Abstract
Blood from late fetal and newborn mice is similar to umbilical cord blood obtained at birth in human beings, an important source of stem cells for clinical transplantation. The mouse model is useful because long-term functions can be readily assayed in vivo. To evaluate the functions of hematopoietic precursors in the blood and other tissues of late fetal and newborn mice, short- and long-term multilineage repopulating abilities were measured in vivo by competitive repopulation. Manipulations that might affect cell function, such as enrichment, tissue culture, or retroviral marking, were avoided. Hematopoietic stem cell functions of late fetal or newborn blood, liver, and spleen, were assayed as myeloid and lymphoid repopulating abilities relative to standard adult marrow cells. Donor cells from these tissues as well as adult control donor marrow cells were all of the same genotype. Cells from each donor tissue were mixed with portions from a pool of standard adult “competitor” marrow distinguished from the donors by genetic differences in hemoglobin and glucosephosphate isomerase. After 21 to 413 days, percentages of donor type myeloid and lymphoid cells in recipient blood were measured to assay the functional abilities of donor precursors relative to the standard. These relative measures are expressed as repopulating units, where each unit is equivalent to the repopulating ability found in 100,000 standard adult marrow cells. Thus, measures of repopulating units do not compare single cells but overall repopulating abilities of donor cell populations. Relative functional abilities in 1 million nucleated cells from late fetal or newborn blood were several times less than those found in adult marrow, but far more than in normal adult blood, and appeared to include long-term functional primitive hematopoietic stem cells (PHSC) similar to those in marrow. To estimate functional abilities of individual PHSC, variances among large groups of identical recipients were analyzed using both the binomial model and competitive dilution, a new model based on the Poisson distribution. The data best fit the hypothesis that individual PHSC from adult marrow, late fetal blood, or newborn blood each produce similar fractions of the total lymphoid and erythroid cells found in the recipient for many months.
MOUSE MODELS are useful for basic research and for answering clinical questions that are impractical to address in human systems. Such models have proven particularly valuable in defining primitive hematopoietic stem cells (PHSC), the precursors that continuously regenerate all renewable myeloid and lymphoid cells, as well as a variety of other cell types, throughout the life span. PHSC are important in basic research on cell differentiation and proliferation; moreover, PHSC are essential for long-term success in clinical bone marrow (BM) transplantation.
Human umbilical cord blood at birth is currently becoming an important clinical source of PHSC.1-6 Thus, it is vital to test PHSC functions in cord blood to evaluate it as a PHSC source. However, there are three major problems in interpreting studies of hematopoietic stem cells from human cord blood.
First, human stem cells are frequently measured as myeloid cell colonies over a few weeks, because such assays are convenient. Unfortunately colony-forming cells are far more differentiated than PHSC. Thus, their numbers will only correlate with PHSC function if these two disparate cell types happen to be affected in a correlated fashion by the variable tested. This explains why colony-forming cell assays often fail to predict PHSC function.7-11 Additionally, colony-forming cells can be readily separated from PHSC.12-16 Testing the generation of colony-forming cells measures PHSC differentiation to produce colony-forming cells, but not PHSC proliferation to produce PHSC; the latter is required for long-term function.
Second, colony-forming cell assays are limited to myeloid cell types, while PHSC repopulate both the lymphoid and myeloid systems.17-22 In fact, the same PHSC produce T cells, B cells, granulocytes, macrophages, platelets, and erythrocytes proportionally, with high correlations between all these cell types.17,23 By comparison, myeloid-specific precursors fail to produce sufficient numbers of descendants for correlations within myeloid cell lineages to be greater than correlations between myeloid and lymphoid cells.17 23
Third, long-term assays are required to show long-term function, the most distinctive characteristic of PHSC. Even showing both lymphoid and myeloid function is not adequate to identify a cell as a PHSC, because most precursors that repopulate both lymphoid and myeloid lineages soon after transplantation are short-lived.15-20 The length of time that such short-term multilineage precursors function appears to be proportional to the life span of the species. Thus, short-term precursors disappear 3 to 4 months after transplantation in mice,15-20 but persist for 1 to 4 years in cats.24 This proportion implies that human short-term multilineage precursors might function for 10 years or more, making it impossible to know that PHSC have been successfully transplanted in human beings until recipients have been observed for more than a decade. Because the human life span is so long, tests administered over a substantial fraction of the life span are impractical.
Differences between short-term and long-term multilineage repopulating precursors are deterministic, because they can be separated using antigenic markers.15,16 Unfortunately, the markers used to enrich PHSC cannot be trusted to predict PHSC function, because cells carrying all the markers associated with long-term reconstitution proliferate greatly in vivo16 or in vitro25 without a proportionate increase in PHSC function. Thus, the only definitive assay for PHSC is to measure their long-term repopulating function in vivo,26 and this is most practical in mouse models.
Just as many mouse systems are models for analogous human systems, late fetal and newborn mouse blood provides a useful model for human umbilical cord blood at birth. The current study uses competitive repopulation to assay PHSC function in the following donors: late fetal or newborn blood, liver, or spleen, and adult BM. Donor cells are mixed with equivalent doses of genetically distinguished standard “competitor” BM cells, and grafted into irradiated recipients; percentages of donor type lymphoid and myeloid cells in the recipients measured donor cell function relative to the standard.23
During testing, precursor cells are not overstressed by excessive stimuli because sufficient cells from the standard BM are used to maintain recipient health. Adequate numbers of niches are available because we have shown that numbers of functioning PHSC increase proportionally to numbers of BM cells grafted over a range of 0.2 to at least 8 million.23 Grafted as mixtures, donor cells are compared with standards in the same normal environment. Nevertheless, even small differences between donor and standard cell functions are detected because the total differentiation pathway is assayed, and small differences in responses to stimuli or in proliferation rates are amplified with each proliferative cycle. Standards and donors are congenic so they do not interact or damage each other; they only differ in the genetic markers used to distinguish their descendants.23 Thus, the intrinsic factors that allow test cells to be analyzed using the competitive repopulation assay are the same factors that allow them to produce large fractions of the differentiated cells in multiple lineages over the long term, and donor cell functional abilities are tested rigorously while the hematopoietic system regenerates in transplant recipients. To have normal repopulating ability, donor PHSC and all of their descendants must produce differentiated cells as well as the standard cells do for many months.23
Concentrations of repopulating units in nucleated cell populations in mouse models of umbilical cord blood were several-fold lower than those in adult BM, but tended to increase relative to adult BM for as long as they were followed, up to 413 days after transplantation, (about half of a mouse's life expectancy). This ability to provide long-term reconstitution may be clinically significant.
