection of telomerase in ex vivo expansion culture to levels as high as seen in most tumor specimens7 and tumor cell lines7,29 was unexpected, as telomere shortening was occurring under these conditions. Ex vivo expansion of CD34+ cells with a combination, but not single cytokines, induced elevated levels of expression of hyper and hypophosphorylated retinoblastoma (RB) protein, CDK2, p34CDC2, and cyclin A, which are distinctly associated with the progression of cells into S phase.20-25,34 35 BrdU analysis of CD34+ cells showed 1.6% in CB and less than 10% in PB CD34+ cells in cycle at the time of harvest, which increased to 40% to 50% within 72 hours of expansion using cell cycle promoting cytokines and correlated with cell cycle protein and telomerase upregulation. In accordance with this observation, we show that baseline telomerase activity was reduced in noncycling FL and CB CD34+ cells compared with slightly more actively cycling PB CD34+ cells (7% to 8% in S-phase) and, in particular, in BM CD34+ cells (30% to 40% in S-phase).
Recent studies have established the role of the interplay of hematopoietic cytokines including IL-1, IL-3, IL-6, KL, Flk-L, and G-CSF in ex vivo expansion systems for various therapeutical applications.26-28 Stem cell self-renewal, as measured by increase in numbers of long-term culture initiating cells, can be achieved, in particular, with KL and Flk-L cytokine combinations.36-38 Synergistic growth promoting interactions have been reported on CD34+ cells from different sources such as CB, PB, and BM.26-28,36-44 In the absence of growth factors, CD34+ cells undergo apoptosis.26-28 Single cytokines preserve cells in expansion cultures and block apoptotic death, but do not induce noncycling progenitors into cycle, whereas cytokine combinations result in progression of cells into DNA synthesis within 72 hours and induction of cell cycle proteins.25-28 We found that Flk-L in combination with KL exhibited no or little stimulating activity in proliferation and telomerase increase, although preventing apoptosis, whereas in the presence of other stimulatory cytokines (KF36EG), the highest cell expansion and telomerase upregulation was observed. Although our results show that telomerase is upregulated with proliferating inducing cytokines, telomerase was not upregulated only as a function of cell expansion. KL + IL-1 or KL + IL-3 induced considerably less CD34+ cell expansion as compared with K36EG, however, both dual combinations resulted in a pronounced increase in telomerase activity as a function of cell cycle progression. Our results are in line with others18 19 indicating that telomerase is present in rapidly expanding cells, upregulated at cell cycle entry as cells progress through S-phase, and repressed in quiescent G0 cells.
Recently, telomerase activity has also been described in other somatic cells as in nonactivated and activated T and B lymphocytes.4,45,46 T cells were found to upregulate telomerase on mitogenic stimulation using IL-2 or phytohemagglutinin (PHA).4 In hematopoietic cells, telomerase activity has been attributed to more mature subsets (CD34+71+45+) rather than to more primitive progenitors with CD34+ 71low45low phenotype or to CD34− cells.16 We confirmed these observations using CD38 as a marker to select for primitive (CD38−) and more mature CD34+ cell subsets (CD38+). Telomerase was found in actively cycling CD34+/CD38+ cells exceeding levels of CD34− cells and of quiescent CD34+/CD38− cells. While our data illustrates that telomerase in CD34+/CD38− can be induced to levels that were lower than detected in the CD34+/CD38+ compartment, we cannot exclude the possibility that the CD34+ cells were differentiated progeny of CD38− cells induced on cytokine stimulation. Cell expansion analysis of CD38+ and CD38− cells, however, clearly showed that telomerase was highly expressed where greatest proliferation and cell expansion takes place.
After 3 to 4 weeks in ex vivo expansion culture, telomerase activity declined, which suggests that telomerase may decrease with the reduction of cell renewal and expansion potential. From primary and secondary selected CD34+ cells, similar telomerase activity in both fractions was found, peaking in week 1 and decreasing thereafter. Secondary CD34+ cells, however, showed a reduced ability to upregulate telomerase activity and to proliferate after 1 week of expansion compared with primary CD34+ cells, which strongly suggests that CD34+ cells lose telomerase activity and may undergo replicative aging on cell proliferation. These results are in line with previous reports showing that telomerase activity decreases with age in normal donor lymphocytes.4 According to our results, telomerase is downmodulated in CD34+/CD38− subsets representing noncycling, quiescent progenitors, in cells on cytokine withdrawal when apoptosis is induced or using negative regulators as TGF-β, a G1/S blocker and inhibitor of cytokine driven early progenitor expansion. Whereas differentiation inducing agents such as ATRA consistently showed to downregulate telomerase activity during induced differentiation in cancer cells lines at all effective concentrations,29 we found telomerase activity in hematopoietic cells downmodulated at high ATRA concentrations, but increased cell expansion and telomerase activity over and above cytokines alone with lower ATRA concentrations. Although by trypan blue exclusion we observed no toxic effect due to high ATRA concentrations used, we cannot completely exclude that ATRA may have also induced apoptosis in a subset of hematopoietic cells and the downregulation of telomerase activity.
