Abstract
Although the cause(s) of clinical drug resistance in non-Hodgkin's lymphomas (NHL) is unknown, in vitro studies suggest that abnormalities of the p53 gene, bcl-2 overexpression, and low tumor proliferation rates may increase chemotherapy resistance. We analyzed tumor tissue from 75 patients with relapsed/refractory NHL (Working Formulation A through H) for p53 mutation/overexpression (abnormality), bcl-2 expression, and tumor proliferation and correlated them with multiple clinical characteristics, response to therapy, disease-free survival, and overall survival (OS). All tumor biopsy specimens were obtained within 6 weeks of treatment with EPOCH (infusional etoposide, vincristine, and doxorubicin and bolus prednisone and cyclophosphamide) chemotherapy. Overall, 16 (21%) tumors had a p53 abnormality. Of 13 tumors with overexpression, mutations were confirmed by sequence analysis in 11, and, in 44 tumors without overexpression, 3 showed mutations. A multivariate analysis showed that tumors with a p53 abnormality were more likely to be drug resistant than tumors with normal p53 (56% v 17%; P2 = .008) and to have a shorter median progression-free survival (PFS; 2.1 v 8.2 months; P2 = .008) and OS (11.7 v 21.5 months; P2 = .038), respectively. The presence of a p53 abnormality did not correlate with any clinical characteristic, bcl-2 expression, or tumor proliferation. A significant correlation was found between low tumor proliferation and drug resistance in a univariate (P2 < .006) but not multivariate analysis. Patients with tumor proliferation of less than 80% were significantly more likely to have no response to therapy (31% v 6%) or to fail to achieve a complete response (16% v 44%) and tended to have shorter PFS and OS than did patients with higher proliferation. No significant association was found between bcl-2 expression and drug resistance, PFS or OS, although patients with intermediate-grade histologies and high bcl-2 expression tended to be drug resistant as compared with low level expressors (P2 < .065). Of interest was the finding of a significant association between high bcl-2 and low tumor proliferation (P2 = .0045). In studies that have found an association between high bcl-2 expression and short PFS, bcl-2 may have been a surrogate for low tumor proliferation. Further studies are warranted to examine this question. These results suggest that p53 mutation and low tumor proliferation, but not bcl-2, may be important causes of clinical drug resistance in NHL.
MOST PATIENTS WITH non-Hodgkin's lymphoma (NHL) ultimately develop resistance to chemotherapy and die of disease. Recently, significant efforts have focused on identifying and understanding mechanisms of drug resistance in NHL to aid the development of more effective therapy. Additionally, the identification of molecular/biological markers of drug resistance may allow the development of a prognostic index based on actual measures of drug resistance.
Accumulating evidence suggests that abnormalities in the control of the cell cycle and programmed cell death (apoptosis), a final common pathway through which cytotoxic agents may exert their lethal effects, can lead to drug and/or radiation resistance.1,2 Abnormalities in specific cell cycle control genes, such as p53, and regulators of apoptosis, such as p53 and bcl-2, are commonly found in lymphoid neoplasms.3-5 Overexpression of bcl-2 protein is observed both in follicular lymphomas, in which bcl-2 has usually undergone a translocation t(14; 18), as well as in other subtypes of lymphoma that lack the translocation.3 The experimental findings that transfection of bcl-2 into murine lymphoid cells confers resistance to nitrogen mustard and camptothecin by inhibiting apoptosis suggests that bcl-2 overexpression may confer clinical drug resistance in lymphomas.6 In contrast to bcl-2, p53 arrests cells exposed to DNA-damaging agents in G1 to allow DNA repair or, if essential repairs are not possible, promotes apoptosis.7 Experimentally, loss of p53 function produces cellular resistance to alkylating and topoisomerase-II drug classes, suggesting that loss of p53 function in lymphomas may cause drug resistance.1 2
These observations led to the hypothesis that bcl-2 and p53 play a role in the development of drug resistance in lymphoma. However, no studies have examined the clinical relationship of bcl-2 expression or p53 mutation to drug resistance in lymphomas. Although several studies assessed the association between bcl-2 expression and disease-free survival (DFS), they reached conflicting conclusions.3,8-11 Furthermore, studies have shown an association between p53 mutation/overexpression and decreased survival, but they could not assess whether mutated p53 was a marker of drug resistance or another biological feature of the tumor.4,9 12
Abnormalities in cell cycle control pathways can also affect cellular proliferation. For example, suppression of the apoptotic-enhancing effect of deregulated c-myc by either bcl-2 or mutated p53 allows expression of an unopposed proliferative signal.13,14 Such changes in tumor proliferation may have a significant impact on the sensitivity of tumors to chemotherapy, as shown in vitro for phase- and cycle-specific agents.15-17 Although several studies have assessed the clinical relationship between tumor proliferation and survival in patients with NHL, they reached contradictory conclusions and none assessed the relationship between tumor proliferation and drug resistance.18 19 Moreover, the association between tumor proliferation and bcl-2 expression and p53 mutation have not been previously determined, and possible interactions potentially confound the interpretation of results. In the present study, we examined the relationship of these markers with one another and assessed their relationship to drug resistance and to progression-free survival (PFS) and overall survival (OS) in patients with relapsed NHL.
MATERIALS AND METHODS
Patients and staging.A total of 109 consecutively treated patients were eligible for this study. However, based on the availability of material, fixed paraffin-embedded tissues could only be obtained for analysis from 75 patients. This study was approved by the Institutional Review Board of the National Cancer Institute and informed consent was obtained according to the Declaration of Helsinki. Eligible patients had confirmed relapsed or refractory low-grade, progressed low-grade, or de novo intermediate-grade NHL (confirmed by one of us, E.S.J.; Table 1); measurable disease; and a nonreactive test for human immunodeficiency virus. All patients had a tissue biopsy performed within 6 weeks of study entry and were subsequently treated with EPOCH (infusional etoposide, vincristine and doxorubicin, and bolus prednisone and cyclophosphamide) chemotherapy, as previously described.20 Patients with low-grade lymphoma were eligible if they clinically required treatment, whereas patients with intermediate-grade lymphoma were treated immediately on relapse from or failure of a previous regimen. Initial staging included a patient history; a physical examination; standard blood tests including lactate dehydrogenase (LDH; normal range, 113 to 226 U/L); computed tomography of the chest, abdomen, and pelvis; and bilateral bone marrow biopsy specimens. Sites of disease were restaged every two cycles. Therapy was continued until the patient had stable or progressive disease over the course of two treatment cycles or until the patient was two cycles past a complete remission (CR). Patient responses were evaluated 3 weeks after the last cycle of EPOCH chemotherapy.
CR was defined as the normalization of physical findings and radiologic abnormalities and the finding that the relevant laboratory data and bone marrow were free of disease 4 weeks after the last cycle of chemotherapy. Patients were considered to have a partial response (PR) if they had at least a 50% reduction in the sum of the products of the greatest cross-sectional diameters of measurable lesions without evidence of new lesions or a greater than 25% increase in an individual lesion over one treatment cycle. Patients not fulfilling the above criteria were considered to have had no response to EPOCH.
p53, bcl-2, and MIB-1 immunohistochemistry.Immunohistochemical studies were performed on paraffin-embedded tissue sections with monoclonal antibodies that included the D07 antibody against wild-type/mutant p53 (Dako, Carpenteria, CA), the MIB-1 antibody against the Ki-67 nuclear proliferation antigen (Immunotech, Westbrook, ME), and an antibody against the bcl-2 protein (Dako clone 124; Dako). All immunohistochemical analyses were performed in the Laboratory of Hematopathology, National Cancer Institute, and were reviewed by two investigators (J.T-F. and M.R.). Deparaffinized slides were first submitted to an antigen-retrieval procedure. In brief, the slides were placed in a microwavable pressure cooker containing 1,500 mL of 10-mmol/L citrate buffer at pH 6.0 and were microwaved (Model R4A80; Sharp Electronics, Mahwah, NJ) for 40 minutes at 700 W. Immunohistochemistry was then performed on an automated immunostainer (Ventana Medical System, Inc, Tucson, AZ) using the manufacturer's paraffin-slide protocol.
Tissues were scored as p53-positive if greater than 10% of the tumor cells had nuclear staining and as p53-negative if ≤10% stained. A control slide containing p53-positive cells was used to confirm the adequacy of the immunohistochemistry. To control for differences in bcl-2 staining between tissue samples, T cells were used as internal controls and were identified by CD3 staining of serial slides. If there was no bcl-2 staining of the control T cells, the tumor sample was not included in the study. Bcl-2 staining was scored on a scale from 0 to 3 as follows: 0, tumor cells were negative but interspersed reactive T cells were positive; 1, tumor cells stained lighter than T cells; 2, tumor cells stained equal to T cells; 3, tumor cells stained stronger than T cells. MIB-1 staining was scored as a mean percentile of 200 to 300 tumor cells averaged over 3 high-powered fields (hpf; 40×) or 600 cells using a grid ocular objective and was reported as 0 to 100%.
p53 denaturing gradient gel electrophoresis (DGGE).High molecular weight genomic DNA was extracted from frozen tissue or from deparaffinized tissue sections using a commercial DNA extraction kit (Stratagene Cloning Systems, La Jolla, CA). Two hundred nanograms of genomic DNA was mixed with 40 pmol of each primer and 75 nmol of each deoxynucleotide triphosphate in 50 μL of polymerase chain reaction (PCR) buffer (10 mmol/L Tris HCL, [pH, 8.3], 50 mmol/L KCL, 1.5 mmol/L MgCL2, and 0.01% gelatin), and 1.5 U of Taq DNA polymerase was added to each sample after heating at 94°C for 3 minutes (“Hot Start”). PCR was performed at 94°C for 1 minute, at 55°C for 1 minute, and at 72°C for 1 minute plus 1 second of extension per cycle, for 35 cycles. Except for the exon 8 primers, primers flanking p53 exons 5-8 were the same as those described by Beck et al.21 Exon 8 primers were as follows: forward, 5′ CCTATCCTGAGTAGTGGTAAT-3′; and reverse, 5′(GC)AAGTGAATCTGAGGCATAAC-3′. One primer of each pair was synthesized with an added 40-bp GC-rich sequence (GC clamp) at the 5′ end. The GC clamps provide high-temperature melting domains at one end of each PCR product, rendering the remainder of the exon sequence accessible to analysis by DGGE. Five microliters of each amplified sample was subjected to electrophoresis on 1.5% agarose gels to determine whether amplification occurred; the remainder was used for the DGGE analysis. Gels were cast using the Model 475 Gradient delivery system (Bio-Rad Laboratories, Hercules, CA), and products were analyzed using a D-gene gel electrophoresis apparatus (Bio-Rad Laboratories). Forty-five microliters of amplified sample was mixed with 8 μL of loading dye, loaded onto 7.5% polyacrylamide gels, and electrophoresed at 150 V at 60°C under the appropriate predetermined denaturing conditions. Except for the gradient conditions for exon 5 and 6, all other p53 exons were analyzed under the same gradient conditions described by Beck et al.21 Conditions for exons 5 and 6 were as follows: 50% to 65% for 5.5 hours and 35% to 60% for 8 hours, respectively.
p53 sequence analysis.Abnormal bands detected by DGGE were cut from the gel with a sterile razor blade under UV light and placed in 300 μL of Tris-EDTA (pH, 7.4), and the DNA was eluted at 65°C overnight. After ethanol precipitation and resuspension in 20 μL of water, 2 to 3 μL was used for PCR amplification as described above, and 6.5 μL of amplified product was used for sequencing according to the instructions from the Sequenase PCR Product Sequencing Kit (US Biochemical Corp, Cleveland, OH). All samples were sequenced in the forward and reverse directions using the identical primers used for PCR amplification. Samples were denatured for 2 minutes at 75°C, and 3 μL was run on a denaturing 6% polyacrylamide/8 mol/L urea sequencing gel for 3 hours at 65 W. The gels were transferred to Whatman paper (Whatman, Maidenstone, UK) and dried. Autoradiography was performed with Kodak X-OMAT film (Eastman-Kodak, Rochester, NY) at room temperature for 24 to 72 hours.
Statistical analysis.Comparisons between two groups of patients with respect to continuous variables were made using the Wilcoxon rank sum test, and comparisons of discrete variables between groups were performed using the χ2 test, Fisher's exact test, or Mehta's modification of Fisher's exact test as appropriate.22 The correlation between time from initial diagnosis to entry on the present study and tumor proliferation was assessed using a Spearman rank correlation coefficient. Durations of PFS and OS were computed from the on-study date until the date of progression, death, or last follow-up evaluation, as appropriate. The Kaplan-Meier method was used to compute the probability of survival or PFS.23 The Mantel-Haenszel technique was used to compute the significance of the difference between pairs of Kaplan-Meier curves.24 In addition, the Cox proportional hazards modeling technique was used to identify which factors, when simultaneously evaluated, may together affect survival or PFS.25 The resulting model parameters (bi ) were converted to relative risks by computing exp(bi ), where exp(a) = 2.7183a.26 The 95% confidence interval for the relative risk was computed as [exp(biL ), exp(biH )], where biL = bi-1.96 [est. std. Error (bi )] and biH = bi+1.96 [est. std. Error (bi )]. The relative risk indicates the risk associated with dying (or being a PFS failure) while being in a greater risk category as compared with that of being in a lower risk category. Logistic regression analysis was used to identify factors simultaneously associated with response to therapy.27 All P values are 2-sided and denoted by P2.
RESULTS
Clinical characteristics.Tumor tissue obtained within 6 weeks of treatment with EPOCH chemotherapy was available for analysis of drug resistance markers in 75 patients with relapsed NHL. Patient characteristics, divided by p53, bcl-2, and MIB-1 (tumor proliferation) status, are shown in Table 1. The median patient age was 51 years. Clinical characteristics shown to have an adverse prognostic impact in the International NHL Prognostic Factors Project (International Index) included age over 60 years in 24%, elevated LDH level in 57%, Eastern Cooperative Oncology Group performance status of 2 to 4 in 59%, advanced stage in 85%, and multiple extranodal disease sites in 23% of patients.28 Patients had received a median of 2 (range, 1 to 8) prior regimens and 8 (range, 3 to 15) different drugs, and 24 patients (32%) had not responded to their last treatment and 5 had primary refractory disease. Aggressive histologies comprised 80% and low-grade histologies comprised 20% of the series. Of the 60 patients with aggressive histologies, 37 had presented de novo with intermediate-grade lymphoma, whereas 23 had progressed from a low-grade histology (PLG). Diffuse large-cell and large-cell immunoblastic were the predominant histologies, comprising 61% of patients in this series, and 93% of tumors (70) were of B-cell phenotype.
p53 immunohistochemistry and mutational analysis.Tumor tissue was available for analysis by p53 immunohistochemistry in all 75 patients and, for p53 mutations, in 56 patients (75%; Table 2). Overall, 16 tumors (21%) showed evidence of a p53 mutation and/or overexpression (termed p53 abnormality). There was very good concordance between protein overexpression as detected by immunohistochemistry and the presence of an abnormal p53-DGGE result. Of 13 tumors showing p53 overexpression by immunohistochemistry, mutations were confirmed by DGGE and sequence analysis in 11. In the 2 unconfirmed cases, 1 was negative for mutation and there was inadequate material for mutational analysis in the other. We also performed mutational analysis on 44 specimens that did not show p53 overexpression and detected mutations in 3 additional cases. Of these 3 cases, 1 possessed a stop codon at position 192 that may have resulted in an unstable protein undetectable by immunohistochemistry. It is worth noting that all positive cases came from tumors of B-cell phenotype, although there were few non–B-cell cases. In this study, immunohistochemistry was a rapid and reliable method of detecting p53 mutations. Using DGGE as a standard, immunohistochemistry had a sensitivity and specificity of 79% and 98%, respectively.
The abnormally migrating bands detected by DGGE in 14 cases were distributed among exons 5-8 as shown in Table 2. Sequence analysis showed all but 1 to be the result of one or two point mutations. The 1 exceptional case had a 35-bp duplication in exon 5. All of the point mutations occurred in common hot spots and were generally of the missense type, except in 1 case in which the mutation resulted in a stop codon.
As shown in Table 1, we examined the relationship between the presence of a p53 abnormality and patient characteristics on study entry. Although there were no significant associations, the aggressive histological subtypes and refractory tumors tended to have a higher incidence of p53 abnormalities.
Bcl-2 immunohistochemistry.Relative expression levels of bcl-2 were assessed in 69 tumors and were scored as 0, 1, 2, or 3. There was a significant association between the histological subtype and bcl-2 expression (P2 = .003); 93% of low-grade tumors showed bcl-2 expression of 2 or 3, whereas 61% of intermediate-grade tumors had low bcl-2 expression of 0 or 1 (Table 1). We also examined the relationship between patient characteristics on study entry and bcl-2 expression. Patients with more than one extranodal disease site were more likely to have higher bcl-2 expression, an association likely caused by the higher incidence of bone marrow disease in low-grade lymphomas; no other clinical correlations were found. There was no relation between p53 abnormality and bcl-2 expression level (P2 = .68).
Relationship of p53 and bcl-2 to drug resistance and clinical outcome.We assessed the clinical variables associated with EPOCH drug resistance by univariate analysis (Table 3). There was no significant difference among the different histological subsets. Of importance was the relationship of p53 abnormality and bcl-2 expression to drug resistance. Tumors with a p53 abnormality were significantly more likely to be drug resistant than tumors with normal p53 (56% v 17%, respectively; P2 = .003) and to have an overall poorer response to therapy (P2 = .01; Table 4). In contrast, bcl-2 expression had no association with clinical drug resistance (21% v 28%; P2 = .52) or with overall response to therapy (P2 = .42; Table 4). A logistic regression analysis of all variables confirmed the independent association of p53 abnormality (P2 = .008) with drug resistance, along with poor performance status and age (Table 5).
To determine if these results were influenced by our including all histological grades, we performed these same analyses in the intermediate-grade subset alone. p53 abnormality remained significantly associated with drug resistance by both univariate (P2 = .017) and multivariate (P2 = .016) analyses. Of interest in this subset analysis was the finding that patients with high bcl-2 expression tended to be more drug resistant (53%) than those with low expression levels (20%; P2 = .065). We also performed these analyses in the low-grade and progressed low-grade subgroups, and none were significant except for p53 abnormality, which showed borderline (P2 = .057) association with resistance in the low-grade subset, not unexpected results given the relatively small sizes of these subgroups.
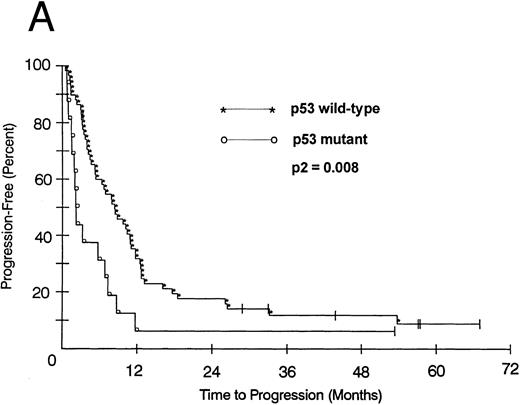
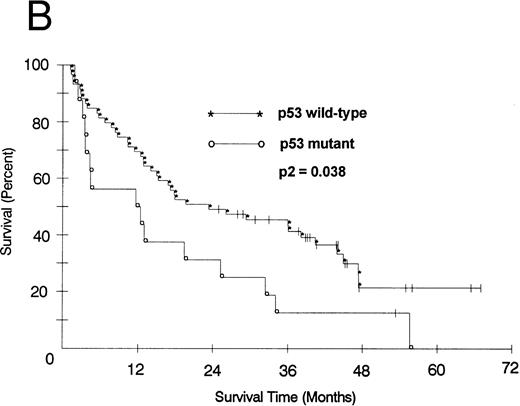
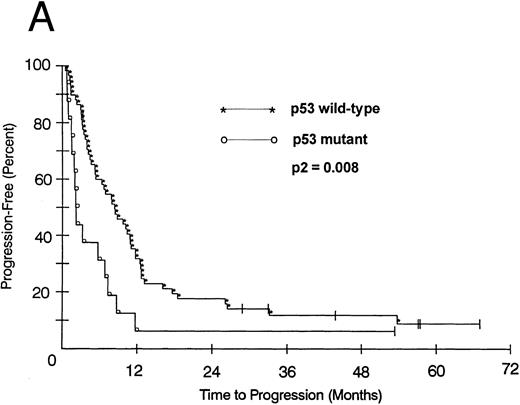
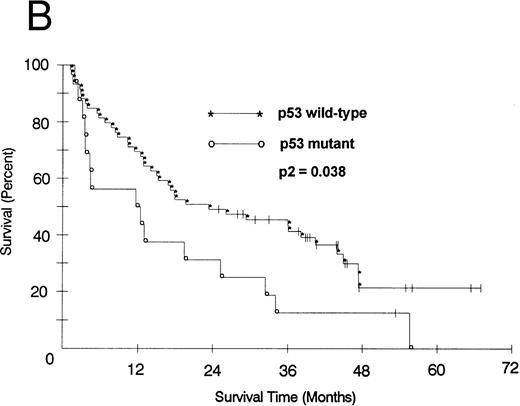
We also looked at the association of all variables on patient PFS and OS. Kaplan-Meier PFS and OS curves with Mantel-Haenszel P values were constructed for all variables and were performed on all patients. Combining all patients, those with a p53 abnormality had a significantly shorter median PFS (2.1 v 8.2 months as compared with p53 wild-type tumors, P2 = .008; Fig 1A). This association remained significant within the low- and intermediate-grade subsets as well (P2 = .026 and .005, respectively). Patients with a p53 abnormality also had a significantly shorter median OS (11.7 v 21.5 months; P2 = .038; Fig 1B), and this association was significant within the subset of patients with intermediate-grade histologies as well (P2 = .027). In contrast, there was no significant association between low (0/1) or high (2/3) bcl-2 expression level and either PFS (7.1 v 6.2 months, respectively) or OS (22 v 17.9 months, respectively). Separate subset analyses within the low- and intermediate-grade subsets were not significant. To assess if there was an interaction between p53 and bcl-2 expression and either PFS or OS, we combined these two variables but found no significant associations other than that accounted for by p53 abnormality.
(A) PFS of 59 patients without p53 abnormalities (*) is compared with that of 16 patients with abnormalities (○). Patients without abnormalities progressed in a median of 8.2 months, as compared with 2.1 months for patients with abnormalities. (B) OS of 59 patients without p53 abnormalities (*) is compared with that of 16 patients with abnormalities (○). Patients without abnormalities died in a median of 21.5 months, as compared with 11.7 months for patients with abnormalities.
(A) PFS of 59 patients without p53 abnormalities (*) is compared with that of 16 patients with abnormalities (○). Patients without abnormalities progressed in a median of 8.2 months, as compared with 2.1 months for patients with abnormalities. (B) OS of 59 patients without p53 abnormalities (*) is compared with that of 16 patients with abnormalities (○). Patients without abnormalities died in a median of 21.5 months, as compared with 11.7 months for patients with abnormalities.
We performed a Cox proportional hazards model to determine which variables were independently associated with PFS and OS. For PFS, p53 abnormality was significantly associated with shorter PFS (P2 = .0014), along with induction failure and poor performance status (Table 6). The presence of a p53 abnormality was also independently associated with a shorter OS (P2 = .036; Table 6). When these same multivariate analyses were restricted to the intermediate-grade subset, p53 abnormality remained associated with PFS (P2 = .0076) and OS (P2 = .02).
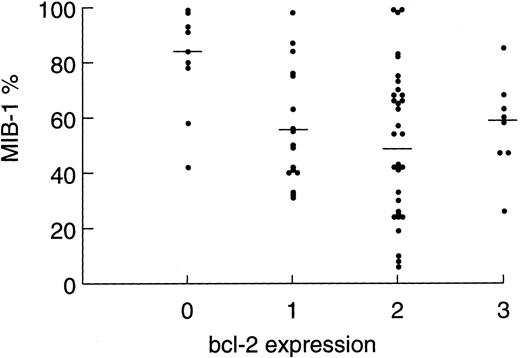
Relationship of MIB-1 expression and clinical drug resistance.MIB-1 detects a nuclear antigen that is present throughout the cell cycle but not in resting (Go ) cells. Thus, it is a measure of tumor growth and is expressed as the percentage of proliferating tumor cells. In the present study, we analyzed tumor specimens from 67 patients (89%) and made correlations between patient variables and tumor proliferation rates. Tumor proliferation correlated with both tumor grade and bcl-2 expression level (Table 1). The mean ± SEM tumor proliferation rates for low-grade (n = 13), progressed low-grade (n = 22), and intermediate-grade NHL (n = 32) were 36.6% ± 6.3%, 50.8% ± 4.4%, and 69.7% ± 4.2%, respectively (P2 = .0002). The association between bcl-2 expression level and tumor proliferation was noteworthy. Tumors expressing no bcl-2 (n = 9) had the highest mean ± SEM tumor proliferation rate of 80.3% ± 6.4%, intermediate (level, 1) expressors (n = 18) had a proliferation rate of 62.1% ± 5.6%, and high (level, 2 or 3) expressors had the lowest proliferation rate of 49.8% ± 3.8%; P2 = .0045). To control for the association between high bcl-2 expression and follicular lymphoma, which have low proliferation rates, we restricted this analysis to the intermediate-grade subset and found a similar result, albeit of borderline significance because of the small patient numbers (P2 = .058). Figure 2 shows the distribution of tumor proliferation and median within each bcl-2 expression level.
Scatter plot showing the distribution of tumor proliferation among tumor samples showing bcl-2 expression levels of 0, 1, 2, and 3. Each point represents an individual patient tumor sample. Bars represent the median within each group (P2 = .009).
Scatter plot showing the distribution of tumor proliferation among tumor samples showing bcl-2 expression levels of 0, 1, 2, and 3. Each point represents an individual patient tumor sample. Bars represent the median within each group (P2 = .009).
As shown in Table 1, there was an association between tumor proliferation and advanced-stage disease, a finding that likely reflects the association of lower tumor proliferation and more advanced-stage disease found in the low-grade lymphomas. We also found that patients with a tumor proliferation rate of less than 80% had received more prior regimens than patients with higher tumor proliferation rates. To assess if patients with lower tumor proliferation rates had had their disease longer than patients with high tumor proliferation rates, we determined the correlation between tumor proliferation and the time from initial diagnosis to entry on the present study and found no association (Spearman rank correlation coefficient = −.03). Thus, this finding indicates that patients with lower tumor proliferation rates had received more prior regimens because they had shorter progression-free periods, suggesting greater drug resistance, as compared with those of patients with higher tumor proliferation rates.
We were interested in exploring whether there was a relationship between the tumor proliferation and drug resistance, PFS, and OS after EPOCH chemotherapy. To compare our results with those of a recent prospective study which showed that tumor proliferation ≥80% was associated with poor survival in aggressive NHL, we performed separate analyses that included all patients or was restricted to the intermediate-grade subset and treated tumor proliferation as either a continuous or a discontinuous (< or ≥80%) variable.16 A significant relationship between tumor proliferation and drug resistance was found. Including all patients, those with tumor proliferation rates of less than 80% were more likely to be drug-resistant (31% v 6%) or to fail to achieve CR (16% v 44%) than patients with higher proliferation (Table 4). These findings were somewhat more significant when tumor proliferation was treated as a continuous variable (P2 = .0062) or restricted to the intermediate-grade subset (P2 = .023). To assess the impact of p53 abnormality on these findings, we excluded all patients with p53 abnormalities and treated tumor proliferation as a continuous variable. As before, the analysis showed that resistant patients had significantly lower tumor proliferation rates than did sensitive patients; nonpartial, partial, or complete responders to EPOCH had mean ± SE tumor proliferation rates of 44.6% ± 6.4%, 53.8% ± 5.0%, and 73.5% ± 5.5%, respectively (P2 = .022). The association of tumor proliferation and drug resistance was not significant in the logistic regression analysis (Table 5).
The effect of tumor proliferation on PFS and OS was less striking. In patients with tumor proliferation rates of less than 80%, the median PFS was 6.3 months, as compared with 8.8 months for patients with proliferation rates ≥80% (P2 = .038). The median OS was 17.8 months in patients with tumor proliferation rates of less than 80% and 34 months in patients with higher proliferation, but this difference was not statistically significant. When the analysis was confined to the intermediate-grade subset, the median PFS and OS tended to be shorter in patients with lower tumor proliferation. None of these findings were significant in the Cox proportional hazards models (Table 6).
DISCUSSION
We undertook the present study of p53, bcl-2, and MIB-1 expression in relapsed NHL to assess the relationship of these markers to clinical drug resistance and clinical outcome. Although there are several published clinical studies on these markers, they have reached conflicting conclusions, and none assessed the relationship of these markers to drug resistance.3,4,8-12,18 19 Furthermore, a confounding variable in interpreting the published reports is that many have analyzed the relationship of individual markers with specific clinical endpoints such as survival and/or DFS and, thus, have not assessed whether the associations are independent of or reflect associations with known clinical prognostic factors. To address this, we examined the relationship of these markers to one another and to patient characteristics to determine their independent association with drug resistance and clinical outcome.
To specifically assess the relationship of these markers to drug resistance, we studied patients with relapsed (61%) or refractory (39%) NHL. We recognize that the heterogeneous nature of NHLs is a potential variable in interpreting the clinical endpoints of PFS and OS because these outcomes may be influenced by factors other than drug resistance, such as prior treatment and response, tumor bulk, performance status, and tumor biology. However, if p53, bcl-2 and tumor proliferation are involved in the fundamental biology of drug resistance in NHL, one might reasonably expect to see their effects across different histological subtypes and patient characteristics. Furthermore, the overlap of histologies and continuum in biology present among many lymphomas classified as low-, progressed low-, or intermediate-grade blurs this categorization.29 Nevertheless, we attempted to control for these variables in several ways. The distribution of p53 abnormalities, bcl-2 expression, and tumor proliferation among patient characteristics was assessed and not found to be associated with any specific clinical features that we believe would significantly bias these results. To control for possible differences among histological subtypes, we correlated all three markers with drug resistance and PFS and OS endpoints in all patients and in the subset of patients with intermediate-grade NHL.
A number of studies have examined the prevalence of p53 mutations in low- and intermediate-grade NHL. In the present study, evidence of p53 abnormality was found in 21% (16 of 75) of cases. However, other studies have reached conflicting conclusions. The first published study detected no mutations in 43 patients with low- or intermediate-grade lymphomas, whereas a recent study of 48 previously untreated patients found mutations in 19% of tumors.4,30 Several studies have associated p53 mutations with advanced-stage disease and histological progression of low-grade lymphomas, suggesting that p53 mutations are a late event in disease evolution.4,5 If correct, patients whose tumors harbor p53 mutations might be expected to have a shorter survival as compared with that of patients without mutations, possibly because of lead time bias. Indeed, two recent studies of mantle cell and aggressive B-cell lymphomas found that p53 overexpression was associated with decreased survival.9,12 The investigators speculated that the shorter survival was due to increased drug resistance, but other potentially important factors such as tumor bulk, performance status, tumor proliferation, and histological subtype (eg, blastic mantle cell) were not assessed.31 In contrast, another study recently assessed the prognostic significance of p53 overexpression in 165 patients with previously untreated diffuse large B-cell lymphomas and found no association with response or survival.10 However, because the investigators did not confirm the presence of p53 mutations by sequence analysis, it is unclear how accurately their immunohistochemical criteria for overexpression identified patients with mutations.
In the present study, we found that patients with p53 abnormalities were significantly more likely to be drug resistant (56%) than patients without abnormalities (17%). Furthermore, no correlations between p53 status and known clinical prognostic factors, bcl-2 expression, or tumor proliferation were found that could account for this result. The association of p53 abnormality with drug resistance was further strengthened by the multivariate analysis that showed it to be an independent predictor of drug resistance. Patients with p53 abnormalities also had significantly shorter PFS and OS. These results suggest that loss of normal p53 function in lymphoma leads to drug resistance. Furthermore, these results indicate that the decreased PFS and OS associated with p53 abnormalities are likely due to the effects of drug resistance and not to an increase in the tumor aggressiveness. Indeed, tumors with p53 abnormalities tended to have lower proliferation rates (Table 1), one indicator of tumor aggressiveness, than did those without abnormalities.
Because the p53 status of tumors will likely become an important clinical parameter in the future, an accurate and inexpensive test will be necessary. Thus, we compared the specificity and sensitivity of p53 immunohistochemistry, using a cutoff of 10%, with those of molecular techniques and found immunohistochemistry to be a reliable method. Of 14 cases with molecularly confirmed mutations, 79% overexpressed p53, and, of 43 cases without overexpression, only 7% had confirmed mutations. Of interest is that 1 of these latter cases had a stop codon mutation that might not be expected to result in overexpression.
We assessed the association of bcl-2 expression with drug resistance and found no association. However, among patients with only intermediate-grade histologies, 53% of those with high bcl-2 expression were drug-resistant, as compared with 20% of those with low bcl-2 expression. Although only of borderline significance, it suggested that high bcl-2 expression may lead to clinical drug resistance. We also noted that patients with high bcl-2 expression tended to have shorter PFS and OS, as compared with those of low-level expressors. These latter results are consistent with several recently published studies. In one study of 151 patients with diffuse large-cell lymphoma, high bcl-2 staining was significantly associated with shorter DFS and OS.8 Two additional studies similarly showed a significant association between high bcl-2 expression and shorter DFS, although, in one of these studies, presence of t(14; 18) had no significance on DFS or OS.10,11 These investigators hypothesized that high bcl-2 protein inhibits apoptosis, thereby producing drug resistance and shorter DFS and OS. However, a potential confounding variable not assessed by these investigators is our observation that bcl-2 expression and tumor proliferation are inversely correlated. Indeed, in the present study, patients with low tumor proliferation were significantly more likely to be drug-resistant and tended to have shorter PFS and OS, as compared with patients with high tumor proliferation. This observation raises the possibility that bcl-2 is a surrogate marker for tumor proliferation and has no independent effect on drug resistance. This latter result is consistent with a recent report showing a significant association between low tumor proliferation and the presence of t(14; 18) in low- and intermediate-grade NHL.32 Because the t(14; 18) generally results in high bcl-2 expression, the level of bcl-2 is most likely the biologically relevant mechanism.
There are conflicting reports regarding the effect of tumor proliferation on clinical outcome in NHL. Miller et al18 reported that tumor proliferation ≥80% was associated with poorer survival in previously untreated patients with aggressive NHL, whereas Hall et al19 found that patients who achieved a good response to chemotherapy were less likely to relapse if they had a tumor proliferation of greater than 80%. Our results are in agreement with those of the latter study and suggest that tumors with low proliferation may be less sensitive to chemotherapy than rapidly proliferating tumors. These findings are also in agreement with experimental observations that high tumor proliferation can be a significant determinant of drug sensitivity.15 16
It is worth noting that, in a previous study, we had quantitatively measured p-glycoprotein (Pgp) RNA, the pump responsible for the multidrug resistance (MDR-1) phenotype, in 32 patients from the present study.33 34 To assess if MDR-1 was a significant cause of drug resistance, we evaluated the relationship of pre- and post-EPOCH MDR-1 levels and their delta with resistance to EPOCH chemotherapy. