Abstract
Plasmin, the primary fibrinolytic enzyme, has a broad substrate spectrum and participates in other biological processes dependent upon proteolytic activity. Consequently, plasmin activity is tightly regulated by plasminogen activators and protease inhibitors. In this study, we examined whether regulation of plasminogen gene expression also might provide a new mechanism for controlling this system. We examined the effects of recombinant human interleukin-6 (rhIL-6), a pleiotropic cytokine, on plasminogen mRNA expression in primary murine hepatocytes and Hep3B human hepatoma cells. In primary hepatocytes, rhIL-6 and hydrocortisone separately increased plasminogen mRNA expression, but hydrocortisone did not markedly enhance the response to rhIL-6. Hep3B hepatoma cells exhibited more modest responses to rhIL-6. We used the polymerase chain reaction to amplify a 1,067-bp fragment of the human plasminogen promoter/5′ flanking region. This fragment was cloned upstream of a luciferase reporter gene. Hep3B cells transiently transfected with this construct provided ∼100-fold higher luciferase activity compared to cells transfected with control plasmids, and luciferase activity was increased ∼4.5-fold when these cells were treated with rhIL-6. Furthermore, mice injected with rhIL-6 exhibited increases in hepatic plasminogen mRNA. Circulating plasminogen levels were significantly higher in the mice injected with rhIL-6 compared to mice injected with saline. Mice injected with lipopolysaccharide (an inducer of IL-6 in vivo) also showed increased hepatic plasminogen mRNA. Thus, plasminogen gene expression can be modulated by rhIL-6, suggesting a new mechanism for regulating biological systems that use plasmin.
THE FIBRINOLYTIC system participates in a plethora of physiological processes because of the broad substrate spectrum of plasmin. Plasmin is the major enzyme responsible for dissolution of fibrin at both intravascular and extravascular sites.1-4 In addition, the proteolytic activity of plasmin is used in processes requiring cell migration including inflammation,5-7 metastasis,8-10 and in tissue remodeling8,10 including angiogenesis,11 neurite outgrowth,12 and wound healing.11 13
Plasmin activity is tightly regulated because of the multiple consequences of activation of this system. Key control mechanisms for regulating the activity of the fibrinolytic system are modulation of the synthesis of plasminogen activators, their inhibitors, the substrate/regulatory molecule, fibrinogen, and modulation of cell surface expression of receptors for urokinase and plasminogen. It is well established that expression of genes encoding fibrinogen,14,15 PAI-1,16-19 tissue plasminogen activator,17,18 urokinase,20,21 and the urokinase receptor22,23 are regulated by molecules induced during local inflammatory responses and/or during the systemic acute phase response to tissue injury, neoplastic growth, or infection. Early reports suggested that plasminogen also might be an acute phase reactant.24 More recent studies also support this concept. Plasminogen levels are strongly positively correlated with C-reactive protein and consequently with an acute phase response in patients with angina pectoris.25,26 Also, plasminogen levels are elevated following distance running,27,28 embolic occlusion of the renal vein,29 and other surgical trauma30 and in smokers.31 However, although motifs mediating the tissue specificity of the plasminogen gene recently have been identified,32 plasminogen gene regulation has not been addressed in detail in the literature.
Induction of the acute phase response causes release of the acute phase mediator, interleukin-6 (IL-6),14 which increases expression of the fibrinolytic substrate precursor, fibrinogen.33 The cDNA sequence of the human plasminogen gene has been elucidated34 and the promoter and 5′ flanking region contains six consensus IL-6 responsive elements.35 36 Although the presence of such consensus sequences does not necessarily imply that a given gene will respond to IL-6, the possibility exists that IL-6 might mediate a response of plasminogen during an acute phase reaction and/or a local inflammatory response, similar to the effects on other components of the fibrinolytic system.
In the present study, we provide evidence that plasminogen gene expression is increased by rhIL-6 as assessed by a radioimmunoassay for plasminogen and by Northern blotting. Moreover, a 1,067-bp fragment of the plasminogen promoter and 5′ flanking region, cloned upstream of a luciferase reporter gene, provided luciferase activity in Hep3B hepatoma cells. The luciferase activity was increased when these cells were cultured in the presence of recombinant human IL-6 (rhIL-6), consistent with transcriptional regulation of plasminogen gene expression. Finally, in in vivo experiments, we found that injection of mice with rhIL-6 resulted in increased circulating plasminogen and increased expression of hepatic plasminogen mRNA.
MATERIALS AND METHODS
Cells.Hep3B human hepatocarcinoma cells (passage 76 to 100) were kindly provided by Dr Barbara Knowles (Jackson Laboratories, Bar Harbor, ME). The Hep3B cells and primary murine hepatocytes (isolated as described below) were grown and maintained in Eagle's minimal essential media (EMEM; Biowhittaker, Walkersville, MD) supplemented with 2 mmol/L glutamine (Biowhittaker), 100 U/mL penicillin, and 100 μg/mL streptomycin (Biowhittaker) and 10% fetal calf serum (FCS; Gemini Bioproducts, Calabasas, CA). The breast carcinoma MCF-7 cells (American Type Culture Collection, Rockville, MD) were grown in RPMI media supplemented with 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin, and 10% FCS.
Primary hepatocytes were isolated using a modification of a published method.37 Briefly, C57BL/6J female mice (4 to 6 weeks old) were anesthetized with metofane (Pitman-Moore, Mundelein, IL). The hepatic vein was perfused with 20 mL of perfusion buffer (10 mmol/L HEPES, pH 7.4, 137 mmol/L NaCl, 4.69 mmol/L KCl, 1.17 mmol/L KH2PO4 , 0.65 mmol/L MgSO4 and 1.0 mmol/L EGTA) at 37°C followed by 10 mL of enzyme solution (100 mmol/L HEPES, pH 7.6, 66.7 mmol/L NaCl, 6.7 mmol/L KCl, 4.8 mmol/L CaCl2 , 0.1 mg/mL hyaluronidase type IV [Sigma, St Louis, MO], 0.5 mg/mL collagenase type I [Sigma]). The perfused liver was then excised and gently abraded into a cell suspension with 4 mL of phosphate-buffered saline (PBS) (9.5 mmol/L NaPO4 , pH 7.0) (Biowhittaker) containing 0.5% bovine serum albumin (BSA; Calbiochem, San Diego, CA) and centrifuged at 50g for 3 minutes at 22°C. The hepatocyte enriched pellet was resuspended in PBS containing 0.5% BSA and layered over a two step metrizamide (Sigma) gradient (2 mL of 15% metrizamide in 10 mmol/L HEPES, pH 7.6, 75 mmol/L KCl, 1.25 mmol/L CaCl2 , 0.25% BSA; layered over 2 mL of 30% metrizamide in the same buffer) and centrifuged at 1,500g for 40 minutes at 4°C. Hepatocytes, present at the 15% to 30% metrizamide interface, were resuspended in PBS containing 0.5% BSA and centrifuged at 50g for 3 minutes. The pellet was then resuspended in EMEM supplemented with 20% FCS. The hepatocytes were 90% viable as assessed by trypan blue exclusion. Based on cell morphology, the hepatocyte preparations were <3% contaminated with nonparenchymal cells. The hepatocytes were cultured in 150-mm Falcon tissue culture dishes (Fisher, Pittsburgh, PA) for 12 hours in EMEM plus 20% FCS, followed by 24 hours in EMEM plus 10% FCS, followed by 24 hours in EMEM plus 1% BSA before stimulation.
Isolation of RNA and Northern blot analysis.Total cellular RNA was isolated using the guanidine thiocyanate-cesium trifluoroacetate technique.38 For Northern blot analysis, 30 μg of total RNA were denatured at 65°C and electrophoresed through 1.2% agarose (BRL, Gaithersburg, MD) /formaldehyde gels.39 The gels were rinsed twice in RNAase free water, and once with 5 × SSC (0.075 mol/L sodium citrate, pH 7.0, 0.75 mol/L NaCl) and transferred overnight to Genescreen membranes (New England Nuclear, Boston, MA).
Hybridization was performed using either a 1.2-kb human plasminogen Pst I-Sph I cDNA fragment (nucleotides 420-1640)40 (provided by Dr Earl Davie, University of Washington, Seattle), a 1.05-kb murine plasminogen EcoR1-PvuII fragment (nucleotides 1-1048)41 (provided by Dr Sandra Degen, Children's Hospital Research Foundation, Cincinnati, OH), a 1.9-kb human fibrinogen β chain EcoRI-Pst I cDNA fragment (nucleotides 1-1,900) (ATCC), or a 381-bp cyclophilin EcoRI-BamHI cDNA fragment,42 as probe. Probes were labeled with [α-32P] dCTP (3,000 Ci/mmol, Amersham, Arlington, Heights, IL) by the random primer method (using a random primer kit from Stratagene [La Jolla, CA]) to specific activities of >108 cpm/μg. The filters were prehybridized in 50% formamide, 5 × SSPE (0.75 mol/L NaCl, 0.05 mol/L NaH2PO4 , 5.0 mmol/L EDTA, pH 7.4), 5 × Denhardt's solution (0.1% Ficoll [Pharmacia, Uppsala, Sweden], 0.1% polyvinylpyrrolidone [Pharmacia], 0.1%BSA, 1% sodium dodecyl sulfate [SDS]) containing 200 μg/mL salmon sperm DNA (Sigma) for 1 to 8 hours at 42°C. The filters were hybridized at 42°C for 12 to 16 hours in the same buffer containing 25 ng of radioactive probe. The filters were rinsed once for 15 minutes in 2 × SSC at 22°C, once for 15 minutes in 2 × SSC at 42°C, and twice for 15 minutes in 2 × SSC/1.0% SDS at 42°C. In some experiments, a more stringent wash of 0.1 × SSC/0.1% SDS at either 42°C or 60°C for an additional 15 minutes to 1 hour was performed. Filters were exposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for 24 to 72 hours at −80°C using an image intensifying screen. All Northern blots were quantitated by scanning densitometry (LKB Ultrascan XL; Pharmacia). The cyclophilin probe was used as a normalizing probe for a housekeeping (constitutively expressed) mRNA to correct for unequal loading and/or unequal transferring of RNA as in previous studies by others.43-48 Cyclophilin is a highly conserved, ubiquitous cytosolic protein that may be involved in Ca2+-mediated signal transduction49 and has been shown to be essentially unregulated.50
For in vivo studies, C57BL/6J male mice (4 to 6 weeks old) were injected intraperitoneally with either saline, LPS (Sigma), or rhIL-6 (Boehringer Mannheim, Indianapolis, IN) and killed at different time points after being anesthetized with metofane. The concentrations of LPS used for injection included the range used previously to stimulate vitronectin mRNA expression.51 The concentration of rhIL-6 used for injection included the range used previously to stimulate fibrinogen expression.52 Plasma was collected and stored at −80°C and livers were excised and homogenized using a dounce homogenizer. Total RNA from murine liver was isolated by the single-step guanidinium isothiocyanate-phenol-chloroform extraction method.53
Polymerase chain reaction (PCR) amplification and reporter gene constructs.Human genomic DNA was isolated from peripheral blood leukocytes as described54 and used as a template for amplification of the human plasminogen promoter and 5′ flanking region by PCR using previously published plasminogen specific oligonucleotides (−914 to −889, 5′GATCGAATTCCGCAGACATTCCACC3′ and +154 to +124, 5′GGCCAGCAGTGCCCAGAAAGTGGGTCCC3′).34 For amplification, 1 μg of genomic DNA was added to 10 mmol/L TrisHCl, pH 8.4, 50 mmol/L KCl, 2.5 mmol/L MgCl2 , the two oligonucleotide primers, each at 10 μmol/L, dNTP's (Promega, Madison, WI) at 200 μmol/L, and 2.5 U of Taq DNA polymerase (Promega) in a volume of 100 μL. Samples were subjected to 30 cycles consisting of a denaturation step at 94°C for 2 minutes, a 2 minute annealing step at 55°C, and a 2-minute extension step at 72°C. The reaction products were electrophoresed through 1.5% agarose and a single 1,067-bp band was detected. The 1,067-bp fragment was gel purified and cloned into the pPCRII plasmid (Invitrogen, San Diego, CA) using the 3′-T-overhang in the vector, in both orientations. The orientation of these constructs was verified by restriction digestion with HindIII. Double restriction enzyme digests of these constructs with Kpn I and Xho I were performed. The DNA fragments were gel purified and cloned into the Kpn I/Xho I sites of the promoterless pGL2 basic plasmid (Promega) upstream of the luciferase reporter gene in both the correct (pGL2/hPLPR) and reverse (pGL2/hPLPR′) orientations. The entire 1,067-bp fragment within the pGL2/hPLPR construct was sequenced on both strands using the dideoxy chain termination method.55
Transfections.Cells were transfected using lipofectamine reagent (BRL, Bethesda, MD) as described.56 Briefly, 40 μg of plasmid DNA in 2.5 mL serum free EMEM and 125 μL lipofectamine reagent in 2.5 mL serum free EMEM were mixed and added to the cells in 100 mm dishes at 90% confluency. The cells were grown for 12 hours in 5% CO2 at 37°C and rinsed with serum free media before stimulation. Cells were stimulated in either media containing 10% FCS or serum free media containing 0.1% BSA in the presence or absence of rhIL-6. Following stimulation, cells were lysed by three cycles of freeze-thawing and protein concentrations were determined as described.57 Cell extracts were assayed for luciferase activity as described58 using luciferase assay reagents (Promega). Units of luciferase activity are reported as the total number of counts per 10-second readout on a Monolight 2001 luminometer (Analytical Luminescence Laboratory, San Diego, CA) per 300 μg protein.
Radioimmunoassays.The radioimmunoassay for plasminogen was of the double antibody type, using either a rabbit polyclonal antihuman antiserum59 or a rabbit polyclonal antimouse antiserum (kindly provided by Dr Tor Ny, University of Umea, Umea, Sweden) and employing goat antirabbit Ig to achieve precipitation. The assay buffer was 0.04 mol/L borate buffer, pH 8.3, containing 0.024 mol/L NaCl, 19 kallikrein inhibitor U/mL trasylol (Miles, Inc, Kankakee, IL), 0.002 mol/L EDTA, 20 U/mL heparin, and 2% heat-inactivated and BaSO4 -precipitated normal rabbit serum. Glu-plasminogen was purified from either fresh human plasma or from mouse plasma as previously described60,61 and radiolabeled with 125I using the iodogen method.62 125I-Glu-plasminogen was used at a final concentration of 0.7 nmol/L, and all other aspects of the assays were the same as previously described.63
Statistics.Data are reported as mean ± standard error of the mean, unless otherwise indicated. Statistical significance was determined by unpaired Student's t-test.
Reagents.Restriction enzymes were from Stratagene. RNA molecular size standards were from BRL. The pGL2/SV40 plasmid was obtained from Promega. Hydrocortisone was from Sigma. rhIL-6 (specific activity >108 U/μg) was from Boehringer Mannheim. The LPS content of the rhIL-6 was <0.1 endotoxin U/μg of protein according to the limulus amoebocyte assay as determined by the manufacturer.
RESULTS
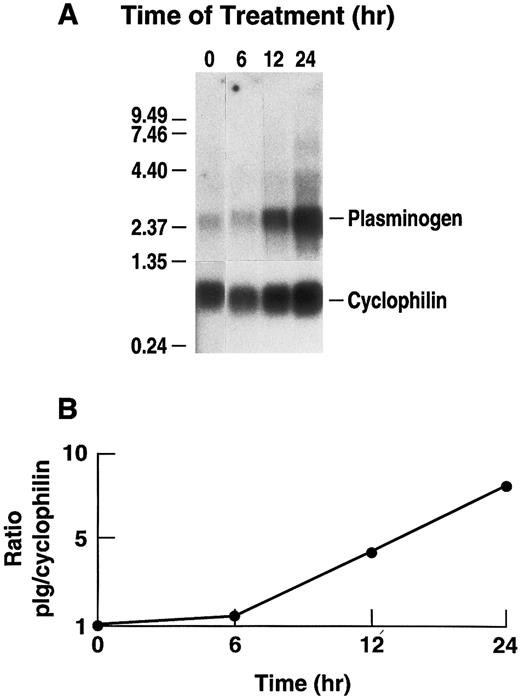
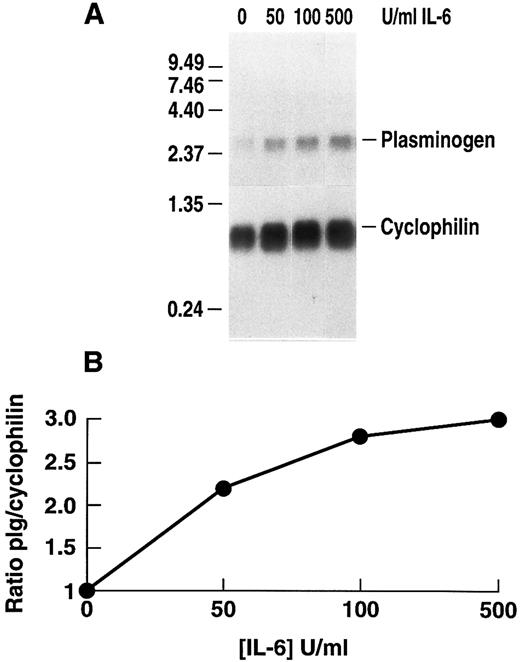
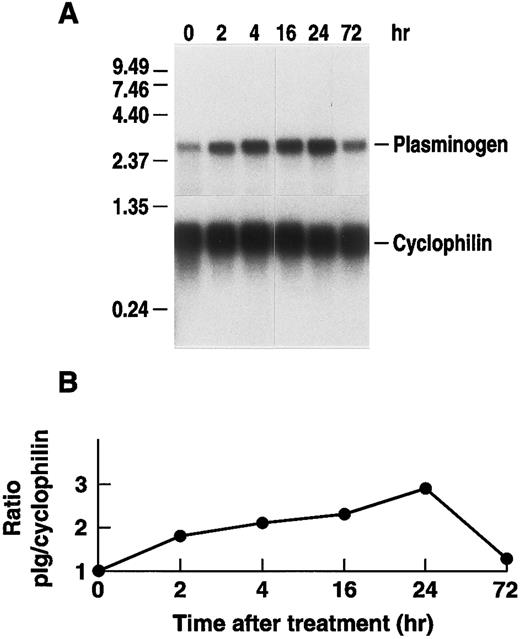
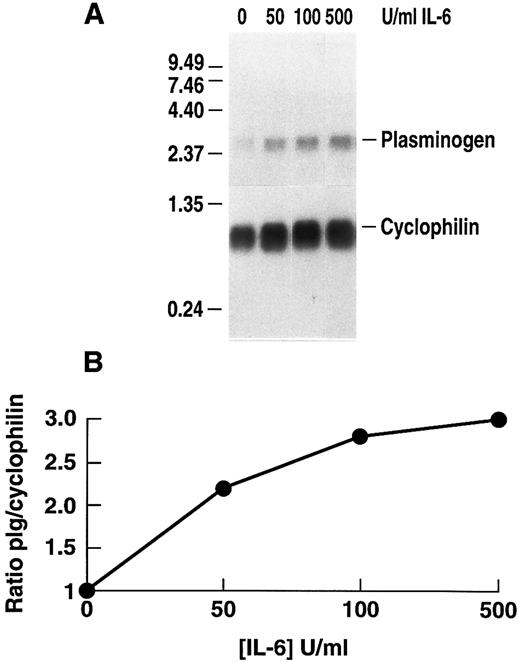
Stimulation of plasminogen mRNA expression in primary murine hepatocytes by rhIL-6.Induction of the acute phase response causes release of the major mediator, IL-6.14 We examined the effects of rhIL-6 on steady state levels of plasminogen mRNA in primary cultures of murine hepatocytes because plasminogen is synthesized predominantly in liver.64-66 Primary hepatocyte cultures were either untreated or incubated with 500 U/mL rhIL-6 for increasing times up to 24 hours. Total RNA was prepared and hybridized with a 32P-cDNA probe for murine plasminogen. The blots were subsequently hybridized with a 32P-cDNA probe for murine cyclophilin as a loading control. The Northern blots were scanned by laser densitometry and the ratios of band intensity for plasminogen mRNA and cyclophilin mRNA were determined at each time point. rhIL-6 increased steady state levels of plasminogen mRNA in the primary hepatocyte cultures in a time-dependent fashion, reaching an ∼eightfold level of stimulation at 24 hours (Fig 1). The dose-dependence of the response was also determined. The primary hepatocytes were cultured with increasing concentrations of rhIL-6 for 24 hours. Maximal stimulation was achieved between 100 and 500 U/mL rhIL-6 (Fig 2). This is similar to the concentration range at which maximal stimulation of antichymotrypsin and haptoglobin are achieved in in vitro hepatoma cell cultures (ie, 50 to 100 U/mL).67 The extent of stimulation observed in different primary hepatocyte preparations was variable, ranging from 3.0-fold to 8.3-fold. The mean increase in plasminogen mRNA levels from 3 different preparations of primary hepatocytes treated with 500 U/mL rhIL-6 for 24 hours was 5.0 ± 1.66. As a positive control, the blots were probed for fibrinogen β chain mRNA expression, which increased 2.3 ± 0.25-fold.
Time dependence of the effect of rhIL-6 on plasminogen mRNA expression in primary murine hepatocytes. (A) Primary murine hepatocytes were isolated as described in Materials and Methods. The cells were grown in serum free EMEM supplemented with 0.1% BSA for 24 hours, followed by incubation with 500 U/mL rhIL-6 for the indicated times in the serum free EMEM plus 0.1% BSA. Total RNA was isolated and Northern blot analysis was performed as described in Materials and Methods using 32P-cDNA probes for murine plasminogen and for murine cyclophilin. Migration of RNA molecular size standards is shown on the left side of the panel. (B) Fold-stimulation of plasminogen mRNA levels was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram in (A). The ratio was normalized to one for untreated cells.
Time dependence of the effect of rhIL-6 on plasminogen mRNA expression in primary murine hepatocytes. (A) Primary murine hepatocytes were isolated as described in Materials and Methods. The cells were grown in serum free EMEM supplemented with 0.1% BSA for 24 hours, followed by incubation with 500 U/mL rhIL-6 for the indicated times in the serum free EMEM plus 0.1% BSA. Total RNA was isolated and Northern blot analysis was performed as described in Materials and Methods using 32P-cDNA probes for murine plasminogen and for murine cyclophilin. Migration of RNA molecular size standards is shown on the left side of the panel. (B) Fold-stimulation of plasminogen mRNA levels was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram in (A). The ratio was normalized to one for untreated cells.
Dose dependence of the effect of rhIL-6 on plasminogen mRNA expression in primary murine hepatocytes. (A) Primary hepatocytes were grown in serum-free EMEM supplemented with 0.1% BSA for 24 hours, followed by incubation with increasing concentrations of rhIL-6 for 24 hours in serum free EMEM supplemented with 0.1% BSA. Total RNA was isolated and Northern blot analysis was performed as in Fig 1. Migration of RNA molecular size standards is shown on the left side of the panel. (B) Fold-stimulation of plasminogen mRNA levels was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram in (A). The ratio was normalized to one for untreated cells.
Dose dependence of the effect of rhIL-6 on plasminogen mRNA expression in primary murine hepatocytes. (A) Primary hepatocytes were grown in serum-free EMEM supplemented with 0.1% BSA for 24 hours, followed by incubation with increasing concentrations of rhIL-6 for 24 hours in serum free EMEM supplemented with 0.1% BSA. Total RNA was isolated and Northern blot analysis was performed as in Fig 1. Migration of RNA molecular size standards is shown on the left side of the panel. (B) Fold-stimulation of plasminogen mRNA levels was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram in (A). The ratio was normalized to one for untreated cells.
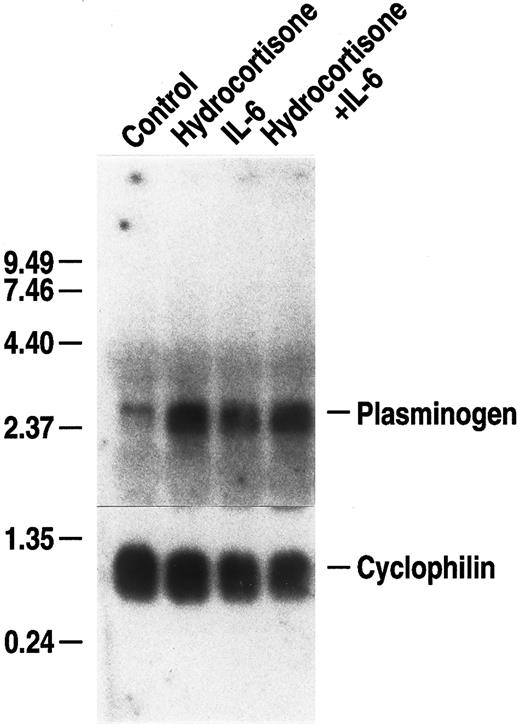
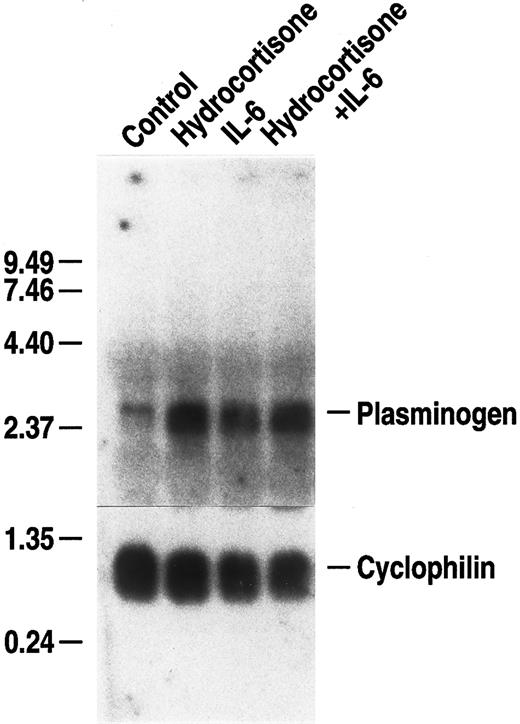
Glucocorticoids are mediators of the acute phase response14 and enhance the IL-6–dependent responses of some genes.68 Therefore, we examined whether hydrocortisone could augment the IL-6–dependent increase in plasminogen mRNA expression. Primary hepatocytes were cultured with either 500 U/mL rhIL-6, 10 μmol/L hydrocortisone, or a combination of these agonists, at the foregoing concentrations, for 24 hours. Hydrocortisone, alone, increased plasminogen mRNA expression to a similar extent as observed with rhIL-6 (3.9-fold and 3.7-fold, respectively) (Fig 3). However, augmentation of stimulation in the presence of both agonists was minimal (4.7-fold). In controls, fibrinogen β chain mRNA expression was increased by both rhIL-6 (3.9-fold) and hydrocortisone (2.7-fold) separately and 4.6-fold by the combination of both agonists (data not shown). Thus, hydrocortisone treatment, alone, increased plasminogen mRNA expression in the primary hepatocytes, but did not markedly augment the rhIL-6–dependent increase in either plasminogen or fibrinogen mRNA expression.
Effect of hydrocortisone on plasminogen mRNA expression in primary murine hepatocytes. Primary murine hepatocytes were grown in serum-free EMEM supplemented with 0.1% BSA for 24 hours, followed by culture for 24 hours in the presence of either 10 μmol/L hydrocortisone, 500 U/mL rhIL-6 or hydrocortisone + rhIL-6 at the foregoing concentrations in serum free EMEM containing 0.1% BSA. Total RNA was isolated and Northern blot analysis was performed as in Fig 1. Migration of RNA molecular size standards is shown on the left side of the panel.
Effect of hydrocortisone on plasminogen mRNA expression in primary murine hepatocytes. Primary murine hepatocytes were grown in serum-free EMEM supplemented with 0.1% BSA for 24 hours, followed by culture for 24 hours in the presence of either 10 μmol/L hydrocortisone, 500 U/mL rhIL-6 or hydrocortisone + rhIL-6 at the foregoing concentrations in serum free EMEM containing 0.1% BSA. Total RNA was isolated and Northern blot analysis was performed as in Fig 1. Migration of RNA molecular size standards is shown on the left side of the panel.
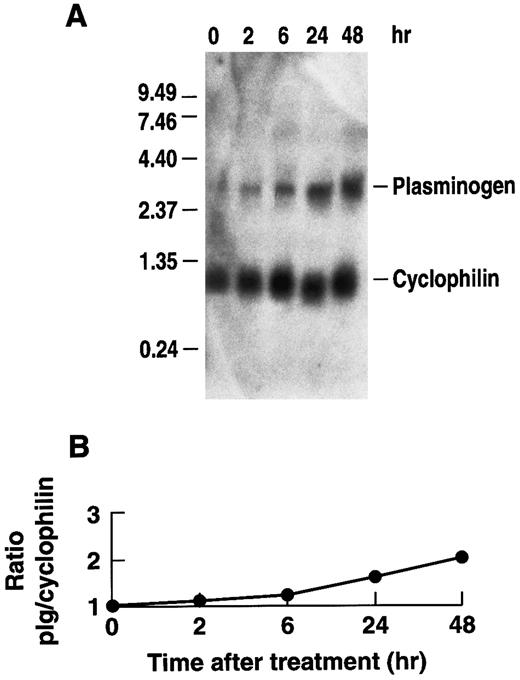
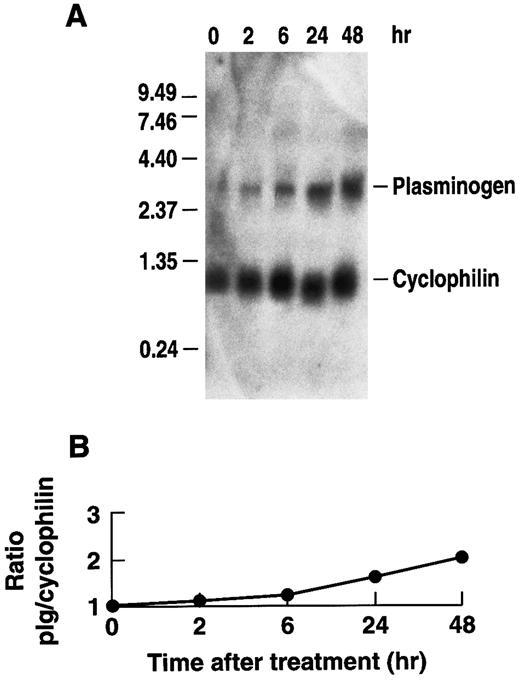
Effect of rhIL-6 on plasminogen mRNA expression in Hep3B hepatoma cells.To determine whether rhIL-6 could also affect plasminogen expression in human cells, the effect of rhIL-6 on plasminogen mRNA expression in Hep3B human hepatoma cells was investigated. The cells were cultured with 500 U/mL rhIL-6 for different times and total RNA was analyzed by Northern blotting with the 32P-cDNA for human plasminogen. A time-dependent increase in plasminogen mRNA levels was observed in Hep3B cells in response to rhIL-6 treatment (Fig 4). The ∼twofold increase was more modest than observed with the primary hepatocytes. Nonetheless, the increase in plasminogen mRNA expression in Hep3B cells was consistently observed in seven separate experiments and was statistically significant. The mean fold-stimulation for plasminogen was 2.1 ± 0.4 over basal ([n = 7] [P = .031, compared to basal] [t = −2.964]). When the same blots were probed for fibrinogen, the mean fold-stimulation was 3.0 ± 0.32 over basal ([n = 4] [P = .001 compared to basal] [t = −6.664]). Secretion of plasminogen in response to rhIL-6 was measured in conditioned media of Hep3B cells by radioimmunoassay. Since the rabbit antihuman plasminogen antiserum, used in the radioimmunoassay, cross-reacts with bovine but not rabbit plasminogen, cells were grown in the presence of normal rabbit serum instead of fetal bovine serum for this experiment. The plasminogen levels were normalized to total secreted protein as a control for any potential effects of IL-6 on protein synthesis in general. The total amount of secreted protein was unchanged in the presence of rhIL-6. When cells were stimulated every 24 hours with 500 U/mL rhIL-6 over a 10-day period, the conditioned media contained 141 ± 34 μg plasminogen/g secreted protein (n = 6), compared to 65 ± 17 μg plasminogen/g secreted protein in the untreated cells (n = 6) (P < .001).
Time dependence of the effect of rhIL-6 on plasminogen mRNA expression in Hep3B cells. (A) Hep3B cells were grown in serum-free EMEM supplemented with 0.1% BSA for 24 hours, followed by culture in the presence of 500 U/mL rhIL-6 in serum free EMEM containing 0.1% BSA for the indicated times and total RNA was prepared. The lane marked 0 represents mRNA from cells cultured in the absence of rhIL-6 for 48 hours. Northern blot analysis was performed using a 32P-cDNA human plasminogen fragment and the 32P-cDNA murine cyclophilin fragment. Migration of RNA molecular size standards is shown on the left. (B) Fold-stimulation of plasminogen mRNA level was determined as the ratio of plasminogen/cyclophilin hybridization band intensity as determined by laser densitometry. The ratio was normalized to one for untreated cells.
Time dependence of the effect of rhIL-6 on plasminogen mRNA expression in Hep3B cells. (A) Hep3B cells were grown in serum-free EMEM supplemented with 0.1% BSA for 24 hours, followed by culture in the presence of 500 U/mL rhIL-6 in serum free EMEM containing 0.1% BSA for the indicated times and total RNA was prepared. The lane marked 0 represents mRNA from cells cultured in the absence of rhIL-6 for 48 hours. Northern blot analysis was performed using a 32P-cDNA human plasminogen fragment and the 32P-cDNA murine cyclophilin fragment. Migration of RNA molecular size standards is shown on the left. (B) Fold-stimulation of plasminogen mRNA level was determined as the ratio of plasminogen/cyclophilin hybridization band intensity as determined by laser densitometry. The ratio was normalized to one for untreated cells.
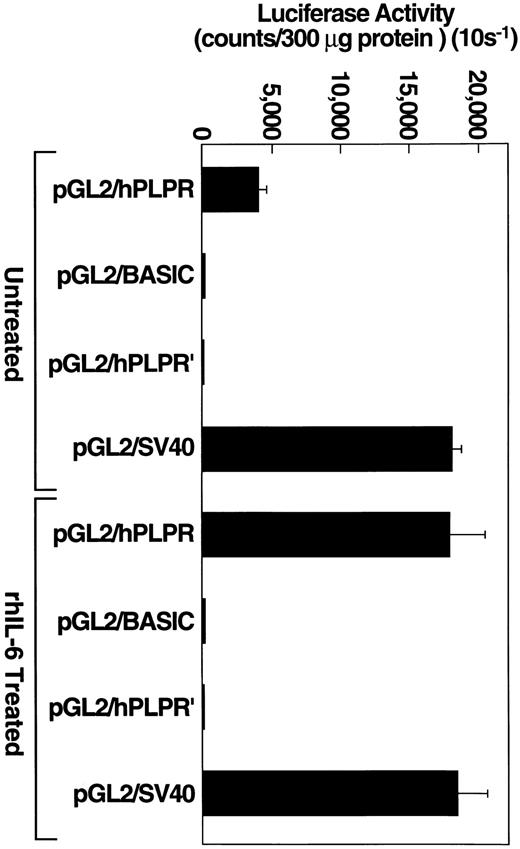
Transcriptional regulation of plasminogen gene expression.To determine whether the plasminogen promoter and 5′ flanking region could respond to rhIL-6, we amplified a 1,067-bp fragment (nucleotides −914 to +154, relative to the transcription initiation site35 ) of the human plasminogen gene using PCR as described previously.34 The fragment was cloned upstream of the pGL2 firefly luciferase reporter plasmid yielding the pGL2/hPLPR construct. Sequencing of the plasminogen promoter and 5′ flanking region contained within the pGL2/hPLPR construct revealed seven nucleotide substitutions compared to the published DNA sequence for plasminogen.34 Nucleotide substitutions were identified at −468 (A→G), −412 (C→T), −388 (A→G), −244 (A→G), −220 (C→A), −17 (T→G), and +10 (C→T) relative to the transcription initiation site (+1) for human plasminogen.35 To examine whether these differences could be attributed to PCR errors, we determined whether the same substitutions were present in the product of a second PCR reaction, performed with the identical primers and conditions used to obtain the pGL2/hPLPR product. All of the seven substitutions were present, also, in this second PCR product, suggesting that the differences were consistent with polymorphisms present in the genomic DNA of our donor and not to the fidelity of the Taq polymerase during amplification.
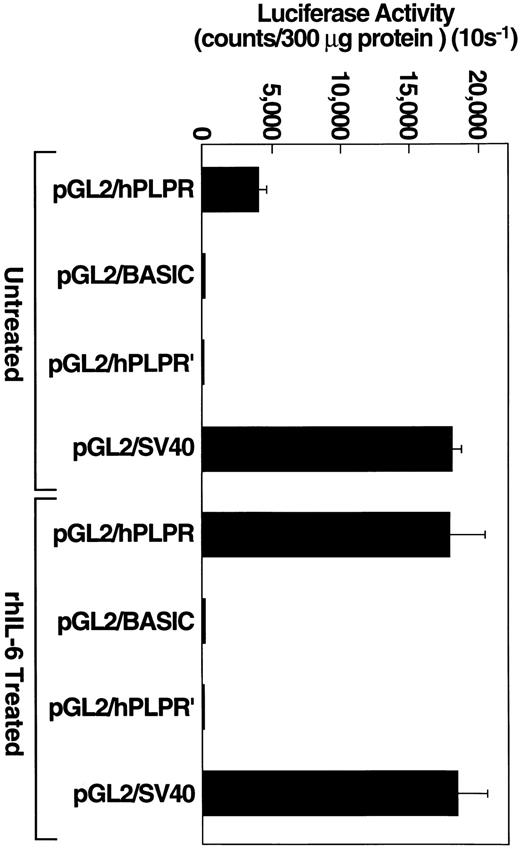
To determine whether this construct could drive luciferase activity, Hep3B cells were transiently transfected, separately, with one of three constructs: the pGL2/hPLPR construct, under the control of the plasminogen promoter, the pGL2/hPLPR′ construct, which is identical to pGL2/hPLPR except that the 1,067-bp plasminogen 5′ flanking region is cloned in the reverse orientation, or the pGL2 basic plasmid, which lacks an eukaryotic promoter or enhancer sequence. Transfection efficiency was monitored with the positive control pGL2/SV40 plasmid, which contains an SV40 promoter/enhancer and expresses high levels of the luciferase reporter protein. Hep3B cells transiently transfected with the pGL2/hPLPR construct expressed luciferase activity which was approximately 100-fold higher than that provided by the pGL2/hPLPR′ control construct (Fig 5). As a control to demonstrate that a cell that does not express plasminogen could not drive the plasminogen promoter, we transiently transfected cells of the MCF-7 breast carcinoma line with the pGL2/hPLPR construct. The MCF-7 cells, which did not express detectable plasminogen mRNA by Northern analysis (data not shown), provided background luciferase activity when transfected with the pGL2/hPLPR construct (40 counts per 10 s/300 μg protein), similar to MCF-7 cells transfected with the pGL2/hPLPR′ or pGL2 basic plasmid (60 counts per 10 s/300 μg protein). MCF-7 cells transfected with a pGL2/RSV promoter construct, as a control for the transfection method, provided 570-fold higher luciferase activity than cells transfected with either the pGL2/hPLPR′ or the pGL2 basic constructs. Therefore, the pGL2/hPLPR construct exhibited the cell specificity expected for the plasminogen promoter region.
rhIL-6 stimulates pGL2/PLPR promoter activity in Hep3B cells. Hep3B cells, grown to approximately 90% confluence in 100-mm dishes, were transiently transfected with the indicated plasmid constructs using 40 μg of plasmid DNA and 125 μL lipofectamine reagent. Cells were grown in EMEM containing 10% FCS and cultured in the presence of either media alone or 500 U/ml rhIL-6 for 24 hours in EMEM containing 10% FCS. Protein concentrations of cell lysates were determined and an appropriate volume of lysate was added to 100 μL of luciferase assay reagent (Promega) so that 300 μg of protein were assayed for each experimental condition. Luciferase activity was measured and expressed as counts/300 μg (10 s−1) protein. Error bars represent standard deviation.
rhIL-6 stimulates pGL2/PLPR promoter activity in Hep3B cells. Hep3B cells, grown to approximately 90% confluence in 100-mm dishes, were transiently transfected with the indicated plasmid constructs using 40 μg of plasmid DNA and 125 μL lipofectamine reagent. Cells were grown in EMEM containing 10% FCS and cultured in the presence of either media alone or 500 U/ml rhIL-6 for 24 hours in EMEM containing 10% FCS. Protein concentrations of cell lysates were determined and an appropriate volume of lysate was added to 100 μL of luciferase assay reagent (Promega) so that 300 μg of protein were assayed for each experimental condition. Luciferase activity was measured and expressed as counts/300 μg (10 s−1) protein. Error bars represent standard deviation.
To test whether cells transfected with pGL2/hPLPR could respond to rhIL-6, Hep3B cells were transiently transfected with either pGL2/hPLPR, pGL2 basic, pGL2/hPLPR′ or pGL2/SV40 and grown in the presence of rhIL-6 (500 U/mL) for 24. The cells transfected with pGL2/hPLPR provided a 4.5-fold stimulation of luciferase activity compared to untreated cells (Fig 5). The cells transfected with either pGL2 basic, pGL2/hPLPR′ or pGL2/SV40 did not exhibit enhanced luciferase activity compared to cells grown in the absence of rhIL-6 (Fig 5), demonstrating the specificity of the responsiveness of the pGL2/hPLPR construct to rhIL-6. Thus, the response of the Hep3B cells transfected with pGL2/hPLPR to rhIL-6 is consistent with transcriptional regulation of plasminogen gene expression by rhIL-6.