Long-term functions of individual PHSC are difficult to estimate, as they may be affected by abnormal stimuli when recipient health is not fully maintained by adequate grafts of normal BM cells. Measures of variances among recipients given identical cell mixtures19 23 were used with the healthy recipients in the current study to estimate functional abilities of individual PHSC in normal populations. Long-term PHSC repopulating abilities appeared to be similar whether they were from adult BM or from cord blood models.
MATERIALS AND METHODS
Mice.Male B6 (C57BL/6J-HbbsGPi 1b) mice that were 3 to 6 months of age were used in these studies as lethally irradiated recipients and as donors of standard competitor BM. B6-HbbdGpi 1a congenic mice, prepared as detailed previously,17,20 23 were used as late fetal, newborn, and adult control donors. All mice were produced and maintained at The Jackson Laboratory (Bar Harbor, ME), which is fully accredited by the American Association for the Accreditation of Laboratory Animal Care. Mice were fed Wayne Lab Blox (Harlan Teklad, Madison, WI) or NIH-31 (4% to 5% fat diets), and were housed four per cage in environmentally controlled animal rooms free of known mouse pathogens. Details of the animal husbandry are published by The Jackson Laboratory (Bar Harbor, ME 04609) in its brochure, the Handbook on Genetically Standardized Jax Mice; quarterly updates are published in the Jackson Laboratory brochure, Animal Health and Genetic Quality Control Report.
Donor treatment and competitive repopulation.Mice were set up for timed pregnancy by observing the mated female every 24 hours after she was caged with a male. Day 1 of pregnancy began 24 hours after the observation of a vaginal plug. In experiments 1 and 2 late fetal (19 day fetal) tissues were collected from females whose plugs were observed at the same time as plugs in females with newborn offspring in the same experiment; tissues were collected from newborn mice before they were 1 day old.
Cell samples of both genders were pooled for each tissue at each age. Late fetal or newborn blood was collected by decapitating the animal in Iscove's Modified Dulbecco's medium (Sigma, St Louis, MO) containing 0.95% sodium citrate (IMDM + NaCit). Numbers of mice pooled were: experiment 1 — 2 fetal and 2 newborn livers, 9 fetal and 26 newborn mice for blood; experiment 2 — 2 fetal and 2 newborn livers, 15 fetal and 10 newborn spleens, 34 fetal and 51 newborn mice for blood. Liver, spleen, thymus, testes, and mesenteric lymph node samples were collected by removing each organ and homogenizing it in either IMDM or IMDM + NaCit with a ground glass tissue homogenizer (Bellco Glass Inc, Vineland, NJ). Liver cells from late fetal and newborn mice in both experiments contained large amounts of fatty tissue that could not be easily separated from the other cells. Clumps of tissue were filtered from liver cell suspensions, suggesting that substantial numbers of cells may have been lost. Addition of NaCit seemed to reduce clumping, resulting in increased numbers of liver cells in experiment 2. However, actual numbers of cells in livers may have been higher than those reported. Because some liver PHSC may have been lost, total numbers of PHSC in late fetal and newborn livers should be considered minimum values for both experiments. BM cells from femurs and tibias of adults were suspended in IMDM by flushing through a 1-mL disposable syringe pressed gently against the bottom of a 4-mL test tube to break up clumps, as detailed previously.23 Cell suspensions were filtered through nylon mesh to remove debris.
In all cases nucleated cells were counted using a model ZBI Coulter Counter (Coulter Electronics, Inc, Hialeah, FL) after erythrocytes were lysed using standard lysing agents for white blood cell counting. Standard (B6 type) competitor BM came from a single pool providing a reproducible standard for all donors in each experiment. Preliminary tests showed that each tissue type required a different ratio of donor to standard cell numbers for optimal results (donor percentages between 30 and 70), and appropriate numbers of donor cells from each tissue were mixed with corresponding portions from the standard pool. Details of cell numbers for each experiment are given in the table legends.
Recipients were B6 male mice lethally irradiated with 1,100 rads from a 137Cs gamma source (Shepard Mark 1; Shepard & Associates, Glendale, CA) at a dose rate of 180 rads/min 2 to 6 hours before transplantation. Each recipient was warmed and given 0.5 mL of the cell mixture injected intravenously through the lateral tail vein.
Percentages of donor type cells in recipients.After time periods ranging from 21 to 413 days (detailed in the tables), blood was taken from each recipient through the orbital sinus in standard heparinized hematocrit tubes; platelets, granulocytes and lymphocytes, or lymphocytes and erythrocytes were separated by density gradient centrifugation. Details of the separations, and of the hemoglobin (Hb) and glucosephosphate isomerase (GPI) electrophoretic techniques, have been described previously.17,20,21 23 All gels were scanned using a Helena Laboratories (Beaumont, TX) Cliniscan 2 scanning densitometer. GPI gels were scanned while still moist and without removing the staining overlay.
Data analyses.Percentages of donor type cells are given as mean ± SD. Statistical significances of differences between organs were not calculated because ratios of donor-to-standard cells had to be varied to adjust for different functional abilities; therefore, comparisons of percentages would not have been meaningful. Values for the different donors were adjusted relative to the standard in each experiment by calculating repopulating units (RU). It is important to note that each RU is not an individual PHSC, but a unit measuring overall repopulating abilities of donor cell populations. Each RU is equivalent to 1 × 105 adult B6 BM cells from the standard competitor pool in that experiment. The numbers of RU in the injected dose of donor cells were calculated as follows23:
where, for example, if 10 × 105 standard cells were used, then RU(standard) = 10; and if % donor type cells is called P, then P = 100RU(donor)/(RU(donor) + 10), and by rearrangement, RU(donor) = 10P/(100 − P).
Functional abilities of individual PHSC were estimated by two methods. In the first, numbers of RU were compared with numbers of PHSC estimated using the binomial model as detailed previously,17,20,21 23 and outlined in the caption of Table 5. Correlation coefficients and covariances were calculated using the StatView II (v 1.04; Abacus Concepts, Berkeley, CA) program on a Macintosh SE/30 personal computer (Apple Computer, Inc, Cupertino, CA).
Estimated PHSC Concentrations in Fetal and Newborn Blood
| Time in . | Calculated Statistics . | Fetal Blood . | Newborn Blood . | Control BM . |
|---|---|---|---|---|
| Recipient (d) . | . | . | . | . |
| 118 | E2:L2 r | .78 (20) | .83 (18) | .87 (19) |
| Est Conc/1065-150 | 1.8 | 5.7 | 9.3 | |
| RUave/PHSC | 1.4 | 1.0 | 2.1 | |
| 245 | E3:L3 r | .91 (18) | .82 (18) | .96 (18) |
| Est Conc/106 | 1.8 | 3.3 | 4.8 | |
| RUave/PHSC | 1.6 | 2.2 | 4.6 | |
| 413 | E4:L4 r | .88 (15) | .72 (15) | .94 (13) |
| Est Conc/106 | 2.0 | 6.7 | 3.6 | |
| RUave/PHSC | 1.5 | 1.1 | 3.7 | |
| 118-245 | E2:E3 r | .87 (18) | .82 (18) | .93 (18) |
| L2:L3 r | .74 (18) | .82 (18) | .92 (18) | |
| 245-413 | E3:E4 r | .99 (15) | .32 (14)5-151 | .98 (13) |
| L3:L4 r | .98 (15) | .46 (13)5-151 | .99 (13) |
| Time in . | Calculated Statistics . | Fetal Blood . | Newborn Blood . | Control BM . |
|---|---|---|---|---|
| Recipient (d) . | . | . | . | . |
| 118 | E2:L2 r | .78 (20) | .83 (18) | .87 (19) |
| Est Conc/1065-150 | 1.8 | 5.7 | 9.3 | |
| RUave/PHSC | 1.4 | 1.0 | 2.1 | |
| 245 | E3:L3 r | .91 (18) | .82 (18) | .96 (18) |
| Est Conc/106 | 1.8 | 3.3 | 4.8 | |
| RUave/PHSC | 1.6 | 2.2 | 4.6 | |
| 413 | E4:L4 r | .88 (15) | .72 (15) | .94 (13) |
| Est Conc/106 | 2.0 | 6.7 | 3.6 | |
| RUave/PHSC | 1.5 | 1.1 | 3.7 | |
| 118-245 | E2:E3 r | .87 (18) | .82 (18) | .93 (18) |
| L2:L3 r | .74 (18) | .82 (18) | .92 (18) | |
| 245-413 | E3:E4 r | .99 (15) | .32 (14)5-151 | .98 (13) |
| L3:L4 r | .98 (15) | .46 (13)5-151 | .99 (13) |
Numbers of recipients are given in parentheses after “r.” Analyses of 13 to 20 recipients of fetal or newborn blood or control BM that are detailed in the caption of Table 3. Total numbers of PHSC are estimated using the binomial equation: N = P (100 − P) ÷ [covariance] where P = mean % donor type, and the covariance = (r) × (SD1 × SD2). Such estimates of numbers using covariance become more meaningful as r approaches 1.00 and the SDs approach the same value. The r values are Pearson's product moment correlation coefficients relating percentages of donor type erythrocytes (E) and lymphocytes (L) in the same recipients sampled at time points 2, 3 and 4, or 2 successive sampling times (2 to 3; 3 to 4) for either E or L.
Concentrations of PHSC in blood donors are estimated by subtracting estimated numbers of BM standards (half the number estimated for BM controls) from total numbers of PHSC, and adjusting to conc./106 by multiplying by 2. RUave/PHSC is the average number of RU calculated from lymphocytes and erythrocytes in Table 2 divided by the estimated PHSC concentration (both per 106 donor cells).
These correlation coefficients are not significant ( P > .05), probably because of confusion in sample order.
In the second method, competitive dilution19 tested the hypothesis that individual PHSC from fetal or newborn blood produce large fractions of the differentiated cells in multiple lineages over the long term in the same fashion as PHSC from adult BM. We modeled three cases in which each PHSC within the donor population repopulated 0.5, 1, or 2 times as well as each PHSC within the standard population. The probability that a recipient is repopulated by i precursor cells is given by the Poisson equation:
where m (the average multiplicity) is the mean number of donor precursor cells in the sample injected into the recipient. Use of this equation assumes that precursor cells are randomly distributed in the BM cell suspension and each precursor cell has an equal opportunity to repopulate irradiated mice.
Average numbers of PHSC in the standard pool were estimated by standard limiting dilution procedures, solving for “m” after determining the probability or proportion of mice that do not receive a donor precursor cell (negative samples), which is represented by the zero term (i = 0) of the Poisson distribution:
From the measured average donor percentages in recipients with both donor and standard cells, we calculate the average PHSC number required if donor PHSC repopulate 0.5, 1, or 2 times as well as standard PHSC. For each average injection dose of donor and standard PHSC, we use the Poisson equation to estimate the probabilities of various numbers of donor and standard PHSC. These two sets of probabilities are combined in a matrix to calculate the probabilities that a recipient will receive each combination of particular donor and standard PHSC numbers. The combined matrix probabilities are then multiplied by the number of recipient samples that are to be modeled (in our case 16 to 18 in the three populations tested) giving the expected number of recipients containing each combination of donor and standard PHSC numbers.
RESULTS
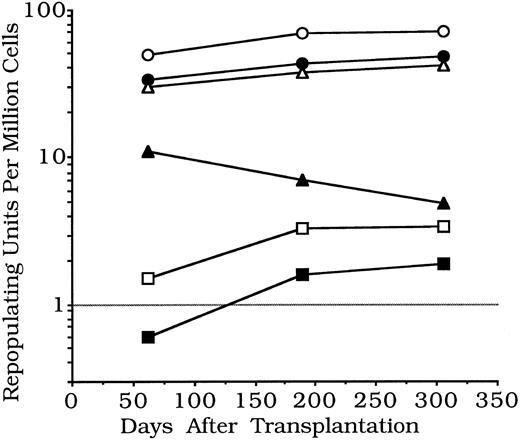
Experiment 1.In experiment 1, donor cells were blood and liver from 19-day fetal or from newborn B6-Hbbd Gpi 1a mice, and BM from B6-Hbbd Gpi 1a adults (Table 1). Cells from each donor tissue were mixed with portions from the same B6 standard BM cell pool, given to B6 recipients, and then sampled after 62, 189, and 306 days. Repopulating unit concentrations in fetal or newborn blood were lower than in adult BM, but repopulating unit concentrations in fetal and newborn liver were higher; both tended to increase from 62 to 189 days, while repopulating abilities of adult BM controls tended to decrease (Fig 1).
Relative Repopulating Abilities of Late Fetal and Newborn Blood and Liver Cells
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | |||
|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Blood . | Liver (1) . | Liver (2) . | . |
| 62 | PE | 59 ± 1 | 56 ± 5 | 46 ± 8 | 65 ± 4 | 30 ± 10 | 53 ± 5 |
| RUE* | 1.4 | 38 | 0.6 | 56 | 34 | 11 | |
| PL | 60 ± 3 | 49 ± 6 | 47 ± 10 | 60 ± 6 | 24 ± 7 | 51 ± 5 | |
| RUL* | 1.5 | 29 | 0.6 | 45 | 25 | 10 | |
| 189 | PE | 76 ± 8 | 62 ± 2 | 70 ± 1 | 72 ± 7 | 35 ± 13 | 44 ± 5 |
| RUE* | 3.2 | 49 | 1.7 | 77 | 43 | 7.9 | |
| PL | 77 ± 5 | 56 ± 3 | 68 ± 4 | 68 ± 6 | 29 ± 9 | 38 ± 6 | |
| RUL* | 3.3 | 38 | 1.5 | 64 | 33 | 6.1 | |
| 306 | PE | 74 ± 6 | 65 ± 3 | 75 ± 7 | 74 ± 8 | 36 ± 12 | 34 ± 1 |
| RUE* | 2.8 | 56 | 2.2 | 85 | 45 | 5.2 | |
| PL | 80 ± 7 | 57 ± 4 | 69 ± 5 | 66 ± 4 | 33 ± 11 | 32 ± 10 | |
| RUL* | 4.0 | 40 | 1.6 | 58 | 39 | 4.7 | |
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | |||
|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Blood . | Liver (1) . | Liver (2) . | . |
| 62 | PE | 59 ± 1 | 56 ± 5 | 46 ± 8 | 65 ± 4 | 30 ± 10 | 53 ± 5 |
| RUE* | 1.4 | 38 | 0.6 | 56 | 34 | 11 | |
| PL | 60 ± 3 | 49 ± 6 | 47 ± 10 | 60 ± 6 | 24 ± 7 | 51 ± 5 | |
| RUL* | 1.5 | 29 | 0.6 | 45 | 25 | 10 | |
| 189 | PE | 76 ± 8 | 62 ± 2 | 70 ± 1 | 72 ± 7 | 35 ± 13 | 44 ± 5 |
| RUE* | 3.2 | 49 | 1.7 | 77 | 43 | 7.9 | |
| PL | 77 ± 5 | 56 ± 3 | 68 ± 4 | 68 ± 6 | 29 ± 9 | 38 ± 6 | |
| RUL* | 3.3 | 38 | 1.5 | 64 | 33 | 6.1 | |
| 306 | PE | 74 ± 6 | 65 ± 3 | 75 ± 7 | 74 ± 8 | 36 ± 12 | 34 ± 1 |
| RUE* | 2.8 | 56 | 2.2 | 85 | 45 | 5.2 | |
| PL | 80 ± 7 | 57 ± 4 | 69 ± 5 | 66 ± 4 | 33 ± 11 | 32 ± 10 | |
| RUL* | 4.0 | 40 | 1.6 | 58 | 39 | 4.7 | |
In experiment 1, percentages of erythrocytes (PE) of donor type or lymphocytes of donor type (PL) are given as mean ± SD; n = 3 to 4 in each group, except n = 14 to 16 for Liver (2). Recipients were given the following doses: Blood, 2 × 105 B6 standard BM cells, mixed with 2.0 (19 day fetal) or 2.75 (newborn) × 106 B6-HbbdGpi 1a donor nucleated blood cells; Liver, 3 × 106 B6 standard BM cells, mixed with 1 × 106 cells from B6-HbbdGpi 1a 19 day Fetal Liver or Newborn Liver (1), except recipients marked Newborn Liver (2) were given 8 × 105 B6 standard BM cells, mixed with 1 × 105 B6-HbbdGpi 1a newborn liver cells; Controls, 2 × 106 donor and standard adult BM cells, because doses of 1 to 4 million cells per recipient are standard when measuring repopulating abilities of fresh BM.23 Controls for recipient repopulation received 2 × 105 B6-HbbdGpi 1a adult BM cells only (values for PE, PL were 98 ± 4, 100 after 62 days, and 100, 100 at the two later time points, with n = 4.
RU per 106 donor cells injected, where 1.0 RU = repopulating ability of 1.0 × 105 standard cells.
Changes with time in stem cell functional abilities in mouse models of umbilical cord blood — experiment 1. Pooled erythroid and lymphoid repopulating units per 106 nucleated cells are shown. Repopulating units are not measures of single cells but of repopulating ability relative to standard BM. Each unit is equivalent to repopulating abilities of 100,000 standard BM cells. Thus, relative functional abilities per million cells of late fetal and newborn blood are several times less than found in adult BM, while values for late fetal or newborn liver are several times more. Fetal and newborn values tended to increase, and adult BM values to decrease, from 62 to 306 days (details in Table 1). (○), Newborn liver (1); (•), fetal liver; (▵), newborn liver (2); (▴), adult marrow; (□), fetal blood; (▪), newborn blood.
Changes with time in stem cell functional abilities in mouse models of umbilical cord blood — experiment 1. Pooled erythroid and lymphoid repopulating units per 106 nucleated cells are shown. Repopulating units are not measures of single cells but of repopulating ability relative to standard BM. Each unit is equivalent to repopulating abilities of 100,000 standard BM cells. Thus, relative functional abilities per million cells of late fetal and newborn blood are several times less than found in adult BM, while values for late fetal or newborn liver are several times more. Fetal and newborn values tended to increase, and adult BM values to decrease, from 62 to 306 days (details in Table 1). (○), Newborn liver (1); (•), fetal liver; (▵), newborn liver (2); (▴), adult marrow; (□), fetal blood; (▪), newborn blood.
Total repopulating units in fetal and newborn blood or liver are estimated in Table 2. Total numbers of cells were counted precisely, with care taken to avoid counting red blood cells. Numbers of nucleated blood cells obtained from late fetal or newborn mice are doubled to account for cells not washed out of the decapitated mice and for those in the placenta. The liver had more than 100 times the total repopulating units of the blood in late fetal or newborn mice.
Total Repopulating Abilities of Late Fetal and Newborn Blood and Liver Cells
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | |||
|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Blood . | Liver (1) . | Liver (2) . | . |
| 62 | RUtot | 2.1 | 289 | 1.1 | 435 | 257 | 3,270 |
| 189 | RUtot | 4.6 | 376 | 3.0 | 608 | 327 | 2,100 |
| 306 | RUtot | 4.7 | 413 | 3.5 | 620 | 365 | 1,470 |
| No. cells × 106 per organ per individual | 1.4 | 9 | 1.9 | 9 | 9 | 300 | |
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | |||
|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Blood . | Liver (1) . | Liver (2) . | . |
| 62 | RUtot | 2.1 | 289 | 1.1 | 435 | 257 | 3,270 |
| 189 | RUtot | 4.6 | 376 | 3.0 | 608 | 327 | 2,100 |
| 306 | RUtot | 4.7 | 413 | 3.5 | 620 | 365 | 1,470 |
| No. cells × 106 per organ per individual | 1.4 | 9 | 1.9 | 9 | 9 | 300 | |
To give total repopulating units, RUtot, each mean RU per million cells (Table 1) was multiplied by the numbers of cells per organ per individual given above.
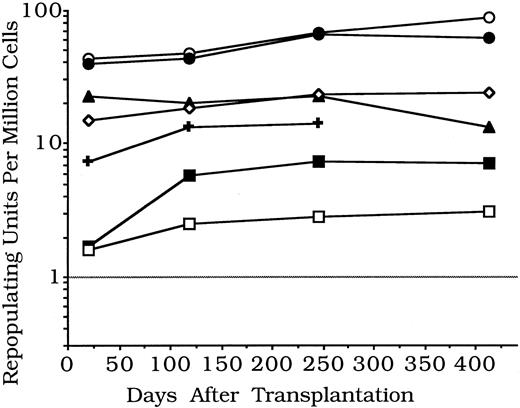
Experiment 2.Experiment 2 (Table 3) repeated experiment 1 using blood, liver, or spleen cells from late fetal or newborn B6-Hbbd Gpi 1a donors. Cells from each organ and from control adult B6-Hbbd Gpi 1a BM were each mixed with portions from the same B6 standard BM cell pool. Recipients were sampled after 21, 118, 245, and 413 days. As compared with adult BM, concentrations of repopulating units were lower in fetal or newborn blood, higher in liver, and about the same in spleen. Again, repopulating unit concentrations tended to increase with time for late fetal and newborn cells; concentrations in adult BM changed little with time (Fig 2).
Relative Repopulating Abilities of Late Fetal and Newborn Blood, Liver, and Spleen Cells
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Spleen . | Blood . | Liver . | Spleen . | . |
| 21 | PP | 17 ± 12 | 41 ± 15 | 39 ± 7 | 24 ± 10 | 31 ± 1 | 51 ± 1 | 64 ± 5 |
| RUP* | 0.8 | 28 | 6.4 | 1.3 | 26 | 10 | 18 | |
| PL | 32 ± 20 | 47 ± 7 | 37 ± 13 | 30 ± 16 | 45 ± 12 | 55 ± 11 | 71 ± 6 | |
| RUL | 1.9 | 35 | 5.9 | 1.7 | 33 | 12 | 24 | |
| PG | 38 ± 21 | 62 ± 16 | 51 ± 10 | 35 ± 15 | 71 | 74 ± 2 | 73 ± 6 | |
| RUG | 2.5 | 65 | 10 | 2.2 | 98 | 28 | 27 | |
| 118 | PE | 37 ± 39 | 56 ± 4 | 63 ± 4 | 65 ± 26 | 57 ± 5 | 72 ± 5 | 72 ± 33 |
| RUE | 2.3 | 51 | 17 | 7.4 | 53 | 26 | 26 | |
| PL | 40 ± 28 | 48 ± 3 | 50 ± 4 | 53 ± 24 | 52 ± 5 | 57 ± 3 | 61 ± 21 | |
| RUL | 2.7 | 37 | 10 | 4.5 | 43 | 13 | 16 | |
| 245 | PE | 46 ± 40 | 68 ± 9 | 67 ± 7 | 70 ± 32 | 68 ± 8 | 74 ± 5 | 72 ± 35 |
| RUE | 3.4 | 85 | 20 | 9.3 | 85 | 25 | 26 | |
| PL | 37 ± 36 | 56 ± 10 | 51 ± 12 | 59 ± 34 | 58 ± 8 | 67 ± 4 | 66 ± 33 | |
| RUL | 2.3 | 51 | 10 | 5.8 | 55 | 20 | 19 | |
| 413 | PE | 49 ± 42 | 68 ± 9 | — 3-151 | 75 ± 33 | 75 ± 5 | 80 ± 6 | 60 ± 47 |
| RUE | 3.8 | 85 | — | 12 | 120 | 40 | 15 | |
| PL | 38 ± 39 | 54 ± 13 | — | 53 ± 30 | 62 ± 4 | 61 ± 12 | 54 ± 39 | |
| RUL | 2.5 | 47 | — | 4.1 | 65 | 16 | 12 | |
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Spleen . | Blood . | Liver . | Spleen . | . |
| 21 | PP | 17 ± 12 | 41 ± 15 | 39 ± 7 | 24 ± 10 | 31 ± 1 | 51 ± 1 | 64 ± 5 |
| RUP* | 0.8 | 28 | 6.4 | 1.3 | 26 | 10 | 18 | |
| PL | 32 ± 20 | 47 ± 7 | 37 ± 13 | 30 ± 16 | 45 ± 12 | 55 ± 11 | 71 ± 6 | |
| RUL | 1.9 | 35 | 5.9 | 1.7 | 33 | 12 | 24 | |
| PG | 38 ± 21 | 62 ± 16 | 51 ± 10 | 35 ± 15 | 71 | 74 ± 2 | 73 ± 6 | |
| RUG | 2.5 | 65 | 10 | 2.2 | 98 | 28 | 27 | |
| 118 | PE | 37 ± 39 | 56 ± 4 | 63 ± 4 | 65 ± 26 | 57 ± 5 | 72 ± 5 | 72 ± 33 |
| RUE | 2.3 | 51 | 17 | 7.4 | 53 | 26 | 26 | |
| PL | 40 ± 28 | 48 ± 3 | 50 ± 4 | 53 ± 24 | 52 ± 5 | 57 ± 3 | 61 ± 21 | |
| RUL | 2.7 | 37 | 10 | 4.5 | 43 | 13 | 16 | |
| 245 | PE | 46 ± 40 | 68 ± 9 | 67 ± 7 | 70 ± 32 | 68 ± 8 | 74 ± 5 | 72 ± 35 |
| RUE | 3.4 | 85 | 20 | 9.3 | 85 | 25 | 26 | |
| PL | 37 ± 36 | 56 ± 10 | 51 ± 12 | 59 ± 34 | 58 ± 8 | 67 ± 4 | 66 ± 33 | |
| RUL | 2.3 | 51 | 10 | 5.8 | 55 | 20 | 19 | |
| 413 | PE | 49 ± 42 | 68 ± 9 | — 3-151 | 75 ± 33 | 75 ± 5 | 80 ± 6 | 60 ± 47 |
| RUE | 3.8 | 85 | — | 12 | 120 | 40 | 15 | |
| PL | 38 ± 39 | 54 ± 13 | — | 53 ± 30 | 62 ± 4 | 61 ± 12 | 54 ± 39 | |
| RUL | 2.5 | 47 | — | 4.1 | 65 | 16 | 12 | |
In experiment 2, percentages of donor type platelets, lymphocytes or granulocytes (PP, PL, or PG); or erythrocytes and lymphocytes (PE, PL) are given as mean ± SD; n = 13 to 20 recipients per group with fetal or newborn blood, or control BM and 3 to 4 in other groups, except 2 to 4 at 413 days. Recipients were given the following doses: Blood, 2 × 105 B6 standard BM cels, mixed with 5 × 105 B6-HbbdGpi 1a donor nucleated blood cells from 19 day fetal or newborn mice; Liver, 4 × 106 B6 standard BM cells, mixed with 1 × 106 B6-HbbdGpi 1a donor 19 day fetal or newborn liver cells; Spleen, equal numbers of B6 standard BM, mixed with B6-HbbdGpi 1a donor cells, 1.52 × 106 with late fetal or 1.8 × 106 with newborn spleen cells; Controls, 2 × 106 donor and standard adult BM cells.
RU per 106 donor cells injected, as in Table 1. In addition, recipients of 2 or 7.6 × 106 B6-HbbdGpi 1d nucleated blood cells from adults, plus 2 × 105 standard BM cells, had donor percentages averaging 20 to 29, so RU per 106 averaged 0.01 to 0.03 with n = 2 to 4.
All recipients died.
Changes with time in stem cell functional abilities in mouse models of umbilical cord blood — experiment 2. Pooled myeloid or erythroid and pooled lymphoid repopulation values per 106 nucleated cells are given. Values for late fetal and newborn blood are several times less than for adult BM, while values for late fetal and newborn spleen are similar to BM, and values for late fetal or newborn liver are several times more. Fetal and newborn values tended to increase, and adult BM values to decrease, from 21 to 413 days (details in Table 3). (○), Newborn liver; (•), fetal liver; (▴), adult marrow; (⋄), newborn spleen; (✚), fetal spleen; (▪), newborn blood; (□), fetal blood.
Changes with time in stem cell functional abilities in mouse models of umbilical cord blood — experiment 2. Pooled myeloid or erythroid and pooled lymphoid repopulation values per 106 nucleated cells are given. Values for late fetal and newborn blood are several times less than for adult BM, while values for late fetal and newborn spleen are similar to BM, and values for late fetal or newborn liver are several times more. Fetal and newborn values tended to increase, and adult BM values to decrease, from 21 to 413 days (details in Table 3). (○), Newborn liver; (•), fetal liver; (▴), adult marrow; (⋄), newborn spleen; (✚), fetal spleen; (▪), newborn blood; (□), fetal blood.
Total concentrations of repopulating units were estimated from cell numbers in late fetal and newborn blood, liver, and spleen (Table 4). Both late fetal and newborn livers averaged 9 million cells in experiment 1, while they averaged 35 million and 22 million cells, respectively, in experiment 2. Some of this increase may have occurred because techniques to dissociate the livers were improved, as detailed in Materials and Methods. However, it is also possible that liver cell numbers are highly variable at this stage of murine development. As in experiment 1, numbers of repopulating units in both blood and spleen were two orders of magnitude less than those in the liver. Preliminary measures (data not shown) gave several times the levels of repopulating ability in newborn BM as those found in spleens, with no detectable repopulating activity in newborn thymus, testes, or mesenteric lymph nodes. Thus, the liver contained most of the repopulating ability at birth.
Total Repopulating Abilities of Late Fetal and Newborn Blood, Liver, and Spleen Cells
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Spleen . | Blood . | Liver . | Spleen . | . |
| 21 | RUtot | 2.1 | 1,408 | 2.7 | 1.4 | 959 | 10.7 | 6,780 |
| 118 | RUtot | 2.8 | 1,514 | 4.8 | 4.7 | 1,073 | 12.9 | 5,970 |
| 245 | RUtot | 3.1 | 2,288 | 5.3 | 6.0 | 1,523 | 16.6 | 6,690 |
| 413 | RUtot | 3.1 | 2,218 | — 4-150 | 6.6 | 1,994 | 17.0 | 4,050 |
| No. cells × 106 per organ per individual | 1.1 | 35 | 0.4 | 0.8 | 22 | 0.7 | 300 | |
| Time (d) . | Amount Donor . | Late Fetal: . | Newborn . | Control BM . | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | Blood . | Liver . | Spleen . | Blood . | Liver . | Spleen . | . |
| 21 | RUtot | 2.1 | 1,408 | 2.7 | 1.4 | 959 | 10.7 | 6,780 |
| 118 | RUtot | 2.8 | 1,514 | 4.8 | 4.7 | 1,073 | 12.9 | 5,970 |
| 245 | RUtot | 3.1 | 2,288 | 5.3 | 6.0 | 1,523 | 16.6 | 6,690 |
| 413 | RUtot | 3.1 | 2,218 | — 4-150 | 6.6 | 1,994 | 17.0 | 4,050 |
| No. cells × 106 per organ per individual | 1.1 | 35 | 0.4 | 0.8 | 22 | 0.7 | 300 | |
Numbers of precursors.Numbers of precursors in fetal or newborn donor blood cells, or control donor BM cells, were estimated using the binomial formula (Table 5). In each case, groups of 18 to 20 recipients were given identical mixtures containing 5 × 105 nucleated blood cells from fetal or newborn donors mixed with 2 × 105 adult BM standard cells; additional recipients were given 2 × 105 adult BM cells mixed with 2 × 105 standard cells. All standards were from the same pool of congenic cells. Each group of recipients represented 18 to 20 independent but equal sized samples of a mixture of donor and standard cells. If samples contained high numbers of precursors, they represented the whole population well, so all samples would have a similar number of precursor of each type, and SD would be small. However, if each sample contained few precursors, percentages of donor and standard type precursors would vary greatly, and SD would be high.
If each precursor contributes equally, variances (SD squared) are inversely proportional to the mean number of precursors in the sample, as represented by the binomial equation (Table 5, caption). If all precursors do not contribute equally, estimates of precursor numbers represent those that contribute most to the differentiated cell populations.27 We substitute erythrocyte:lymphocyte covariance for variance, as the extremely high correlations between these cell types suggest that they are descended proportionally from common precursors. Importantly, use of covariances eliminates contributions to the variance from random error.17,20,21 23
Variances, shown as SDs in Table 3, tend to increase greatly in control BM, and somewhat in blood samples between 21 and 118 days. Apparently, precursor concentrations are much higher at 21 than at 118 days. Most of the early precursors no longer contribute to differentiated cell populations by 118 days, and strong correlations between 118 to 245, and 245 to 413 days suggest that by 118 days, lymphocytes and erythrocytes are descended from PHSC that function for much of the lifespan (Table 5), as expected from true PHSC.17-22
Repopulating abilities per PHSC.Estimates of precursor numbers were compared with estimates of repopulating units by giving average RU per PHSC. These ranged from 1.0 to 2.2 in fetal or newborn blood, and 2.1 to 4.6 in control BM (Table 5). Thus, PHSC from fetal or newborn blood have similar, or slightly lower, repopulating abilities compared with those from adult BM. Importantly, there is no sign of a loss in functional activity of fetal or newborn blood PHSC relative to adult BM PHSC during 413 days, about half the lifespan of a normal mouse (Fig 2).
Competitive dilution procedures were also used on the data from experiment 2. Because the same standard pool was mixed with fetal blood, newborn blood, and adult BM, limiting dilution statistics were used to estimate standard PHSC frequencies. If less than 10% of the differentiated cells were of the standard type, we considered that no standard PHSC had been received, and that death meant the recipient had received neither standard nor donor PHSC. Proportions of recipients with no standard PHSC were 7 of 20, 5 of 18, and 8 of 19 in the three groups. Solving for the zero term of the Poisson distribution, these proportions give average standard PHSC frequencies of 1.05, 1.28, and 0.86, with a grand average of 1.05 standard PHSC per recipient.
Focusing on fetal blood, 10 of 20 recipients failed to receive donor PHSC, for an average frequency of 0.69 donor PHSC in the injection dose. From the average donor percentage in recipients repopulated by both donor and standard PHSC, the frequency of donor PHSC would be nearly the same value, 0.73, if donor and standard PHSC contributed equally. The data were ordered from lowest to highest donor percentages, and observed values subtracted from values produced by the competitive dilution model. Sums of absolute values for all differences were 80 using the model in which donor and standard PHSC contributed equally, while sums of differences were much higher, 192 and 240, using models in which donor PHSC contributed 0.5 and 2 times as much as standard PHSC.
Models using newborn blood or adult BM donors were very similar to each other, and both best fit the model that all PHSC contribute equally, although the model that donor PHSC contribute 0.5 as much fits almost equally well. Only 1 of 18 and 2 of 19 recipients had no donor PHSC, giving frequencies of 2.89 and 2.25 donor PHSC per recipient of newborn blood and adult BM, respectively; however, limiting dilution techniques become less accurate when such small percentages are negative. From average donor percentages in recipients repopulated by both donor and standard PHSC, frequencies were 1.57 and 1.71 if donor and standard PHSC contributed equally, and these values were used in the model. The data were ordered from lowest to highest donor percentages, and observed values were subtracted from modeled values. For newborn blood and adult BM, respectively, sums of absolute values for all differences were minimal, 162 and 191, when donor and standard PHSC were modeled as contributing equally. Differences were only a little higher, 171 and 205, when modeled donor PHSC contributed 0.5 times as much as standard PHSC; differences were much higher, 342 and 355, when modeled donor PHSC contributed 2 times as much.
DISCUSSION
Nucleated cells from cord blood are now used for clinical reconstitution.1-3 They have the great advantage that they cause no stress for the donor. Although they can proliferate and produce high numbers of colony-forming cells in vitro,4-6 this does not mean that they will successfully produce large fractions of the differentiated cells in multiple lineages over long time periods in vivo. Long-term functional abilities of precursor cells from newborn blood were directly tested here for the first time by using mouse models. Myeloid and lymphoid cell production by the tested precursor cells was followed during about half of the lifespan. Precursor cells in blood from late fetal and newborn mice showed no lack of long-term function; in fact, they tended to produce increasing proportions of lymphocytes and erythrocytes when followed for up to 306 and 413 days compared with adult BM standards (Tables 1 and 3; Figs 1 and 2). Precursor cells in adult blood had 0.01 to 0.03 RU per 106 nucleated cells. This was less than 1% of the functional ability shown by late fetal and newborn blood cells, which had 2.8 ± 0.6 (n = 6) and 7.2 ± 3.0 (n = 6) RU/106 nucleated cells, respectively, giving mean ± SD for results averaged over 118 to 413 days (Table 3).
Nucleated cells in blood from late fetal and newborn mice appear to contain true PHSC, with strong correlations between lymphocytes and erythrocytes indicating that the same precursors produce these cells proportionally, as is observed with adult BM (Table 5). High correlations over time between 118 and 413 days suggest that the same precursors function for at least half the murine lifespan (Table 5). Thus, precursors in late fetal and newborn blood repopulate multiple lineages for long time periods, as do BM PHSC.
The data here support the clinical use of cord blood grafts, despite the fact that cord blood mouse model cells contain far less total repopulating units than do adult BM cells or fetal liver cells in mice. After 4 months or more, cord blood model cells average 4.0 RU and 4.4 RU in Tables 2 and 4, respectively. Thus, the contents of a single sample should provide sufficient repopulating ability to fully maintain a recipient, because recipients are repopulated reliably and maintain health for about 12 months (half of a mouse life span) if they receive 2 to 4 RU.17 23 Of course, definitive evaluations of clinical cord blood grafts require long-term studies in human beings.
Because others have reported nonoverlapping differences between functional abilities of adult and fetal stem cells, repopulating abilities per PHSC were estimated using PHSC concentrations from the binomial model. These ranged from 1 to 2.2, similar to values usually expected in BM and about half the value found in control BM in this study (Table 5). The hypothesis that individual PHSC from fetal and newborn blood have equal repopulating ability to those in the adult BM standard was more rigorously tested using the competitive dilution model. This directly compared the distribution of differentiated cells expected in groups of recipients if relative repopulating abilities in donor cells were 0.5, 1, or 2 times those in standard cells. Actual data and modeled data were most similar in each case for the model in which donor and standard PHSC each produced similar large fractions of the differentiated cells in multiple lineages over the long term. Results were most clear-cut for fetal blood PHSC; for PHSC from both newborn blood and adult BM donors, the best fit models had donor PHSC repopulating between 0.5 and 1 times as well as standard PHSC. Numbers of donor PHSC were higher than optimal for the model in the latter cases; however, the similar results with newborn blood and adult BM suggest that individual PHSC from both types of donor contribute equally. This does not contradict evidence of functional heterogeneity for individual PHSC in other systems, but suggests that the populations of PHSC in mouse cord blood models have similar functional ranges as those in adult mouse BM, at least in B6 strain mice. Genetic differences in relative functional abilities of fetal and adult stem cells might be important and are vital to define in both mice and human beings.
The liver is the primary site of blood production during fetal life, but by birth it must perform adult functions. A recent report suggests that there is a precipitous decline in liver PHSC frequency in the 15 day fetus because hemopoiesis is shifting to other organs.28 We tested the hypothesis that the newborn liver is no longer a major PHSC source; this might be expected after beginning postnatal liver function. This hypothesis is not correct, because the concentration of long-term repopulating activity in the newborn liver is several times higher than that of adult BM (Tables 1 and 2) and increases relative to adult BM with time (Figs 1 and 2). When the total PHSC contents are estimated, the newborn liver is the dominant source of repopulating cells (Tables 2 and 4).
In conclusion, the late fetal and newborn mouse blood model predicts that the PHSC in human umbilical cord blood cells should have long-term multilineage repopulating abilities similar to PHSC found in the BM. In the mouse, concentrations of repopulating cells tend to be 2 to 4 times higher in adult BM than in late fetal or newborn blood.
ACKNOWLEDGMENT
The authors thank Avis Silva, Karen Davis, and Bee Stork for their excellent technical assistance.
Supported by National Institutes of Health Grants No. DK25678, HL46536, AG10838, and AG11643.
Address reprint requests to David E. Harrison, PhD, The Jackson Laboratory, 600 Main St, Bar Harbor, ME 04609-0800.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal