Abstract
The effect of short-term coincubations of Epstein-Barr virus (EBV) with mononuclear cells on the synthesis of leukotrienes (LT) by monocytes was investigated. Although treatment of mononuclear cells with EBV alone had no significant effect on LT synthesis by monocytes, the preincubation of mononuclear cells with EBV before the further stimulation of the cells with either the ionophore A23187, the chemoattractant formyl-Met-Leu-Phe, or the phagocytic particles zymosan strikingly enhanced the formation of both LTB4 and LTC4 above the levels of synthesis observed with the stimuli alone. Such priming effect of EBV on LT synthesis was maximal after 15 minutes of preincubation of mononuclear cells with EBV and slowly declined at longer preincubation times; the priming effect of EBV was observed both in Hank's Balanced Salt Solution and plasma. The effect of EBV was abolished by prior treatment of viral particles by heat or by antibody raised against the glycoprotein gp350 of the viral envelope, but not by UV irradiation of the viral particles. Exposure of mononuclear cells to EBV was shown to strongly enhance the activation of the 5-lipoxygenase and the release of arachidonic acid induced upon cell stimulation with a second agonist. The release of arachidonic acid by the EBV-treated mononuclear cells was inhibitable by arachidonyl trifluoromethyl ketone, an inhibitor of the 80-kD cytosolic phospholipase A2 . Furthermore, EBV was shown to rapidly increase (maximum effect within 15 minutes) the levels of phosphorylated form of the cytosolic phospholipase A2 (as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis), a process related to the activation of this enzyme. These data show that the interaction of EBV with monocytes upregulates the formation of important lipid mediators of inflammation.
THE INFLAMMATORY process is an important immune mechanism involved in the control of infectious agents and constitutes one of the first lines of defense against microbial invasion. In viral infections, the rapid onset of inflammation and the activation of effective cellular immune responses against the invading agent limit the spread of the virus during the first stages of infection.
Leukotrienes (LTs) constitute an important family of mediators of the immune response. LTs such as LTB4 and LTC4 are potent proinflammatory lipidic compounds produced by the dioxygenation of arachidonic acid via the 5-lipoxygenase pathway.1,2 Although the production of LTs was originally observed in polymorphonuclear leukocytes,1 it is accepted that monocytes are the main source of arachidonic acid metabolites among peripheral blood mononuclear cells.2,3 The immune functions of LTB4 are not fully understood; however, its potent chemotactic activity for phagocytes (ie, neutrophils, monocytes, and macrophages) constitutes its most documented biologic activity. Additional effects of LTB4 on immune functions, such as stimulation of suppressor T lymphocytes, induction of interferon γ (IFNγ) and interleukin-2 (IL-2) by T cells, and induction of IL-1 by monocytes, have been reported.4-7 Modulation of LTB4 synthesis may then affect the regulation of the immune response and impact on the spread of the virus.
There are previous observations of modulatory effects of virus or viral envelope proteins on the arachidonic acid cascade. Wahl et al8 reported that human mononuclear cells release cyclooxygenase products as well as LTB4 and LTC4 upon the addition of the envelope glycoprotein gp120 of human immunodeficiency virus-1 (HIV-1), an effect that involved signaling through the CD4 molecule, and may contribute to the regulatation of CD8+ T cells in HIV pathology. It was also suggested that LTB4 may be involved in the recruitment of neutrophils in different HIV-related lung diseases.9 Recently, Nokta et al10 reported that HIV-1–induced tumor necrosis factor α (TNFα) regulates arachidonic acid release and prostaglandin E2 synthesis in mononuclear phagocytes. In patients affected by pneumonia or bronchiolitis, the enhanced concentration of LTC4 in respiratory tract secretions was related to the presence of respiratory syncytial virus (RSV),11,12 and viral particle antibody complexes were found to strongly increase the synthesis of LTC4 in vitro.13 It was also shown that RSV, which binds to human normodense eosinophils, does not directly induce LTC4 synthesis in these cells but primes the cells for enhanced response to a subsequent challenge; these data suggest that the enhanced LTC4 synthesis in the respiratory tract may be involved in the development of persistent airway hyperreactivity after viral infections.14 In addition, in vitro studies have shown that Simian-virus-40 infection enhances LTA4 hydrolase expression in fibroblasts.15 Although additional studies are needed to further characterize the effect of viruses on the arachidonic acid cascade, it can be argued that the release of LTs at sites of primary viral infections may play some roles in the evolution of viral infections and the pathogenesis of viral diseases.
We have previously shown that Epstein-Barr virus (EBV) binds to human monocytes and exerts important modulatory effects on inflammatory cytokine synthesis.16-18 Indeed, upon EBV interaction with monocytes, we observed that IL-1 and IL-6 gene transcription was activated, whereas that of TNFα was inhibited. In the present study, we report that EBV primes monocytes for an increased synthesis of LTB4 and LTC4 after stimulation with a second agonist. These data provide new insights on the effects of EBV on the inflammatory response.
MATERIALS AND METHODS
Preparations of peripheral blood mononuclear cells (PBMCs).Venous blood was obtained from healthy donors using heparin as anticoagulant and PBMCs were prepared by dextran sedimentation of whole blood and centrifugation on Ficoll-Hypaque cushions (Pharmacia Biotech Inc, Baie d'Urfé, Québec, Canada) as previously described.19 The final cell suspensions (serum-free Hanks' Balanced Salt Solution [HBSS], 107 cells/mL) contained ∼10% monocytes and less than 1% neutrophils. Viability, estimated by the trypan blue-dye exclusion test, was greater than 99% in all preparations.
Preparation of enriched monocyte suspensions.For the preparation of enriched monocyte suspensions, large amounts of leukocytes were obtained by leukopheresis from normal volunteers, and PBMCs were prepared as described above. Monocytes were separated from PBMCs using the commercial magnetic cell sorting system MACS (Becton Dickinson, Mountain View, CA) as previously described.20 Briefly, undesired cells (ie, T and B lymphocytes) were labeled with superparamagnetic particles coated with anti-CD2, anti-CD3, or anti-CD19 monoclonal antibodies (MoAbs; Becton Dickinson) according to the manufacturer's instructions. Labeled cell suspensions were then passed through a steel wool column inserted into a magnet. The monocytes (unlabeled) were eluted, while the labeled cells (T and B cells) were retained in the column. The purity of the collected cell populations was evaluated by cytofluorometry using anti-CD14 MoAbs (Becton Dickinson). The final enriched monocyte suspensions (serum-free HBSS, 107 cells/mL) contained 75% to 85% monocytes and viability was greater than 99% as estimated by the trypan blue-dye exclusion test. In some experiments, enriched monocyte suspensions containing 70% to 85% monocytes were obtained by centrifugation of PBMCs over Percoll gradients, as previously described.21 The percentage of contaminating platelets, as evaluated by cytofluorometry, was less than 6% in all cell preparations.
Virus preparation.Preparations of EBV strain B95-8 were obtained as previously described.19,22 Briefly, B95-8 cells (mycoplasma-free tested) were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). When cell viability was 20% or less, cell culture supernatants were harvested and filtered through a 0.45-μm pore size filter (cellulose acetate; Corning, Oneonta, NY), and viral particles were purified by differential ultracentrifugation. Virus stocks were resuspended in RPMI-1640 and stored at −80°C until use. Viral titers were determined as previously described22 and adjusted to 107 transforming units/mL (TFU/mL). When indicated, virus inactivation was performed by heating (56°C for 60 minutes), by UV irradiation (265 nm for 60 minutes), or by using a neutralizing MoAb (72A1) raised against the gp350 glycoprotein of the viral envelope, as previously described.18
Incubation conditions.Cell suspensions were incubated in serum-free HBSS containing 10 mmol/L HEPES and 1.6 mmol/L CaCl2. Cells were preincubated with or without infectious EBV (107 TFU/mL) and stimulated with fMLP (1 μmol/L for 10 minutes), with zymosan (0.1 mg for 30 minutes), or with ionophore A23187 (75 nmol/L for 1 or 5 minutes) under conditions specified in the figure legends. In some experiments, preincubations with EBV were performed in homologous heparinized plasma. At the indicated times, reactions were stopped by the addition of 2 vol of a mixture of methanol/acetonitrile (50/50, vol/vol) containing 12.5 ng/mL each of prostaglandin B2 (PGB2 ) and 19-hydroxy-PGB2 as internal standards for high-performance liquid chromatography (HPLC) analysis; the denatured reaction mixtures were stored overnight at −20°C. Incubation mixtures containing plasma were denatured using 4 vol of the same mixture of organic solvents. Arachidonyl trifluoromethyl ketone (AACOCF3) was purchased from Calbiochem (San Diego, CA). fMLP, zymosan, and A23187 were obtained from Sigma Chemical Co (St Louis, MO). AACOCF3, A23187, and fMLP were used in solution in dimethylsulfoxide (DMSO); zymosan was boiled 30 minutes in distilled water, washed twice, and resuspended in 0.9% NaCl at 2 mg/mL.
Assay of 5-lipoxygenase activity in intact cells.The activation of 5-lipoxygenase was measured by the enzyme-catalyzed transformation of exogenous 15(S)-hydroperoxy-5,8,11,13(Z,Z,Z,E)-eicosatetraeonic acid (15-HpETE) into 5(S),15(S)-dihydroxy-6,8,11, 13-(E,Z,Z,E)-eicosatetraenoic acid (5,15-DiHETE) as previously described.23 Briefly, 3 μmol/L of 15-HpETE was added along with the agonist or its diluent (dimethylsulfoxide, 0.1% final concentration) to all suspensions and then incubated for 5 minutes. The incubation was stopped and the samples were processed and analyzed as described below for 5-lipoxygenase products. The reaction product, 5,15-DiHETE, was detected at 229 nm; the limit of detection was 1 ng.
Analysis of 5-lipoxygenase products.The denatured samples were centrifuged at 2,000g for 20 minutes to remove the precipitated material, the organic solvent content of samples was reduced to 50% by evaporation under a stream of nitrogen, and LTB4 , LTC4 , or 5,15-DiHETE was analyzed by reverse-phase (RP) HPLC using an on-line extraction procedure described previously24 and UV detection.