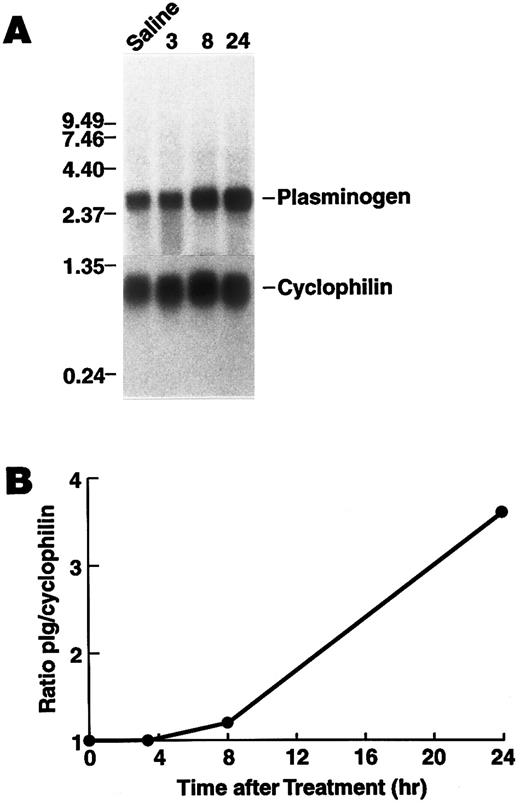
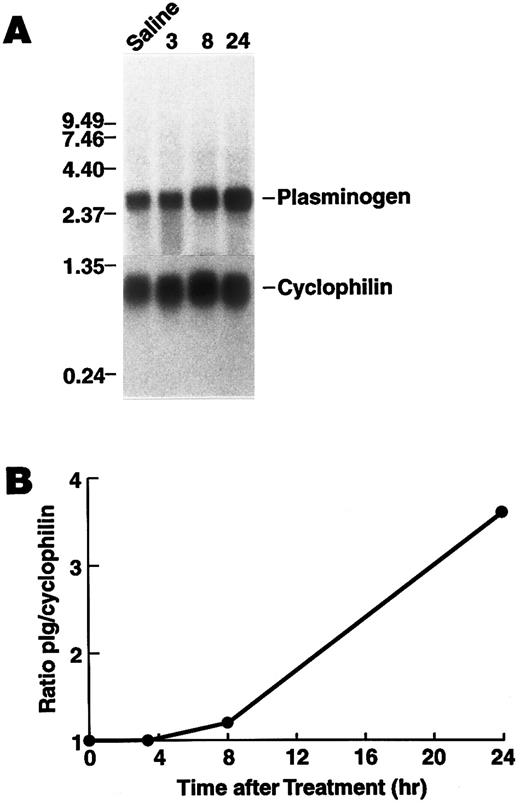
Regulation of plasminogen mRNA expression in vivo.To examine the effect of rhIL-6 on hepatic plasminogen mRNA expression in vivo we determined whether steady state levels of hepatic plasminogen mRNA were altered in C57BL/6J mice, following an intraperitoneal injection of 4,000 U rhIL-6. (This dose [∼133 U/g] is similar to the dose [200 U/g], which has been shown to increase hepatic mRNA levels for α2 -macroglobulin, fibrinogen, cysteine proteinase inhibitor, and α1 -acid glycoprotein when injected into rats52 ). Northern blot analysis showed that plasminogen mRNA expression increased in a time-dependent manner, achieving a 3.6-fold stimulation (range between experiments, 1.8- to 3.6-fold) at 24 hours compared with saline injected control animals (Fig 6). As a positive control fibrinogen β chain mRNA increased 2.8-fold at this time point. Furthermore, the concentration of plasminogen in plasma was higher in mice injected with 20,000 U rhIL-6 (2.28 ± 0.40 μmol/L, [n = 6]) compared to saline injected control animals (1.65 ± 0.27 μmol/L [n = 6]P = .009).
Time dependence of the effect of rhIL-6 injection on hepatic plasminogen mRNA expression in mice. (A) C57BL/6J male mice were injected intraperitoneally with either saline (T = 0) or 4,000 U rhIL-6 and killed at the indicated times. Livers were procured and total RNA was extracted and subjected to Northern blot analysis using the 32P-cDNA probes for murine plasminogen and for murine cyclophilin. The time course points were determined at least twice. Each lane represents RNA isolated from an individual representative animal. Migration of RNA molecular size standards is shown on the left. (B) Fold-stimulation was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram in (A). The ratio was normalized to one for the untreated condition.
Time dependence of the effect of rhIL-6 injection on hepatic plasminogen mRNA expression in mice. (A) C57BL/6J male mice were injected intraperitoneally with either saline (T = 0) or 4,000 U rhIL-6 and killed at the indicated times. Livers were procured and total RNA was extracted and subjected to Northern blot analysis using the 32P-cDNA probes for murine plasminogen and for murine cyclophilin. The time course points were determined at least twice. Each lane represents RNA isolated from an individual representative animal. Migration of RNA molecular size standards is shown on the left. (B) Fold-stimulation was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram in (A). The ratio was normalized to one for the untreated condition.
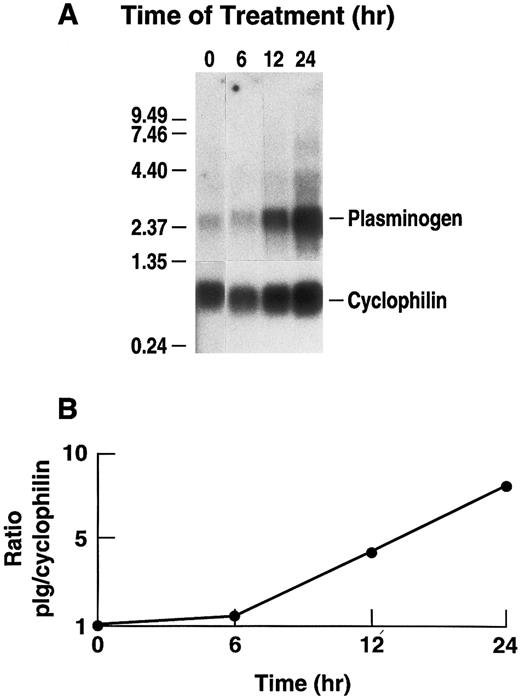
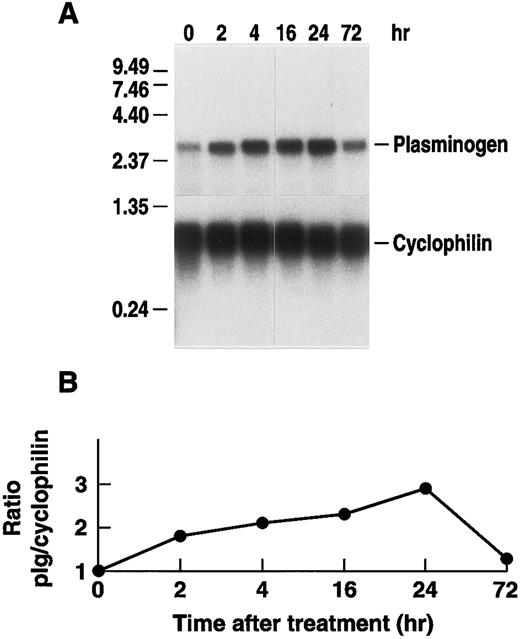
Several cytokines, including IL-6, increase substantially in the plasma of mice following intraperitoneal injections of LPS.69 Therefore, we determined whether changes in steady state levels of hepatic plasminogen mRNA could be elicited in a time and dose-dependent manner in C57BL/6J mice receiving an intraperitoneal injection of LPS. Northern blot analysis, revealed that plasminogen mRNA production increased in a time-dependent manner following intraperitoneal injection of 50 μg LPS, achieving a maximal 2.9-fold increase (range between experiments, 2.1- to 2.9-fold) at 24 hours and returning to base line by 72 hours (Fig 7). As a control for another responsive gene, these samples showed a fourfold increase in steady state levels of vitronectin mRNA.51 Similarly, Northern blot analysis revealed that plasminogen mRNA production increased in a dose-dependent manner. A 50% increase in plasminogen mRNA was detected when mice were injected with 10 μg LPS and we observed a 2.5-fold increase in plasminogen mRNA production after 24 hours when mice were injected with the maximum dosage of 100 μg LPS (data now shown). Under this condition, vitronectin mRNA expression was increased fourfold.
Time dependence of the effect of LPS injection on hepatic plasminogen mRNA expression in mice. (A) Mice were injected intraperitoneally with either saline or 50 μg LPS and killed at the indicated times. Livers were harvested and total RNA was extracted and subjected to Northern blot analysis using the 32P-cDNA probes for murine plasminogen and for murine cyclophilin. The time course points were determined at least twice. Each lane represents RNA isolated from an individual representative animal. Migration of RNA molecular size standards is shown on the left. (B) Fold-stimulation was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram. The ratio was normalized to one for the untreated condition.
Time dependence of the effect of LPS injection on hepatic plasminogen mRNA expression in mice. (A) Mice were injected intraperitoneally with either saline or 50 μg LPS and killed at the indicated times. Livers were harvested and total RNA was extracted and subjected to Northern blot analysis using the 32P-cDNA probes for murine plasminogen and for murine cyclophilin. The time course points were determined at least twice. Each lane represents RNA isolated from an individual representative animal. Migration of RNA molecular size standards is shown on the left. (B) Fold-stimulation was determined as the ratio of plasminogen/cyclophilin hybridization band intensity determined by laser densitometric scanning of the autoradiogram. The ratio was normalized to one for the untreated condition.
DISCUSSION
In this study, we have shown plasminogen gene regulation, as assessed by Northern blotting and by transfection studies with the plasminogen promoter/5′ flanking region cloned upstream of the luciferase reporter gene. We examined the regulation of plasminogen antigen and of steady state levels of plasminogen mRNA by a mediator induced during the acute phase response, IL-6. Increases in plasminogen gene expression, assessed by radioimmunoassay and Northern blotting, were observed in primary murine hepatocytes and in the human hepatoma cell line, Hep3B, when cultured with rhIL-6. Moreover, a 1,067-bp fragment of the 5′ flanking region of the plasminogen gene cloned upstream of the luciferase reporter provided luciferase activity when transfected into Hep3B cells. The luciferase activity was increased when these cells were cultured with rhIL-6, consistent with transcriptional regulation of plasminogen gene expression by rhIL-6. Furthermore, mice injected with rhIL-6 exhibited significantly higher levels of hepatic plasminogen mRNA compared to control mice injected with saline.
The present study is consistent with an earlier report suggesting that plasminogen might be an acute phase reactant.24 More recent studies also support this concept. Plasminogen levels are strongly positively correlated with C-reactive protein and consequently with an acute phase response in patients with angina pectoris.25,26 Also, plasminogen levels are elevated following distance running,27,28 embolic occlusion of the renal vein,29 and other surgical trauma30 and in smokers.31 Plasminogen levels also appear to be regulated in other physiological and pathophysiological states. For example, plasminogen is decreased by ingestion of a high–complex carbohydrate low-fat diet70 or Gemfibrozil (a lipid lowering fibric acid derivative) treatment71 and plasminogen activity and antigen are increased in patients with systemic lupus erythematosus.72
In our study, plasminogen gene expression in vitro was increased in response to two mediators of the systemic acute phase response, IL-652,68 and the corticosteroid analog, hydrocortisone.14,73 The primary hepatocytes were considerably more responsive than the Hep3B human hepatoma cell line. Further studies will be necessary to determine whether this reflects a difference between primary versus passaged cells or is due to a difference in responsiveness between species. Furthermore, mice injected with either rhIL-6 or LPS (an inducer of IL-669 ) exhibited increased plasminogen expression compared to saline injected mice. Because both IL-6 and LPS can induce the expression of other cytokines, these in vivo experiments cannot be unequivocally interpreted as a pure IL-6 effect. Nonetheless, they are consistent with the concept of IL-6–dependent regulation of plasminogen expression in vivo. The extent of the increase in plasminogen expression was similar to that exhibited by other known acute phase proteins including human complement C3, rat fibrinogen, rat haptoglobin, and rat α1 acid glycoprotein.14 74-76 Alterations in plasminogen levels could provide a mechanism to compensate for depletion of plasminogen in disseminated intravascular coagulation, which is frequently associated with the acute phase response.
The DNA sequence of the 5′ flanking region of the plasminogen gene contains consensus transcriptional regulatory elements whose presence is compatible with the results of this study. Two sequence elements of CTGGGA that are found in many acute phase reactant genes and may be involved in the transcriptional regulation of these acute phase proteins, are present in the plasminogen 5′ flanking region34,77,78 and six consensus sequences for responsive elements for IL-6 have been noted also.35,36 Also, a potential glucocorticoid responsive element is present between nucleotides −820 to −806 (GGAACAATGCTTTCT) in the plasminogen promoter/5′ flanking region. Twelve of the 15 bp are identical to the consensus sequence (AGAACAN3TGTTCT) characterized by Straehle et al.79
In this study we detected seven base pair substitutions consistent with polymorphisms present in the 5′ flanking region of the plasminogen gene. Only one of these differences (−17) was present within a putative IL-6 responsive element and the T→G substitution is still compatible with the consensus sequence for an IL-6 responsive element.80 Three of these substitutions are also present in members of the apo(a) gene family, a family of genes exhibiting extensive homology to plasminogen and plasminogen related genes. Specifically: the G at −244 is present in the sequence of apo(a) I36; the G at −17 is present in apo(a) II81 and the T at +10 is present in apo(a) I and II as well as the plasminogen related genes A and B.81 Interestingly, Suzuki et al82 identified other nucleotide substitutions in the promoter and 5′ flanking regions of the apo(a) gene, which appear to be involved in transcriptionally regulating expression of the apo(a) gene. Therefore, it is interesting to speculate that the promoter and 5′ flanking regions of the plasminogen genes characterized by Peterson et al34 and by us may exhibit different transcriptional activities.
Future studies are aimed at understanding the consequences of increased plasminogen expression in plasmin-mediated biological and pathological processes as diverse as fibrinolysis, tissue remodeling, cell migration, inflammation, and metastasis.
ACKNOWLEDGMENT
We thank Shari Olsen for excellent secretarial assistance, Patty Fowler for assistance in isolating primary hepatocytes, Dr Barbara Knowles for providing Hep3B cells, Dr Earl Davie for providing the human plasminogen cDNA, Dr Sandra Degen for providing the murine plasminogen cDNA, Dr Tor Ny for providing the rabbit antimurine plasminogen antisera and Dr James Zapf (The Scripps Research Institute) for helpful discussions.
Supported by the National Institutes of Health Grants No. HL-45934 and HL-38272 (L.A.M.), HL-50398 (R.J.P.), and HL-50704 (D.S.); California Tobacco-Related Disease Research Program (4KT-0192, D.S.) and the Department of Veterans Affairs. G.R.J. is the recipient of an Individual National Research Service Award HL09055. This work was done during the tenure of Established Investigatorship awards from the American Heart Association (R.J.P.), and from the American Heart Association and SmithKline Beecham (L.A.M.).
Address reprint requests to Lindsey A. Miles, PhD, Department of Vascular Biology (VB-1), The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037.