Abstract
The adhesion of neutrophils (polymorphonuclear leukocytes [PMNs]) to immobilized fibrinogen/fibrin is mediated by β2-integrins. However, the influence of physiologic flow conditions on neutrophil adhesion to these surfaces is poorly defined. In this report, the effect of flow and neutrophil activation on adhesion to immobilized fibrinogen and fibrin was examined. For the evaluation of (the distribution of ) neutrophil adhesion, real-time video-assisted microscopy and custom-made software were used. Under flow conditions, adherent neutrophils appeared to support the subsequent margination of other neutrophils, thereby enhancing the adherence of these cells to fibrin. Consequently, neutrophils adhered in clusters, especially at higher shear stresses (eg, cluster index 1.4 at shear 80 mPa). Preactivation of PMNs with fMLP (10−7 mol/L) or 4β-phorbol, 12-myristate, 13-acetate (PMA; 100 ng/mL) resulted in approximately 50% inhibition of adhesion to fibrin and a more random distribution (cluster index <0.5). L-selectin antibodies or neuraminidase treatment of PMNs also inhibited adhesion and clustering, indicating a role for L-selectin. Under static conditions, no clustering appeared and PMN activation with fMLP or PMA caused threefold and sevenfold increased adhesion, respectively. Under these conditions, anti–L-selectin antibodies or neuraminidase did not affect adhesion. These results indicate that, under flow conditions, adherent neutrophils support adhesion of flowing neutrophils by L-selectin–mediated cell-cell interactions. Preactivated neutrophils, with lowered L-selectin expression, are less susceptible for this interaction. By this mechanism, adhered leukocytes can modulate the recruitment of leukocytes to the vessel wall at sites of inflammation.
THE ABILITY OF NEUTROPHILS to adhere to various substrates and cells is crucial for their function in host defense. During an inflammatory response, neutrophils (polymorphonuclear leukocytes [PMNs]) bind to (activated) endothelial cells and migrate through the basement membrane towards the site of inflammation. A local inflammatory response can also be initiated on damaged endothelium and the subsequently formed thrombus.1 Fibrinogen, fibrin, and activated platelets present in the thrombus have been shown to function as potent adhesive substrates for PMNs both in vivo2,3 and in vitro.4-6 PMN infiltration of a damaged vascular wall is thought to play a enhancing role in the process of thrombus degradation.7 On the other hand, adhesion of granulocytes to sites of vessel wall damage is considered important in the pathogenesis of several diseases, such as atherosclerosis, deep venous thrombosis, and vasculitis.3,8 9
Different adhesion molecules are involved in the margination and subsequent adhesion of PMNs from the flowing blood to the vessel wall.10,11 The initial rolling interaction under flow conditions is mediated by P-selectin glycoprotein ligand-1 (PSGL-1) and L-selectin present on leukocytes.12,13 These adhesion molecules recognize P- and E-selectin and proteins expressing sialylated Lewis X, which are expressed by endothelial cells.14,15 P-selectin is also expressed by surface-bound activated platelets and strongly promotes rolling adhesion of neutrophils.5 The selectin-mediated rolling interaction is followed by β2-integrin–mediated firm adhesion.16 These integrins mediate firm adhesion by recognizing many different cellular and noncellular ligands.17 On endothelial cells, these ligands include intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which belong to the Ig gene superfamily. Fibrinogen and its clotting product fibrin are well-known ligands for the neutrophil β2-integrins MAC-1 and p150.95.18,19 However, β2-integrins are constitutively expressed on resting PMNs in a low-affinity state. A conformational change induced by activation or divalent cations results in an increased affinity for soluble and immobilized fibrinogen.17,20 21 Studies concerning the interaction of granulocytes with immobilized fibrinogen were mainly performed under static conditions and showed increased adhesion by PMN activation.
Shear forces, such as those occurring under physiologic conditions, are known to strongly influence adhesion to surface-coated proteins and cells.22 23 Therefore, in the present study, the role of neutrophil-neutrophil contact during adhesion to fibrin was examined not only under static, but also under flow conditions. The role of β2-integrins in the adhesion of unstimulated neutrophils to fibrin prompted us to examine the effect of activation. We will show that, in contrast to static conditions, activation of PMNs leads to a decreased adhesion to fibrin under flow conditions. This decrease is explained by a downregulation of L-selectin–dependent cell-cell interaction at the surface upon activation. Interestingly, adherent neutrophils can directly support neutrophil adhesion under flow conditions, thus providing a mechanism for amplifying leukocyte recruitment during the inflammatory process.
MATERIALS AND METHODS
Reagents.Percoll was obtained from Pharmacia (Uppsala, Sweden). N-formyl-methionyl-leucyl-phenylalanine (fMLP), thrombin, and 4β-phorbol, 12-myristate, 13-acetate (PMA) were bought from Sigma (St Louis, MO). Fibrinogen was obtained from Enzyme Research Laboratory (South Bend, IN). Neuraminidase was purchased from Behring (Behringwerke AG, Marburg, Germany). H-D-Phe-Pro-Arg-Chloromethylketone (PPACK) was purchased from Bachem (Bubendorf, Switzerland). Adhesion experiments were performed in HEPES buffer (20 mmol/L HEPES, 132 mmol/L NaCl, 6 mmol/L KCl, 1.2 mmol/L KH2PO4 , 1 mmol/L MgSO4⋅7H2O, 5 mmol/L glucose, 1 mmol/L CaCl2 , pH 7.4). Tissue culture supplies (media, antibiotics, and trypsin) were purchased from GIBCO Biocult (Paisley, UK). All other reagents were of reagent grade.
Monoclonal antibodies (MoAbs).Hybridomas producing the MoAbs 44a (CD11b, 10 μg/mL for blocking studies) and IB4 (CD18, 10 μg/mL for blocking studies) were obtained from the American Type Culture Collection (Rockville, MD). The MoAb LAM1-3 (anti–L-selectin, CD62l, 5 μg/mL for blocking studies) was a kind gift of T. Tedder (Harvard Medical School, Boston, MA).24 The MoAb Leu-8 (anti–L-selectin, CD62l) was bought from Becton Dickinson (San Jose, CA). The MoAb WASP12.2 (anti–P-selectin, CD62p, 1 μg/mL for blocking studies) was purchased from Endogen (Boston, MA). The MoAb CLB-FcR gran/1 (CD16, 10 μg/mL) and fluorescein isothiocyanate (FITC)-labeled goat antimouse-IgG were from the Central Laboratory of The Netherlands Red Cross Blood Transfusion Service (CLB; Amsterdam, The Netherlands).
Neutrophil isolation.Blood was obtained from healthy volunteers from the Red Cross Blood Bank (Utrecht, The Netherlands). Mixed granulocytes were purified from the buffy coat of 500 mL blood, anticoagulated with 0.34% (wt/vol) trisodium-citrate (pH 7.4), as described before.25 In short, mononuclear cells were removed by centrifugation over Ficoll-paque (density, 1.077/mL). The remaining erythrocytes were lysed by incubation in isotonic 115 mmol/L ammoniumchloride-solution (pH 7.4) at 4°C for 20 minutes. After centrifugation, the remaining granulocytes were washed, diluted in HEPES buffer (2 × 106 cells/mL), and kept at room temperature until the start of the perfusion. PMN purity was greater than 95% and the viability, as measured with Trypan-blue exclusion, was greater than 98%. Neutrophil morphology was controlled by light microscopy for the presence of polarized and aggregated cells. The presence of polarized and aggregated cells (>1%) precluded the use of these cell preparations. For blocking experiments, the PMNs were preincubated with MoAbs for 20 minutes at room temperature. PMNs were always prewarmed for 5 minutes at 37°C before the adhesion assays.
Fibrin surfaces.To obtain plasma-fibrin surfaces, coverslips coated with tissue factor-rich ECM were perfused with platelet-poor plasma (PPP; < 104 platelets/μL plasma). Tissue-factor–rich ECM was obtained as described before.26 In short, human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical cord veins (according to Jaffe et al,27 with some minor modifications28 ). Cells of the second passage were subcultured on gelatin-coated coverslips and grown to confluence in 5 to 7 days. HUVECs were then stimulated with the phorbol ester PMA (20 ng/mL; overnight), after which they were removed by exposure to 0.1 mol/L NH4OH for 5 minutes at room temperature. The coverslips with the remaining matrices were washed and stored in phosphate-buffered saline (1:10 vol/vol 0.1 mol/L sodium phosphate buffer in 150 mmol/L NaCl, pH 7.4) at 4°C for a maximum of 3 weeks. PPP was obtained from whole blood of healthy donors and was anticoagulated with 20 U/mL low molecular weight heparin (LMWH; Fragmin; Kabi Vitrum, Stockholm, Sweden). The plasma was obtained by centrifugation (5 minutes at 1,500 RPM at 5°C). The fibrin network was formed by perfusion of the PPP over the ECM-coated coverslips for 10 minutes at a shear rate of 20 s−1 at 37°C. After perfusion, some of the coverslips were fixed (1% paraformaldehyde) and stained with May-Grünwald/Giemsa to determine the percentage of the surface covered with fibrin. The coverage was evaluated by light microscopy using an Quantimet 570C image analysis system (Leica Cambridge LTD, Cambridge, UK). In all cases, the fibrin network formed from plasma fibrinogen (designated plasma fibrin) covered more than 75% of the surface and consisted of thick fibrin fibers.
Pure fibrinogen was coated on a gelatin-coated glass coverslip by incubation with a fibrinogen solution (5 mg/mL in HEPES buffer) for 30 minutes at room temperature. To obtain pure fibrin surfaces, the pure fibrinogen coverslips were incubated with thrombin (1 U/mL HEPES buffer for 10 minutes at 37°C), resulting in degradation and polymerization of the fibrinogen. Thus, a fibrin network without plasma proteins formed under static conditions was obtained. Possible remaining thrombin activity was blocked by incubation with the specific thrombin inhibitor PPACK (H-D-Phe-Pro-Arg-Chloromethylketone; 1 μmol/L in HEPES buffer for 20 minutes at room temperature).
Perfusion experiment and evaluation.Adhesion experiments under steady flow conditions were performed in a transparent perfusion chamber as described before.5 The perfusion chamber has a slit height of 0.3 mm and width of 6 mm and is a modification from the chamber described by Sakariassen et al.29 The chamber contains two circular plugs on which mounted coverslips are exposed to plasma and different cell suspensions. PMNs in suspension (2 × 106 cells/mL in HEPES buffer) were aspirated from a reservoir through plastic tubing, through a valve, and through the perfusion chamber with a Harvard syringe pump (Harvard Apparatus, South Natic, MA). In this way, the flow rate through the chamber could be precisely controlled. The wall shear stress (t) was calculated from the equation t = (6Q⋅h)/(w⋅h2), in which Q is the flow rate, h is the suspending medium viscosity, w is the slit width, and h is the slit height.29 Shear stress can be calculated in units of Pascal or as dyne per square millimeter, in which 1 Pascal equals approximately 10 dynes/cm2. During the perfusion, the flow chamber was mounted on a microscope stage (DM RXE; Leica, Wetzlar, Germany) that was equipped with a B/W CCD-video-camera (Sanyo, Osaka, Japan) coupled to a VHS video recorder. Perfusion experiments were recorded on video tape. Video images were evaluated for the number of adhered cells with a Quantimet 570C image-analysis system (Leica Cambridge LTD). The number of surface-adhered neutrophils per square millimeter was measured after 5 minutes of perfusion at a minimum of 30 randomized fields (minimal area was 3 mm2).5 To measure the distribution of adhered cells over the surface, an application was developed in Quantimet basic. A cluster index was calculated as follows. For each adherent cell, the number of cells in a surrounding area of approximately 1,750 μm2 was measured. In the case of a random distribution, the expected number of cells inside this area could be calculated based on the mean number of surface-adhered PMNs per square millimeter. The cluster index per cell was set to be the difference between the measured and the expected number of cells inside an arbitrary area around the cell. In the equation Cluster Index Per Cell = [m − (X*a/A − 1)], in which m is the measured number of cells in the rectangle area, X is the total number of cells in the image, A is the size of the total image, and a is the size of the rectangular cell-surrounding area. For each experiment, the mean cluster index of a minimal of 500 cells was calculated. Various control tests, using computerized images, showed that the mean cluster index was independent of the number of adhered cells per square millimeter (results not shown). Moreover, a homogeneous/regular distribution of cells resulted in a mean cluster index of approximately 0.
Adhesion assay under static conditions.Adhesion experiments under static conditions were performed by incubating purified neutrophils in suspension (2 × 106 cells/mL in HEPES buffer) in the presence of activators or antibodies for 45 minutes at 37°C on the different surfaces. Nonadherent cells were then removed by washing twice with HEPES buffer, and adherent cells were fixed with 1% paraformaldehyde. The mean number of surface-adhered PMNs/mm2 was measured at a minimum of 30 randomized fields (ie, a surface with a total area >3 mm2).
Immunofluorescence flow cytometry.Surface marker expression by resting and activated neutrophils was determined on a flow cytometer (FACS Vantage; Becton Dickinson & Co, Mountain View, CA). PMNs (2 × 106 cells/mL HEPES buffer) were incubated with different concentrations of fMLP for 5 minutes at 37°C and immediately fixed in ice-cold paraformaldehyde (1% wt/vol). The cells were stained with IB4 (CD18), 44a (CD11b), or LAM1-3 (CD62L, anti–L-selectin). After washing twice, the cells were stained with FITC-labeled goat antimouse-IgG and again washed. The samples were kept on ice in the dark until analysis.
Statistical analysis.Results are expressed as the mean ± standard error (SE). Statistical analysis of the data was performed using a paired Student's t-test for single measurements or the repeated measures analysis of variance (ANOVA) for series of measurements. P values less than .05 were considered to be significant.
RESULTS
Neutrophil adhesion to fibrin under flow conditions.Using the transparent perfusion chamber, video and image analysis equipment, the behavior of flowing neutrophils interacting with a fibrin surface could be studied under continuous flow conditions. As was previously described,30 the number of PMNs adhering to a fibrin-covered surface after 5 minutes of perfusion was optimal at a low shear stress of 20 mPa (1,135 ± 122 PMN/mm2, n = 6). Adhesion rapidly decreased with increasing shear to 120 ± 39 cells/mm2 (n = 6) at shear 320 mPa (Fig 1). During the evaluation of the neutrophil perfusions over fibrin surfaces, it was noted that, at different shears, not only the number of adhering cells changed, but also the distribution of the adhered PMNs on the surface. Under high shear conditions (160 mPa), the formation of clusters of adhered cells, which were oriented in the direction of the flow, could be clearly observed (Fig 2A). As a control, Fig 2B shows a typical example of a random distribution of adhered neutrophils during a perfusion at a low shear stress of 20 mPa. To quantify this shear-dependent distribution of cells on the surface, a cluster index was calculated. This index is defined as the difference between the measured number of cells surrounding an adhered cell and the expected/calculated number of cells based on a random distribution. By this definition, the mean cluster index is independent of the total number of adhered cells (for more details see the Materials and Methods). After 5 minutes of perfusion, a clear correlation between cell clustering and shear stress was observed (Fig 1). The cluster index increased with increasing shear stress from 0.5 ± 0.1 (n = 6) at a shear stress of 20 mPa to a cluster index of 3.1 ± 0.5 (n = 6) at shear 320 mPa. At all shear stresses, the first few cells adhering immediately after the start of the perfusion appeared to be randomly distributed over the surface. Subsequently adhering cells then appeared to bind preferably in close proximity of the previously adhered cells, thus enhancing the cluster-like appearance on the surface. This observation was confirmed by measuring the cluster index at different time points. Indeed, the index measured after 5 minutes was always higher compared with the index after 1 minute of perfusion at all shear stresses tested (measured at 20 and 160 mPa; results not shown).
Neutrophil adhesion and clustering at different shear stresses. Neutrophils were perfused over a plasma fibrin surface at shear stresses ranging from 20 to 320 mPa. An ECM-coated coverslip was incubated with (leukocyte-free) PPP from heparinized blood at a shear stress of 20 mPa (10 minutes at 37°C). Subsequently, the fibrin surface was used for a perfusion with isolated resting PMNs (2 × 106 cells/mL HEPES buffer). After 5 minutes, the total number of adhering neutrophils per square millimeter (•) and the cluster index (○) was determined. The mean values ± SE of four to six experiments are given.
Neutrophil adhesion and clustering at different shear stresses. Neutrophils were perfused over a plasma fibrin surface at shear stresses ranging from 20 to 320 mPa. An ECM-coated coverslip was incubated with (leukocyte-free) PPP from heparinized blood at a shear stress of 20 mPa (10 minutes at 37°C). Subsequently, the fibrin surface was used for a perfusion with isolated resting PMNs (2 × 106 cells/mL HEPES buffer). After 5 minutes, the total number of adhering neutrophils per square millimeter (•) and the cluster index (○) was determined. The mean values ± SE of four to six experiments are given.
Random and clustered distribution of neutrophils adhered to fibrin. Neutrophils were perfused over a plasma fibrin surface as described in Fig 1 and pictures were taken after 0, 1, 3, and 5 minutes of perfusion. (A) Clear cellular clusters, aligned in the direction of the flow, occur after a perfusion at a shear stress of 160 mPa. Under these conditions, the mean cluster index after 5 minutes of perfusion is approximately 1.8. (B) More neutrophils adhered in a more random fashion after a perfusion at a low shear stress of 20 mPa. Under these conditions, the mean cluster index after 5 minutes of perfusion is approximately 0.5. The experiments shown are representative of four additional experiments. The arrow indicates the direction of the flow (equal for all figures).
Random and clustered distribution of neutrophils adhered to fibrin. Neutrophils were perfused over a plasma fibrin surface as described in Fig 1 and pictures were taken after 0, 1, 3, and 5 minutes of perfusion. (A) Clear cellular clusters, aligned in the direction of the flow, occur after a perfusion at a shear stress of 160 mPa. Under these conditions, the mean cluster index after 5 minutes of perfusion is approximately 1.8. (B) More neutrophils adhered in a more random fashion after a perfusion at a low shear stress of 20 mPa. Under these conditions, the mean cluster index after 5 minutes of perfusion is approximately 0.5. The experiments shown are representative of four additional experiments. The arrow indicates the direction of the flow (equal for all figures).
Role of adhesion receptors in neutrophil adhesion and clustering.The adhesion receptors that could play a role in the observed homotypic interaction of neutrophils or in the neutrophil binding to fibrin were studied in more detail. Table 1 summarizes the results of the neutrophil adhesion studies to coverslips coated with pure fibrin (purified fibrinogen incubated with thrombin). Neutrophil adhesion and distribution under flow conditions was determined after 5 minutes of perfusion at a shear stress of 80 mPa. Under control conditions (with no antibodies added), 997 ± 98 neutrophils adhered per square millimeter and a cluster index of 1.1 ± 0.1 (n = 6) was measured. Blocking CD18 and CD11b antibodies inhibited this adhesion under flow conditions by more than 80%. Adhesion on other surfaces, such as pure fibrinogen or plasma fibrin, showed a similar CD18/CD11b dependency (data not shown).30 The antibodies directed against these integrins showed no effect on the cluster index (Table 1). Incubation of the PMNs with the blocking L-selectin antibody LAM1-3 inhibited adhesion to pure fibrin under flow conditions by 55% (Table 1). Interestingly, the cluster index was similarly lowered after incubation with LAM1-3. The ligand of L-selectin is a sialyl Lewis X residue present on glycoproteins expressed on different cell types, including neutrophils. Removal of these sialic acid residues from the PMN surface by treatment with neuraminidase reduced the adhesion under flow conditions to 65% of control values. Again, significantly less cellular clusters were observed. Incubation of the fibrin surface with neuraminidase did not have an effect on PMN adhesion or distribution. These results indicate that a homotypic interaction between neutrophils, mediated by L-selectin, allows more neutrophils to bind to fibrin under flow conditions. Whether this was also the case under static conditions was examined using the same surfaces and antibodies (Table 1). Under static conditions, 76 ± 15 PMN/mm2 adhered to a fibrin-coated surface (equal to 100%). This PMN adhesion was only inhibited significantly by CD18 antibody treatment (58% less adhesion, n = 4). Neither L-selectin nor sialic acid residues seemed to play a role in PMN adhesion to pure fibrin under static conditions.