Analysis of arachidonic acid.Cell suspensions were incubated in serum-free HBSS in the presence or absence of infectious EBV for 20 minutes before stimulation with fMLP or ionophore A23187. The incubations were terminated by the addition of 2 vol of ice-cold methanol containing 10 ng of D8-arachidonic acid per milliliter as an internal standard. Samples were processed as described under incubation conditions and subjected to HPLC analysis, and the HPLC fractions containing arachidonic acid (determined by using 3H-arachidonic acid) were collected. The samples were evaporated under reduced pressure (using a Speed Vac model SVC 100D; Savant Instruments Inc, Farmingdale, NY) and dissolved in 100 μL of acetonitrile. Arachidonic acid was assayed by liquid chromatography-mass spectrometry (LC-MS) using a nebulizer-assisted electrospray (ion spray) interface coupled to a mass spectrometer (API-III; PE Sciex, Thornhill, Ontario, Canada), as described previously.25
Western blot analysis of cytosolic phospholipase A2.Enriched monocyte suspensions were treated or not with EBV for 30 minutes before ionophore A23187 or fMLP stimulation. The reaction was stopped on ice and samples were centrifuged at 500g for 8 minutes at 4°C. The supernatants were kept for 5-lipoxygenase product analysis and the pellets were resuspended in a sucrose buffer containing an antiprotease cocktail (1 mmol/L phenylmethyl sulfonyl fluoride, 10 mg/mL aprotinin and leupeptin) as previously described.26 To obtain subcellular fractions, cells were sonicated (3 times for 20 seconds at 60% Duty cycles, output control set at 3) using a Johns Scientific Inc Ultrasonic Processor (Biotech Scientifique, Montréal, Canada). Under these conditions, 90% to 95% of the cells were disrupted. The sonicates were first centrifuged at 12,000g for 10 minutes at 4°C (pellets referred to as the nucleus-containing fractions), and the resulting supernatants were further centrifuged (180,000g for 25 minutes at 4°C) in a Beckman Airfuge ultracentrifuge (Beckman Instruments Inc, Mississauga, Ontario, Canada). The 180,000g supernatants thus obtained (referred to as the cytosols) and the corresponding pellets (referred to as cellular membranes), as well as the 12,000g pellets, were immediately subjected to electrophoresis on a 5% to 15% gradient sodium dodecyl sulfate-polyacrylamide gel and immunoblotted as described previously.26 Briefly, proteins were transferred for 2.5 hours at 500 mA current setting onto an Immobilon-P polyvinylidene difluoride blotting membrane; transfer efficiency was visualized by Ponceau Red staining. The membranes were soaked for 30 minutes at 25°C in Tris-buffered saline (TBS; 25 mmol/L Tris-HCl, pH 7.60, 0.2 mol/L NaCl, 0.15% Tween 20) containing 5% dried milk (wt/vol) and subsequently exposed to the anti-cytosolic phospholipase A2 (cPLA2 ) antiserum (antiserum MF-142 [generously provided by Dr P. Weech, Merck-Frosst, Montreal, Canada] was used at a dilution of 1:500). The membrane was then washed twice with TBS and treated with a horseradish peroxidase-linked donkey antirabbit antibody. Bound antibodies were shown with the enhanced chemiluminescence (ECL) reagent (Dupont-NEN, Boston, MA) according to the manufacturer's instructions.
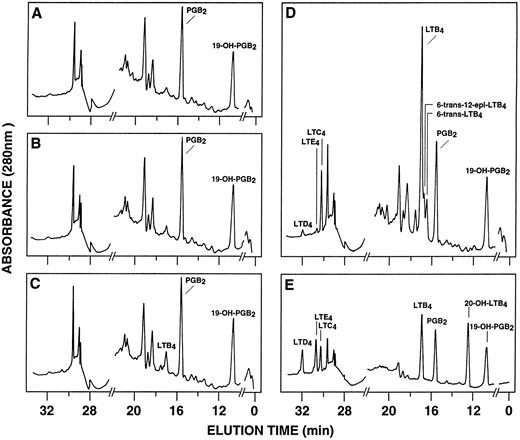
RP-HPLC chromatograms of 5-lipoxygenase products in enriched monocyte suspensions stimulated with EBV and A23187. Enriched monocyte suspensions (5 × 106 cells/0.1 mL) were preincubated in the absence or in the presence of EBV for 30 minutes at 37°C, diluted 10-fold with HBSS, and further incubated with 75 nmol/L A23187 or DMSO for 5 minutes. (A) Untreated cells; (B) cells stimulated with A23187 only; (C) cells stimulated with EBV only; (D) cells pretreated with EBV and stimulated with A23187; (E) synthetic leukotriene standards. Analyses were performed by RP-HPLC as described in the Materials and Methods. Results shown are from one experiment representative of four separate experiments. The amounts of PGB2 and 19-OH-PGB2 (internal standards) in chromatograms (A) through (D) are 12.5 ng.
RP-HPLC chromatograms of 5-lipoxygenase products in enriched monocyte suspensions stimulated with EBV and A23187. Enriched monocyte suspensions (5 × 106 cells/0.1 mL) were preincubated in the absence or in the presence of EBV for 30 minutes at 37°C, diluted 10-fold with HBSS, and further incubated with 75 nmol/L A23187 or DMSO for 5 minutes. (A) Untreated cells; (B) cells stimulated with A23187 only; (C) cells stimulated with EBV only; (D) cells pretreated with EBV and stimulated with A23187; (E) synthetic leukotriene standards. Analyses were performed by RP-HPLC as described in the Materials and Methods. Results shown are from one experiment representative of four separate experiments. The amounts of PGB2 and 19-OH-PGB2 (internal standards) in chromatograms (A) through (D) are 12.5 ng.
RESULTS
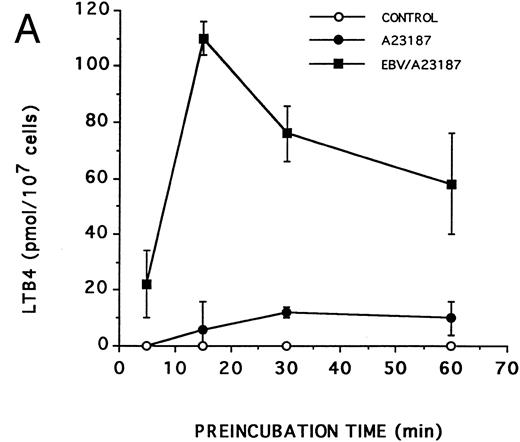
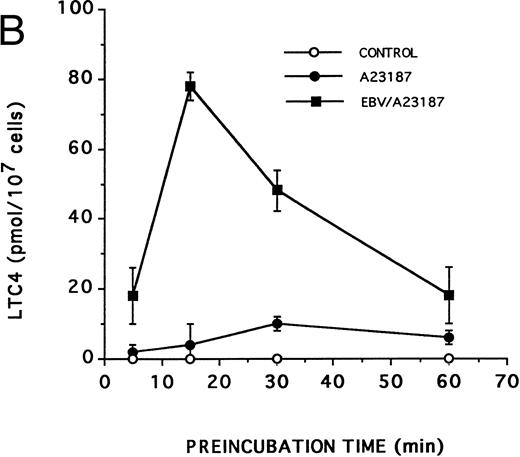
Effect of EBV on LT synthesis.Enriched monocyte suspensions were treated with EBV or A23187 or preexposed to EBV before stimulation with A23187, and LT synthesis was assessed by HPLC. Figure 1A and B show that unstimulated cells and cells stimulated with a low concentration of A23187 (75 nmol/L for 5 minutes) did not produce detectable amounts of LT. Stimulation of the cells with EBV alone resulted in the formation of small but detectable amounts of LTB4 (Fig 1C). In contrast, significant amounts of both LTB4 and LTC4 were formed in cell suspensions preincubated with EBV and stimulated with A23187; the HPLC tracing also shows the presence of small amounts of LTD4 , LTE4 , and the 6-trans-isomers of LTB4 (Fig 1D). The synthesis of 5-HETE was also consistently enhanced in EBV-pretreated cells stimulated with A23187 (not shown). Figure 1E shows the HPLC profile obtained upon analysis of synthetic LT standards. The concentration of A23187 used in these experiments (75 nmol/L) is a threshold concentration for induction of LT synthesis in the enriched monocyte suspensions (data not shown); such low concentration of A23187 was used throughout the present studies to avoid the potential problem that maximal activation by high concentrations of A23187 (0.5 to 2 μmol/L) might mask a stimulatory effect of EBV on LT synthesis. Table 1 shows that stimulation of PBMCs with 75 nmol/L A23187 results in a variable level of LT synthesis in different cell preparations. However, in all experiments, the pretreatment of the cell suspensions with EBV before A23187 (75 nmol/L) stimulation consistently resulted in a strongly enhanced formation of LT relatively to either stimulus alone. We also assessed the ability of EBV to prime for LT synthesis upon cell stimulation with the soluble agonist fMLP and zymosan. Results show that EBV pretreatment strikingly enhances LTB4 synthesis by PBMCs under all stimulatory conditions tested. The ability of EBV to potentiate the production of LT in cells stimulated with A23187 was further characterized in time course studies. Figure 2A and B show that the priming effect of EBV on LTB4 and LTC4 formation was already detectable after 5 minutes of preincubation of enriched monocyte suspensions with EBV. The effect of EBV was maximal after 15 minutes of preincubation, decreased at longer incubation times, but was still apparent at 60 minutes, both for the synthesis of LTB4 and LTC4. In the experiment shown, cell treatment with EBV alone did not result in the detectable formation of LT (data not shown). The time course of the priming effect of EBV was also investigated in plasma. Incubation procedures were as described in the legend to Fig 2, except that 107 PBMCs were preincubated with EBV in 0.1 mL plasma before 10-fold dilution with HBSS and stimulation with 1 μmol/L fMLP for 10 minutes. The kinetics of the priming effect of EBV on LTB4 synthesis in plasma were identical (data not shown) to those observed in HBSS (Fig 2A), with a maximum effect at 15 minutes decreasing at longer preincubation times with the viral particles. Amounts of 5-LO products generated upon fMLP stimulation of EBV-treated PBMC in HBSS (Table 1) and plasma (not shown) were similar.
Kinetics of the priming effect of EBV on the synthesis of LTB4 and LTC4 . Enriched monocyte suspensions (10 × 106 cells/0.1 mL) were preincubated or not with EBV for various periods of time at 37°C, diluted 10-fold with HBSS, and stimulated for 5 minutes with 75 nmol/L A23187. Levels of LTB4 (A) and LTC4 (B) in incubation media were measured by RP-HPLC as described in the Materials and Methods. Values are the mean ± SD of triplicate incubations from one experiment representative of five separate experiments.
Kinetics of the priming effect of EBV on the synthesis of LTB4 and LTC4 . Enriched monocyte suspensions (10 × 106 cells/0.1 mL) were preincubated or not with EBV for various periods of time at 37°C, diluted 10-fold with HBSS, and stimulated for 5 minutes with 75 nmol/L A23187. Levels of LTB4 (A) and LTC4 (B) in incubation media were measured by RP-HPLC as described in the Materials and Methods. Values are the mean ± SD of triplicate incubations from one experiment representative of five separate experiments.
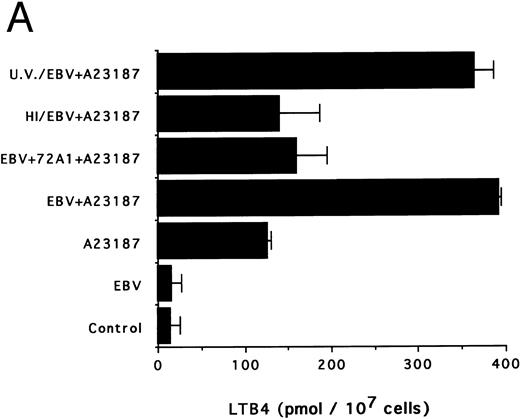
The specificity of the effect of EBV was next investigated. Figure 3A and B show that heat inactivation and pretreatment of viral particles with the neutralizing antibody 72A1 almost completely suppress the priming effect of EBV. Conversely, UV irradiation of EBV, a treatment that inactivates the virus without altering the native particle, had no inhibitory effect on the ability of EBV to prime the monocytes for LTB4 and LTC4 synthesis.
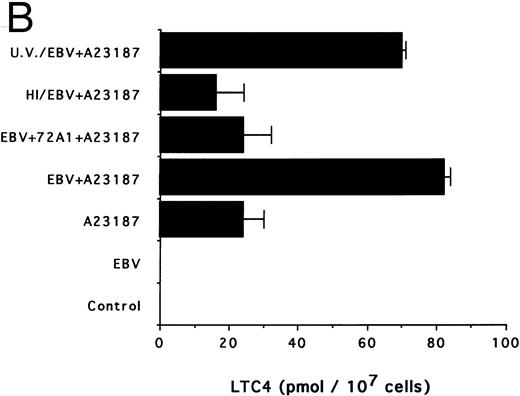
Effects of infectious and inactivated viruses on LTB4 and LTC4 synthesis. Enriched monocyte suspensions (5 × 106 cells/0.1 mL) were preincubated or not with infectious or inactivated viral particles for 30 minutes at 37°C and diluted 10-fold with HBSS before A23187 stimulation (75 nmol/L) for 5 minutes. Analyses of LTB4 (A) and LTC4 (B) contents of incubation media were performed as described in the Materials and Methods. Results are from one experiment (mean ± SD of triplicate incubations) and are representative of three separate experiments. UV/EBV, UV-irradiated EBV; HI/EBV, heat-inactivated EBV; EBV/72A1, EBV neutralized with the MoAb 72A1.
Effects of infectious and inactivated viruses on LTB4 and LTC4 synthesis. Enriched monocyte suspensions (5 × 106 cells/0.1 mL) were preincubated or not with infectious or inactivated viral particles for 30 minutes at 37°C and diluted 10-fold with HBSS before A23187 stimulation (75 nmol/L) for 5 minutes. Analyses of LTB4 (A) and LTC4 (B) contents of incubation media were performed as described in the Materials and Methods. Results are from one experiment (mean ± SD of triplicate incubations) and are representative of three separate experiments. UV/EBV, UV-irradiated EBV; HI/EBV, heat-inactivated EBV; EBV/72A1, EBV neutralized with the MoAb 72A1.
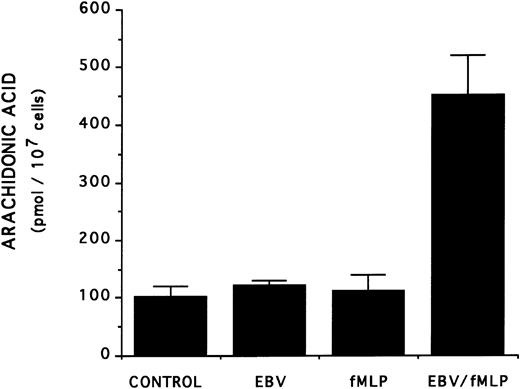
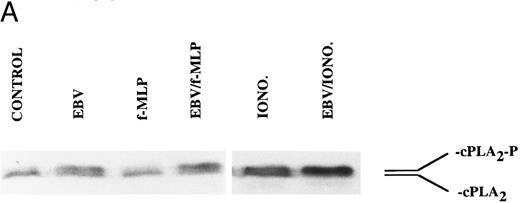
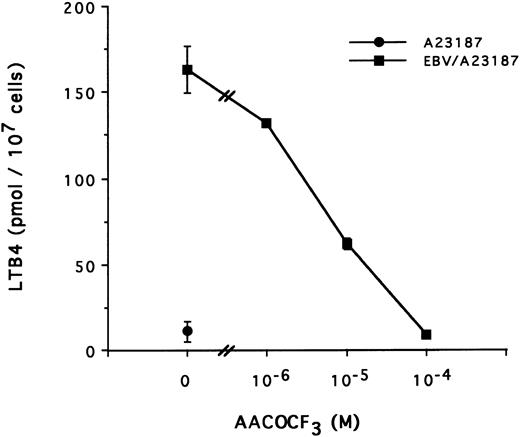
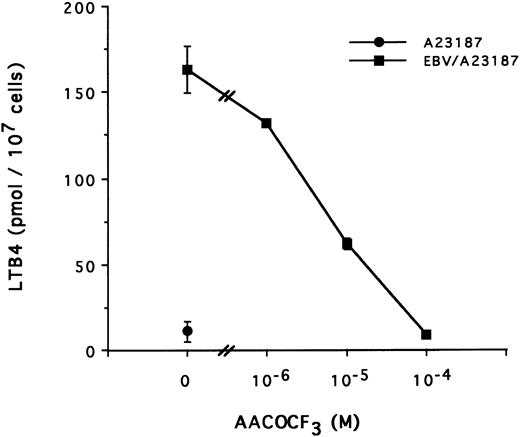
The mechanism of the priming effect of EBV on LT synthesis.To provide insights on the mechanism of the priming effect of EBV on LT synthesis, the effects of EBV on two cellular processes required for LT synthesis, namely the release of arachidonic acid and activation of the 5-lipoxygenase, were investigated. Figure 4 clearly shows that, whereas EBV alone had minimal effects on the release of arachidonic acid from enriched monocyte suspensions, preincubation of the cells with EBV resulted in the enhanced release of substrate for LT synthesis upon stimulation with fMLP. Similar data were obtained using PBMC suspensions and A23187 stimulation (data not shown). The effect of EBV on phosphorylation of the 80-kD cPLA2 , a process involved in the activation of the enzyme,27-30 was investigated. After incubations of enriched monocyte suspensions in various experimental conditions, proteins from the whole cells were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis using an antibody to cPLA2. In all experiments performed, treatment of enriched monocyte suspensions with EBV for a period of 30 minutes resulted in enhanced levels of phosphorylated cPLA2 (Fig 5A, upper band of the doublet)29 as assessed by SDS-PAGE and immunoblot analysis. EBV pretreatment also enhanced the levels of phosphorylated cPLA2 in cells stimulated with fMLP and A23187; increased phosphorylation was detectable after 5 minutes of incubation in the presence of EBV and remained elevated after 15 and 30 minutes (Fig 5B). In separate experiments, subcellular fractions were prepared after incubation of enriched monocyte suspensions with EBV and A23187 and sonication. SDS-PAGE and immunoblot analysis of cPLA2 in the cytosol, membrane fraction, and 10,000g pellet indicated that treatment of the cells with EBV did not alter the subcellular distribution of cPLA2 (data not shown), which was largely cytosolic (≥75%) in the unstimulated and fMLP-stimulated cells. Finally, to further support the involvement of the cPLA2 in the release of arachidonic acid by the EBV-treated mononuclear leukocytes, experiments were performed in the presence of the cPLA2 inhibitor AACOCF3.31 Figure 6 shows that AACOCF3 in the concentration range of 10−6 to 10−4 mol/L dose-dependently inhibited LT synthesis by A23187-stimulated PBMCs with an IC50 of ∼5 μmol/L, which is compatible with an inhibition of cPLA2 (IC50 of 9 and 8 μmol/L reported, respectively, for the inhibition of authentic cPLA232 and of arachidonic acid release from A23187-stimulated U937 cells33 ).
Effect of EBV on the release of arachidonic acid in fMLP-stimulated enriched monocyte suspensions. Enriched monocyte suspensions (2 × 106 cells/0.1 mL) obtained by centrifugation on a Percoll density gradient were incubated or not with EBV (for 20 minutes) at 37°C, diluted 10-fold with HBSS, and stimulated with fMLP (1 μmol/L for 10 minutes). Samples were subjected to RP-HPLC and fractions containing arachidonic acid were collected for quantitation by mass spectrometry as described in the Materials and Methods. Results (mean ± SD) are from one experiment representative of three separate experiments performed in triplicate.
Effect of EBV on the release of arachidonic acid in fMLP-stimulated enriched monocyte suspensions. Enriched monocyte suspensions (2 × 106 cells/0.1 mL) obtained by centrifugation on a Percoll density gradient were incubated or not with EBV (for 20 minutes) at 37°C, diluted 10-fold with HBSS, and stimulated with fMLP (1 μmol/L for 10 minutes). Samples were subjected to RP-HPLC and fractions containing arachidonic acid were collected for quantitation by mass spectrometry as described in the Materials and Methods. Results (mean ± SD) are from one experiment representative of three separate experiments performed in triplicate.
Immunoblot analysis of the effect of EBV on cPLA2 phosphorylation in enriched monocyte suspensions. Enriched monocyte suspensions (5 × 106 cells/0.1 mL) were preincubated or not in the presence of EBV for 30 minutes at 37°C, diluted 10-fold with HBSS, and subsequently stimulated with fMLP (1 μmol/L for 10 minutes) or A23187 (50 nmol/L for 5 minutes). Incubations were stopped on ice and whole cells were processed for SDS-PAGE analysis and immunoblotting of cPLA2 as described in the Materials and Methods. The upper and lower bands of the doublet represent the phosphorylated (cPLA2-P) and nonphosphorylated (cPLA2 ) forms of the cPLA2 , respectively. (A) The data obtained using fMLP and A23187 as second stimuli are derived from separate experiments. (B) Enriched monocyte suspensions (3 × 106 cells/0.1 mL) were preincubated or not in the presence of EBV for the indicated times at 37°C, diluted 10-fold with HBSS, stimulated with A23187 (50 nmol/L for 5 minutes), and processed for analysis of cPLA2 as described above. Experiments shown are representative of three performed.
Immunoblot analysis of the effect of EBV on cPLA2 phosphorylation in enriched monocyte suspensions. Enriched monocyte suspensions (5 × 106 cells/0.1 mL) were preincubated or not in the presence of EBV for 30 minutes at 37°C, diluted 10-fold with HBSS, and subsequently stimulated with fMLP (1 μmol/L for 10 minutes) or A23187 (50 nmol/L for 5 minutes). Incubations were stopped on ice and whole cells were processed for SDS-PAGE analysis and immunoblotting of cPLA2 as described in the Materials and Methods. The upper and lower bands of the doublet represent the phosphorylated (cPLA2-P) and nonphosphorylated (cPLA2 ) forms of the cPLA2 , respectively. (A) The data obtained using fMLP and A23187 as second stimuli are derived from separate experiments. (B) Enriched monocyte suspensions (3 × 106 cells/0.1 mL) were preincubated or not in the presence of EBV for the indicated times at 37°C, diluted 10-fold with HBSS, stimulated with A23187 (50 nmol/L for 5 minutes), and processed for analysis of cPLA2 as described above. Experiments shown are representative of three performed.
Effect of TFK-AA on LTB4 synthesis in EBV-primed PBMC. PBMC (10 × 106 cells/0.1 mL) were preincubated (or not) with EBV for 15 minutes at 37°C, diluted 10-fold with HBSS, and stimulated with A23187 (50 nmol/L for 10 minutes) in presence of increasing concentrations of the cPLA2 inhibitor AACOCF3 added to the cell suspensions 5 minutes before A23187. Results (mean ± SD) are from one experiment representative of three experiments performed in triplicate.
Effect of TFK-AA on LTB4 synthesis in EBV-primed PBMC. PBMC (10 × 106 cells/0.1 mL) were preincubated (or not) with EBV for 15 minutes at 37°C, diluted 10-fold with HBSS, and stimulated with A23187 (50 nmol/L for 10 minutes) in presence of increasing concentrations of the cPLA2 inhibitor AACOCF3 added to the cell suspensions 5 minutes before A23187. Results (mean ± SD) are from one experiment representative of three experiments performed in triplicate.
The effect of EBV on 5-lipoxygenase activation was also investigated. PBMCs were preincubated for 20 minutes in the presence or absence of EBV and then stimulated with a low concentration of A23187 in the presence of 3 μmol/L 15-HpETE (a substrate for the 5-lipoxygenase). The activity of the 5-lipoxygenase was assessed by the measurement of the 5-lipoxygenase–catalyzed conversion of 15-HpETE into 5,15-diHETE. Table 2 shows that, in four separate experiments, EBV consistently enhanced the capacity of the PBMCs to generate the 5-lipoxygenase product 5,15-diHETE. Unstimulated cells or cells treated with EBV only did not form detectable amounts of LTB4 or 5,15-diHETE. As expected, the generation of LTB4 from endogenous substrate (arachidonic acid) was also strongly enhanced.
DISCUSSION
In the present study, we showed that short-term coincubations of monocytes and EBV strikingly enhanced the capacity of monocytes to produce LTB4 and LTC4. By itself, EBV did not cause the synthesis of 5-lipoxygenase products (or to a very small extent only), but primed the monocytes for LT synthesis induced by a second stimuli. The priming effect was specific, as indicated by the complete inhibition of the effect of EBV by prior treatment of the virus suspension with an MoAb (72A1) raised against a glycoprotein of the viral envelope (gp350). Furthermore, the results obtained with heat-inactivated and UV-irradiated EBV indicated that, although structural integrity of the constituents of the viral envelope are required for the priming effect of EBV, infectivity is not a prerequisite. These results suggest that interaction of EBV with the cell membrane is sufficient to induce priming. The binding sites for EBV on the monocytes have not been characterized yet. However, we have previously shown that EBV binding on monocytes and neutrophils was not inhibited by cell treatment with antibodies directed against the CD21 receptor,16,19 which suggested that the gp350 of EBV might bind to a molecule distinct from CD21; such binding could be involved in the EBV priming effect described herein. This hypothesis is in agreement with other reports that EBV binds to cell types that do not express the CD21 receptor.34-37 The priming of LT synthesis by EBV was also observed in plasma, ie, in biologically relevant conditions.
The various studies reported here were performed using either PBMCs or enriched monocyte mononuclear cell suspensions; although these cell preparations contain lymphocytes (and <1% neutrophils), it is assumed that monocytes present in these preparations are essentially responsible for the formation of LT. Indeed, T lymphocytes do not contain the 5-lipoxygenase and, although the 5-lipoxygenase is present in B cells, these do not synthesize LT upon A23187 stimulation.38 Furthermore, lymphocytes are not responsive to the chemotactic peptide fMLP.39 Accordingly, an almost pure (∼99%) lymphocyte preparation was not responsive (in terms of LT synthesis) to EBV and A23187 or fMLP stimulation (data not shown, and Surette et al21 ).
An interesting feature of the effect of EBV observed in the present study is the ability of the virus to prime for LT synthesis induced by a variety of stimuli, including the soluble agonist fMLP, the zymosan particles, and the unspecific stimulus A23187. These data suggest that the priming effect of EBV on LT synthesis occurs at steps downstream to receptor engagement, possibly at the level of the regulation of the activity of enzymes involved in the biosynthesis of LT.
In neutrophils and monocytes, LT synthesis is limited by the availability of arachidonic acid, present in the form of esters in cellular lipids.40,41 Neutrophils and monocytes contain at least two types of PLA2 , the 80-kD cPLA2 and the low molecular weight 14-kD secreted PLA2 (sPLA2 ).42-44 The cPLA2 shows high selectivity for the release of arachidonic acid from the sn-2 position of phospholipids and its activity is enhanced after translocation to membrane structures in a Ca2+-dependent (high nanomolar concentrations) process.26-28 Studies performed with a recombinant cPLA2 in vitro have also shown that the enzyme is activated by serine-phosphorylation (Ser505).29,30 The sPLA2 activation is also Ca2+-dependent, but requires millimolar concentrations of the cation. LT synthesis in phagocytes is also dependent on the activation of the 5-lipoxygenase, which shows little activity in resting cells, but is enhanced in Ca2+-dependent processes upon cell activation.45 In the present study, the putative mechanisms of the priming effect of EBV on LT synthesis have been explored. Our data show that exposure of monocytes to EBV results in enhanced activation of 5-lipoxygenase upon cell activation with a second stimuli; this effect of EBV on 5-lipoxygenase was shown using an exogenous substrate (15-HpETE) that allows the assessment of 5-lipoxygenase activity independently of the effects of the cell treatment on the release of endogenous substrate.23 The mechanism of the effect of EBV on 5-LO activation remains to be elucidated. We also investigated the effect of EBV on substrate availability in the activated monocytes; our data have clearly established that EBV priming does enhance the release of arachidonic acid after cell stimulation. EBV was also found to enhance the cellular levels of phosphorylated cPLA2 (catalytically more active than the nonphosphorylated form29,30 ) in a time course compatible with the observed priming of LT synthesis. Finally, AACOCF3, an inhibitor of cPLA2 ,31 efficiently blocked LT formation in EBV-treated and A23187-stimulated PBMCs. Taken together, these data support the involvement of cPLA2 in the increased release of substrate induced by EBV.
It is noteworthy that the priming effect of EBV on LT synthesis in monocytes shares several of the characteristics of similar processes observed both in human neutrophils and monocytes upon treatment with cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF ) or bacterial lipopolysaccharides (LPS). Indeed, in the neutrophils, GM-CSF primes for LT synthesis induced by soluble agonists, particulate stimuli, and ionophores.46-48 LPS were found to prime neutrophils and monocytes for LT synthesis induced by fMLP.21,25 Such priming effects of GM-CSF and LPS were induced in short-term incubations (15 to 45 minutes) and were transient. Although the detailed mechanisms of these priming effects are still elusive, enhanced arachidonic acid release upon treatment with the second agonist appears to be a common feature of the action of all priming agents. Furthermore, LPS and GM-CSF were shown to induce the phosphorylation and activation of cPLA2 in neutrophils.49,50 Finally, in analogy to the present observations with EBV, we previously reported that GM-CSF causes enhanced 5-lipoxygenase activation after neutrophil activation with a second agonist.46
In summary, our data show that exposure of human blood monocytes to EBV results in a rapid and transient priming of the cells for LTB4 and LTC4 synthesis in a mechanism that may implicate glycoprotein gp350 of the viral envelope and involves stimulatory events both at the level of 5-lipoxygenase activation and arachidonic acid release. LTB4 and LTC4 are important mediators of inflammation, with the former promoting the migration of phagocytes at inflammatory sites, whereas the latter is a potent modulator of vascular tone and permeability. The upregulation of LT synthesis in monocytes by EBV might contribute to trigger and/or potentiate early events in host defense mechanisms against the virus. Studies are in progress to assess the effect of EBV on lipid mediator synthesis (and other cell functions) in other phagocytes, as well as the mechanisms involved.
ACKNOWLEDGMENT
We thank P. Côté for excellent secretarial assistance.
Supported by a grant from the Medical Research Council of Canada (MRC) to J.G. J.G. is a Scholar of the MRC.
Address reprint requests to Jean Gosselin, PhD, Laboratory of Viral Immunology, Centre de recherche en Rhumatologie et Immunologie, CHUL, 2705 boul. Laurier, Room T-1-49, Sainte-Foy (Québec), G1V 4G2 Canada.