Recently, different models for telomerase regulation in cell subsets have been proposed: a “repression and reactivation” model suggesting that somatic cells fail to express telomerase regardless of their proliferative state, that telomerase is repressed in quiescent immortal cells, but may become telomerase positive after their telomeres have progressively shortened.18,19 An “expansion” model suggests that somatic cells such as rapidly proliferating tissue or stem cells with self-renewal capacity are telomerase-competent ‘ab initio,’ may upregulate telomerase as the consequence of reentry into cell cycle, but give rise to more mature, telomerase negative cells.19 Based on our findings, a “cell cycle” model may be suggested, which postulates that telomerase is repressed in quiescent stem cells (CD34+ CD38−), is activated on cell proliferation, expansion, cell cycle entry, and progression into the progenitor compartment (CD34+/CD38+), and is repressed again on terminal cell differentiation (CD34−).
Mean and peak telomere length analysis showed that FL and CB CD34+ cells had longer telomeres compared with PB and BM cells. After 4 to 5 weeks in culture, telomeres were still longer in CB than in adult cells. This suggests that CB has a higher replicative potential than adult PB or BM cells, which combined with their greater expansion potential, would support the value of this stem cell source for allogeneic transplantation. Hematopoietic cells lost per average 73 bp/PD, which is comparable to telomeric DNA loss observed in other somatic cells (50 to 100/PD).7,8 A 2.4-kbp difference between neonatal and adult cells would therefore allow CB cells to go through 33 more PD, reaching proliferative senescence later. Our calculated loss of telomeric DNA in Delta expansion was, however, higher than reported in another ex vivo expansion system.8 Reasons for this difference may be that (1) apoptotic cells in culture are ignored in calculations leading to an underestimation of PDs or that (2) the Delta assay itself is designed to support maximum hematopoietic cell proliferation with greater expansion than other culture systems with associated accelerated telomeric decline. Of note was, however, that telomere length at weeks 1 through 4 of ex vivo expansion declined with a base pair loss of 0.4 kbp per week, although cell expansion and PDs peaked in weeks 1 and 2 so that telomere length decline would have been anticipated greatest in these. Calculation of number of PDs per week showed that CD34+ cells in ex vivo expansion cultures underwent 20 PD in week 1 and 2 compared with 3.5 PD in weeks 3 and 4, with an average base pair loss of 26.3, 83, 210, and 250 per PD in weeks 1, 2, 3, and 4, respectively. Our calculations of total number of PDs almost certainly underestimate the actual number of doublings, which Delta expansion culture initiating cells undergo, as these comprise probably less than 10% of the input CD34+ cell population. Thus, the estimation of base pair loss may be influenced by the different clonal progeny of cells of which the minority are hematopoietic stem cells. Secondly, this estimation of base pair loss may be somewhat effected by the lack of accurate assessment of differentiated cells that have undergone apoptosis. Nevertheless, our results clearly show that excessive loss of base pairs occurs in the last 2 weeks of culture when telomerase is low or undetectable.
Our data illustrate that telomerase is expressed in CD34+ cells at basal levels, is probably not expressed in G0 stem cells, and is upregulated on ex vivo expansion. Positively regulating, proliferation inducing cytokines caused telomerase upregulation in association with entry of cells into cycle closely linked with BrdU incorporation and the induction of cell cycle regulating proteins involved in the G1/S phase progression. Telomere loss was observed in ex vivo expansion cultures of CD34+ cells, in part compensated by telomerase upregulation. Possible reasons for telomere shortening despite telomerase activity are that (1) telomerase stabilizes telomeres in a small cell subset, which is not detected when mass cell cultures are analyzed, (2) that telomerase activity in progenitor cells allows extensive proliferation and cell expansion reducing rather than preventing the number of base pairs lost per cell division, (3) that the level of telomerase permits repair of damaged telomeres and/or (4) that telomerase prevents further telomere shortening only when cells approach the Hayflick limit. Based on our work, we conclude that telomerase activity is upregulated in normal hematopoietic cells following their cytokine-induced entry into cell cycle, which is sufficient to reduce, although not to completely prevent, telomere loss when bulk cell turnover, cell expansion, and massive cell proliferation takes place.
ACKNOWLEDGMENT
We thank Dr K.R. Prowse (Geron Corp, San Francisco, CA) for her expert help in establishing the TRF assay, Dr N.W. Kim (Geron Corp) for providing the alkaline phosphatase protocol, and Dr Dan Levitt (Geron Corp) and Dr Howard Scher (MSKCC, New York, NY) for their helpful discussion of this work.
Supported by Grants No. U9 CA 67842-01 (to M.A.S.M.) and CA 56564 (to R.K.) from the National Institutes of Health, Bethesda, MD; Gar Reichman Fund of the Cancer Research Institute, New York (to M.A.S.M.); Byrne Foundation, New York (to M.A.S.M.); Martin Himmel Fund, New York (to M.A.S.M.); Fondo de Investigacı́on Sanitaria (FIS, Barcelona, Spain) 96/5706 (to J.A.); and Deutsche Forschungsgemeinschaft, Bonn, Germany, 95/319/1-1 (to M.E.).
Address reprint requests to Malcolm A.S. Moore, D Phil, Laboratory of Developmental Hematopoiesis, Memorial Sloan-Kettering Cancer Center, RRL 717-C, 1275 York Ave, Mailbox 101, New York, NY 10021.

![Fig. 3. Telomerase in CD34+/CD38+ and CD34+/CD38− cells. (A) Telomerase activity in CD34+/CD38+ and CD34+/CD38− cells on days 3, 5, and 7 of ex vivo expansion culture. Telomerase was upregulated to a higher extent in CD34+/CD38+ cells compared with the more quiescent CD34+/CD38− population. Both fractions when stimulated with multiple cytokines (K36EG, F36EG, KF36EG) as opposed to KL + IL-6 or KL + IL-6 + sIL-6R resulted in an increased telomerase expression. (B) Telomerase activity at baseline with telomerase confined to CD34+/CD38+ cells and absent in CD34+/CD38− fraction is diplayed. After 1 week of ex vivo expansion, telomerase upregulation using combinations of KL + IL-6 was increased in CD34+/CD38+ cells compared with CD34+/CD38− cells. This was even more pronounced when cytokine combinations of KF36EG were used. Lane 1: CD34+/CD38− fraction at baseline (day 0) [no telomerase activity]; lane 2: CD34+/CD38+ fraction at baseline (day 0) [telomerase activity]; lane 3: CD34+/CD38− fraction, 7 days in ex vivo expansion (day 7) with KL + IL-6; lane 4: CD34+/CD38+, 7 days in ex vivo expansion (day 7) with KL + IL-6; lanes 5 to 7: CD34+/CD38− fraction on day 3 (lane 5), day 5 (lane 6), and day 7 (lane 7) of ex vivo exansion with KF36EG; lanes 8 to 10: CD34+/CD38+ fraction on day 3 (lane 8), 5 (lane 9), and 7 (lane 10) with KF36EG.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.182/5/m_bl_0026f3.jpeg?Expires=1770203029&Signature=LGGF8fmAnF-o7suMyUZdfh8bvDmvwpQ6yOXwn76AC8c3UTeG3a7OsYFoXhIWrwbSUutm5w9aSyyBPK7ore14p-N87PRBSb3pfj7uB5gIPqFx0zHzuzz7OA7DqU2Ckb0FIZWqhafioz9ESPNwLuS5~qOg57kRZ5iZokgEDCw03xUzUC0tHrR3EqqE5NBLwKhNxzM-q4r1wL8FQBEXbvUlmk-K7zAmSgFFRQ2cKVoELhA7-GPIVD7OJZasL9vJkfoIRgNCAxk4u6egwdE~9iPXL5Bn~1-~QXya2tJW0a-5uPGgTbi0yzok0LdUNTPHfGNzrxxsdvoA9m3dz3avSQUdsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Secondary CD34+ and CD34− cells. (A) Telomerase activity at baseline and weeks 1 to 4 of ex vivo expansion in primary CD34+ (•), secondary CD34+ (▪), and CD34− () cells is shown ([A] depicts the relative telomerase activity in percentage of the SK-N-SH neuroblastoma cell line control). After 1 week of culture, telomerase activity was upregulated in primary CD34+ and CD34− cells. In primary CD34+ cells, telomerase activity increased 20-fold (median) compared with baseline levels. Secondary CD34+ cells isolated at week 1 of culture displayed a 1.4-fold telomerase increase compared with primary CD34+ cells, but a sharp decline after 1 week of expansion and no upregulation of telomerase (week 1 → week 2). There was no upregulation of telomerase activity in primary or in secondary CD34+ cells in the following weeks (weeks 2 to 4) of culture. In CD34− cells, telomerase was fivefold to 10-fold lower in the first 2 weeks of expansion compared with primary CD34+ cells and undetectable in weeks 3 and 4. (B) Cumulative population doublings in primary CD34+, secondary CD34+, and CD34− cells are shown. Similarly to the reduced upregulation of telomerase activity in secondary CD34+ cells, cellular expansion was also reduced.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/1/10.1182_blood.v90.1.182/5/m_bl_0026f4.jpeg?Expires=1770203029&Signature=oEzagOPa8cFR2NAnQJw8Q7xgueE-Icb9K93PzktcQ5Q9aYBoIhALfSNXyObBqu4Wdv7wqCYSzM4BtRh3JkKe-5fas4zvzfpr0wnRoF8o2ZOwjdmCPlFBGd2Mgn8pNt3LJMpJEeXyw5hTDTj~0TRfFZ0HFHAp--hZLX9XcWt28PK9MynN4-Z-8RS~I~rP2At4AmgELY6T3lj5WKnqujLEEw8klmh73e5~8hMUkrnWQiN5CQvYrSvZo7kMWVs2Cr7nd5Sh10S6S2z7ooJdp4ktooeYpUXGpbeFdgGYjaqIJiZPBQHncr7~GM2A9RLodp45qFczxaGxlzIraXAbEZOyhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)