Role of Adhesion Molecules in Neutrophil Adhesion to Pure Fibrin
| . | Flow . | Static . | |
|---|---|---|---|
| . | Adhered Cells (% of control) . | Cluster Index . | Adhered Cells (% of control) . |
| . | . | (% of control) . | . |
| Control | 100 (997 ± 98) | 100 (1.1 ± 0.1) | 110 (76 ± 15) |
| CD16 | 98 ± 3 | 119 ± 27 | 90 ± 10 |
| CD18 | 13 ± 3* | 95 ± 29 | 42 ± 16* |
| CD11b | 18 ± 5* | 96 ± 9 | ND |
| L-selectin | 45 ± 8* | 56 ± 7* | 111 ± 12 |
| NA (cells) | 65 ± 10* | 61 ± 10* | 95 ± 20 |
| NA (surface) | 108 ± 10 | 92 ± 12 | 86 ± 13 |
| . | Flow . | Static . | |
|---|---|---|---|
| . | Adhered Cells (% of control) . | Cluster Index . | Adhered Cells (% of control) . |
| . | . | (% of control) . | . |
| Control | 100 (997 ± 98) | 100 (1.1 ± 0.1) | 110 (76 ± 15) |
| CD16 | 98 ± 3 | 119 ± 27 | 90 ± 10 |
| CD18 | 13 ± 3* | 95 ± 29 | 42 ± 16* |
| CD11b | 18 ± 5* | 96 ± 9 | ND |
| L-selectin | 45 ± 8* | 56 ± 7* | 111 ± 12 |
| NA (cells) | 65 ± 10* | 61 ± 10* | 95 ± 20 |
| NA (surface) | 108 ± 10 | 92 ± 12 | 86 ± 13 |
Neutrophil adhesion and distribution to a pure fibrin-coated surface was measured under flow conditions or under static conditions. Fibrin was formed on a gelatin-coated glass coverslip by incubation in a fibrinogen solution (5 mg/mL in HEPES buffer) for 30 minutes at room temperature. Subsequently, incubation with thrombin (1 U/mL HEPES buffer for 10 minutes at 37°C) resulted in the formation of pure fibrin. For adhesion under flow conditions, PMNs (2 × 106 cells/mL HEPES buffer) were perfused at shear stresses of 80 mPa (5 minutes at 37°C). Adhesion to fibrin under static conditions was measured after a PMN incubation for 45 minutes at 37°C (2 × 106 cells/mL HEPES buffer). For blocking studies, PMNs were incubated with antibodies against FcγRIII (CLB FcR gran/1, CD16), β2-subunit (IB4, CD18), MAC-1 (44a, CD11b), or L-selectin (LAM1-3, CD62I) for 30 minutes at room temperature. The fibrin-coated coverslip or the PMNs were incubated with neuraminidase (0.25 U/mL) for 20 minutes at 37°C. Data represent the number of adhering neutrophils per square millimeter or the cluster index as a percentage of control. The absolute control values are given in parentheses (mean ± SE, n = 4 to 6).
Abbreviations: NA, neuraminidase; ND, not determined.
Statistically significant effect of treatment was determined by paired Student's t-test (P < .05).
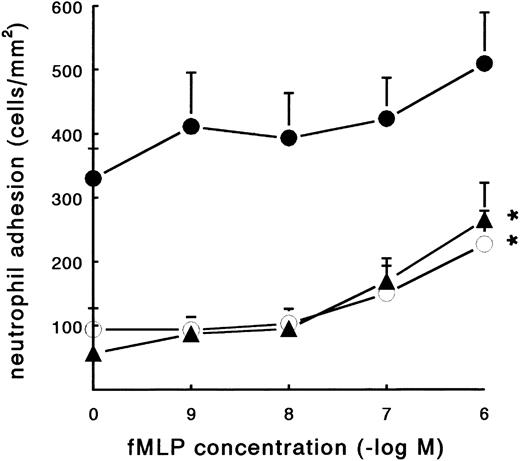
Effect of neutrophil activation on adhesion to fibrin.Activation promotes the binding of neutrophils to soluble fibrinogen by inducing a high-affinity state of the β2-integrins. The bacterial formyl-peptide fMLP was examined for its ability to stimulate the adhesion of PMNs to coated fibrinogen and fibrin. In a static adhesion assay, three different surfaces were used, ie, pure fibrinogen, pure fibrin, and plasma fibrin (Fig 3). After 45 minutes of incubation at 37°C, adhesion of resting PMNs to pure fibrinogen and pure fibrin surfaces was low (57 ± 6 and 94 ± 33 PMN/mm2, respectively; n = 7). The spontaneous neutrophil adhesion to plasma fibrin was 330 ± 47 PMN/mm2 (n = 8). Activation of the neutrophils with fMLP during the incubation showed a clear induction of adhesion (Fig 3). The increased adhesion to pure fibrinogen and pure fibrin surfaces was dose-dependent, with a maximum induction at a concentration of 10−6 mol/L (265 ± 58 and 227 ± 52 PMN/mm2, respectively; n = 6). The effect of fMLP on the adhesion to plasma fibrin was less pronounced and not significant. fMLP-treated neutrophils steadily showed more spreading compared with unstimulated cells.
Effect of fMLP on PMN adhesion under static conditions. Neutrophil adhesion to three different fibrin-coated surfaces was measured under static conditions: pure fibrinogen (▴), pure fibrin (○), and plasma fibrin (•). PMNs were incubated on the surface for 45 minutes at 37°C (2 × 106 cells/mL HEPES buffer) with or without fMLP. Shortly before measuring the total number of adhering PMNs per square millimeter, the surfaces were carefully rinsed to remove nonadherent cells. The data represent the number of adhering neutrophils per square millimeter. The mean values ± SE of six to eight experiments are given. *Significant effect of fMLP activation was determined by repeated measures ANOVA (P < .01).
Effect of fMLP on PMN adhesion under static conditions. Neutrophil adhesion to three different fibrin-coated surfaces was measured under static conditions: pure fibrinogen (▴), pure fibrin (○), and plasma fibrin (•). PMNs were incubated on the surface for 45 minutes at 37°C (2 × 106 cells/mL HEPES buffer) with or without fMLP. Shortly before measuring the total number of adhering PMNs per square millimeter, the surfaces were carefully rinsed to remove nonadherent cells. The data represent the number of adhering neutrophils per square millimeter. The mean values ± SE of six to eight experiments are given. *Significant effect of fMLP activation was determined by repeated measures ANOVA (P < .01).
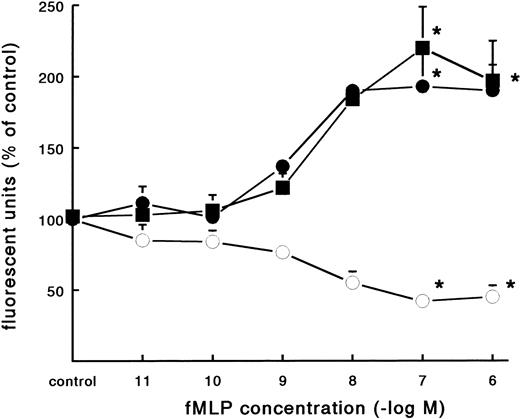
In contrast to the increased adhesion under static conditions after fMLP stimulation, an opposite effect was observed under flow conditions (Fig 4A). Activation of PMNs with different concentrations of fMLP induced a significant decrease of adhesion to fibrin, which was concentration-dependent. The effect was most pronounced on adhesion to surfaces coated with pure fibrin. Here, neutrophil adhesion decreased by 51% (633 ± 129 v 349 ± 74 PMNs/mm2, n = 5) after treatment with fMLP at a concentration of 10−6 mol/L. Using plasma fibrin-coated surfaces, neutrophil adhesion was inhibited by 38% ± 4% (882 ± 78 v 547 ± 73 PMNs/mm2, n = 8) after activation. In addition to the number of adhering cells, the cellular distribution was determined in these experiments. The cluster index decreased from 1.1 ± 0.12 for the unstimulated control to 0.4 ± 0.03 (n = 4) after activation of PMNs with fMLP concentrations of 10−8 mol/L and higher (Fig 4B). A similar dose-response curve occurred when the effect of fMLP on the expression of L-selectin was measured using flow cytometry (Fig 5). L-selectin expression on the neutrophil surface decreased by 50% upon stimulation with fMLP at concentrations of 10−8 mol/L and higher. As expected under the same conditions, CD11b and CD18 expression increased (by 90% and 115%, respectively; n = 4 to 7).
Effect of activation with fMLP on the adhesion of PMNs to fibrin. Neutrophils were perfused over fibrin-coated surfaces at shear stress 80 mPa after preactivation with different concentrations of fMLP (5 minutes at 37°C). (A) The total number of adhering PMNs per square millimeter was measured to pure fibrin (○) and plasma fibrin (•). The data represent the number of adhering neutrophils per square millimeter as a percentage of the (unstimulated) control. Absolute control values are 1,020 ± 56 cells/mm2 for pure fibrin and 921 ± 78 cells/mm2 for plasma fibrin. *A significant effect of fMLP activation was determined for both surfaces by repeated measures ANOVA (P < .05). (B) During the experiments, the cluster index of the cells adhered to pure fibrin (○) was determined as well. The mean values ± SE of four to six experiments are given. *A significant effect of fMLP activation was determined by repeated measures ANOVA (P < .05).
Effect of activation with fMLP on the adhesion of PMNs to fibrin. Neutrophils were perfused over fibrin-coated surfaces at shear stress 80 mPa after preactivation with different concentrations of fMLP (5 minutes at 37°C). (A) The total number of adhering PMNs per square millimeter was measured to pure fibrin (○) and plasma fibrin (•). The data represent the number of adhering neutrophils per square millimeter as a percentage of the (unstimulated) control. Absolute control values are 1,020 ± 56 cells/mm2 for pure fibrin and 921 ± 78 cells/mm2 for plasma fibrin. *A significant effect of fMLP activation was determined for both surfaces by repeated measures ANOVA (P < .05). (B) During the experiments, the cluster index of the cells adhered to pure fibrin (○) was determined as well. The mean values ± SE of four to six experiments are given. *A significant effect of fMLP activation was determined by repeated measures ANOVA (P < .05).
Effect of fMLP on adhesion molecule expression on neutrophils. Neutrophils were incubated with different concentrations of fMLP for 5 minutes at 37°C and surface marker expression was determined using flow cytometry. The mean fluorescence is given as a measure for expression of L-selectin (○), CD11b (•), and CD18 (▪) on the neutrophil surface. The mean values ± SE of four experiments are given. *A significant effect of fMLP activation was determined by repeated measures ANOVA (P < .05).
Effect of fMLP on adhesion molecule expression on neutrophils. Neutrophils were incubated with different concentrations of fMLP for 5 minutes at 37°C and surface marker expression was determined using flow cytometry. The mean fluorescence is given as a measure for expression of L-selectin (○), CD11b (•), and CD18 (▪) on the neutrophil surface. The mean values ± SE of four experiments are given. *A significant effect of fMLP activation was determined by repeated measures ANOVA (P < .05).
The contrasting effects of activation on adhesion under flow compared with under static conditions was also seen using PMA (100 ng/mL) instead of fMLP (Table 2). Under static conditions, PMA induced a sevenfold to eightfold increase of adhesion to a pure fibrin or fibrinogen surface, whereas the adhesion to plasma fibrin increased 2.5-fold. Under flow conditions, adhesion to pure fibrin and plasma fibrin decreased significantly (53% and 39%, respectively) after PMA treatment.
Effect of PMA Activation on Neutrophil Adhesion to Fibrinogen and Fibrin
| . | Plasma Fibrin . | Pure Fibrinogen . | Pure Fibrin . |
|---|---|---|---|
| Static | |||
| Control | 330 ± 47 | 65 ± 8 | 76 ± 15 |
| PMA | 838 ± 122* | 498 ± 56* | 570 ± 120* |
| Flow | |||
| Control | 921 ± 78 | ND | 1020 ± 56 |
| PMA | 561 ± 39* | ND | 479 ± 75* |
| . | Plasma Fibrin . | Pure Fibrinogen . | Pure Fibrin . |
|---|---|---|---|
| Static | |||
| Control | 330 ± 47 | 65 ± 8 | 76 ± 15 |
| PMA | 838 ± 122* | 498 ± 56* | 570 ± 120* |
| Flow | |||
| Control | 921 ± 78 | ND | 1020 ± 56 |
| PMA | 561 ± 39* | ND | 479 ± 75* |
Neutrophil adhesion to fibrinogen/fibrin-coated surfaces was measured under flow conditions or under static conditions. Surfaces with plasma fibrin, pure fibrinogen, and pure fibrin were obtained as described in the Materials and Methods. For adhesion under flow conditions, PMNs (2 × 106 cells/mL HEPES buffer) were perfused at shear stresses of 80 mPa (5 minutes at 37°C). Adhesion to fibrin under static conditions was measured after a PMN incubation for 45 minutes at 37°C (2 × 106 cells/mL HEPES buffer) with or without PMA (100 ng/mL). Data represent the number of adhering PMNs per square millimeter (mean ± SE, n = 3 to 6).
Abbreviation: ND, not determined.
Statistically significant effect of PMA activation was determined by paired Student's t-test (P < .05).
DISCUSSION
The recruitment of neutrophils to sites of a damaged vessel wall plays an important role in thrombus degradation and in the pathophysiology of atherosclerosis, vasculitis, and deep venous thrombosis. In studies of leukocyte adhesion to endothelial cells, flow and shear forces have been shown to be important factors in modulating the adhesion response. Under flow conditions, selectins are required for the first interaction of leukocytes with endothelial cells, followed by a tighter adhesion mediated by integrins.31 Using an in vitro model for a damaged vessel wall, we and others previously described a similar sequence of rolling and static adhesion of PMNs to thrombi containing fibrin and platelets.5,30,32 P-selectin expressed on adhered platelets was shown to mediate rolling adhesion, particularly at high shear stresses (>200 mPa or 2 dyne/mm2 ). The presence of fibrin together with platelets in a thrombus could support adhesion by MAC-1 (CD11b/CD18)-mediated interactions.30 For adhesion of PMNs to fibrin and fibrinogen surfaces without platelets, a role for selectins was not anticipated, because no L-selectin ligand or P-selectin is present on fibrinogen. Indeed, little adhesion and no rolling on the fibrin surface was seen at high shear stresses, whereas MAC-1 blocking antibodies almost totally abolished adhesion.23,30 On the other hand, close evaluation of the neutrophil adhesion behavior in these perfusions showed a typical interaction of flowing cells with surface-bound cells. This interaction can be described as neutrophils rolling over fibrin-adhered neutrophils. Particularly at higher shear stresses (80 to 320 mPa), these neutrophil-neutrophil interactions clearly resulted in the reduction of the velocity of some flowing cells. Subsequently, these slow cells could then adhere to fibrin, often in close proximity and directly downstream of previously attached cells. In this manner, adhered neutrophils seemed to support the arrest of newly arriving cells and cellular clusters oriented in the direction of the flow could be formed (Fig 2). Similar observations, using surfaces coated with purified PSGL-1, were reported very recently in rapid communication by Walchek et al.33 Once adhered to the surfaces, the cells became highly shear-resistant and no spontaneous detachment was observed. The observations that, at higher shear stresses, almost all cells adhered after contact with and in close proximity of adhered cells and that no detachment could be observed lead us to conclude that the cell-cell contact was very important in the recruitment of neutrophils and the formation of clusters.
The typical adhesive behavior described might be partially explained by a disturbance of the laminar flow behind attached cells. Immediately downstream of bound cells, the disturbed streamlines could result in a locally decreased shear stress. A similar rheologic mechanism was suggested for PMNs rolling in a row on an artificial P-selectin–rich surface.34 However, our results with L-selectin antibodies and neuraminidase treatment provide evidence for an alternative mechanism by which surface-bound neutrophils promote neutrophil adhesion in an L-selectin–dependent fashion. Indeed, blocking of L-selectin caused a decrease in the number of adhering cells and resulted in a more random distribution. This was also achieved by neuraminidase treatment of the PMNs, but not of the fibrin surface. These findings show that rheologic changes downstream of individual cells were of relatively little importance. It can be concluded that the partial inhibition of the PMN adhesion induced by L-selectin antibodies and neuraminidase treatment is caused by the blockade of cell-cell interactions. L-selectin is known to be important for leukocyte binding to endothelial cells as well as for leukocyte-leukocyte interactions. For example, under certain experimental conditions, neutrophil homotypic aggregation and neutrophil rolling over a monolayer of adhered neutrophils are mediated by L-selectin.35,36 For this latter process, L-selectin expressed by the rolling cells, and not by the surface-bound cells, was required. The precise nature of the ligand(s) of L-selectin present on PMNs remains to be defined but is most likely comprised in part by PSGL-1 and the sialylated Lewis X moiety on these cells that is sensitive to neuraminidase treatment.33 37
The decreased adhesion under flow conditions observed after PMN activation with fMLP (and PMA) is an additional argument for the role of L-selectin in adhesion, because L-selectin is shed upon activation (Fig 4A and Table 2). Indeed, a strong correlation is present between L-selectin expression and PMN clustering (Figs 4B and 5). The proadhesive influence of homotypic PMN-PMN interactions at fibrin surfaces works exclusively for adhesion under flow conditions, when interaction dynamics and cellular movement are restrictive factors. Accordingly, adhesion of neutrophils to fibrin under static conditions was not affected by selectin antibodies or by the shedding of the L-selectin ligand from the cell surface. As was also found by other investigators, neutrophil activation caused increased adhesion under static conditions through the activation of integrins (Fig 3 and Tables 1 and 2).21
Using three different fibrinogen/fibrin surfaces under static conditions (Fig 3 and Table 2), a difference in PMN adhesion occurred between the surfaces that was not observed under flow conditions. A fibrin surface formed from heparinized plasma showed a threefold enhanced adhesion of resting neutrophils compared with pure fibrinogen or fibrin. Spontaneous activation by plasma factors such as complement factors present on the surface could induce the enhanced adhesion.38,39 This would also explain the less clear effect of additional activation by fMLP or PMA on the adhesion to fibrin formed from plasma fibrinogen (Fig 3 and Table 2). Furthermore, on the plasma fibrin surface, additional integrin ligands, such as factor X, thrombospondin, or C3bi, are present, which could enhance adhesion. In previous studies, we showed that the presence of plasma proteins in the fibrin network did not affect neutrophil adhesion under flow conditions.36
Neutrophil adhesion to fibrin itself under flow conditions was largely mediated by β2-integrins. Because integrins have to be in an activated state to bind fibrinogen with a high affinity, this also appears to be true for fibrin. Indeed, in our experiments under flow conditions, PMNs adhered to fibrin or fibrinogen with a high affinity. Once cells were bound, they became shear-resistant for shear stresses up to 1,000 mPa (data not shown). These observations indicate an activation event during adhesion. The change in shape of adherent PMNs observed after 5 to 10 minutes is further indication that the cells were activated. Whether this activation is induced by the contact with the fibrin network, by other PMNs, or by the sudden exposition to shear is unclear and is currently under investigation. Another explanation could be the L-selectin–mediated cell-cell contact. In this respect, cross-linking of L-selectin by MoAbs has been shown to be involved in neutrophil intracellular signaling and activation.40,41 Simon et al42 proposed that cross-linking of L-selectin results in a cell signal that directly stimulates β2-integrin adhesive responses.
In this report, we describe a new regulatory mechanism for PMN adhesion under flow conditions. The L-selectin–mediated interaction between flowing and adherent PMNs promotes PMN adhesion to a fibrinogen or fibrin surface. The homotypic cell-cell interaction might also be involved in the adhesion under flow conditions of other leukocytes to other surfaces such as endothelial cells and platelets. This positive feedback mechanism seems important for the recruitment of unstimulated neutrophils to sites that do not provide rolling receptors or ligands. An intriguing consequence of this process is that activation of flowing leukocytes will lead to impaired adherence because L-selectin is shed from the surface. Therefore, activation of leukocytes in the peripheral blood may lead to a loss of adhesive properties to a broad range of surfaces and substrates under physiologic flow conditions. However, cells that have been activated during or after (rolling) adhesion exhibit a strong resistance against shear-induced detachment. We have described for the first time the importance of L-selectin for PMN adhesion to surfaces that are ligands for integrin receptors.
Supported by Dutch Heart Foundation Grant No. 92.106 and by Glaxo Wellcome BV, The Netherlands.
Address reprint requests to L. Koenderman, PhD, Department of Pulmonary Diseases, Room F.02.333, University Hospital Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal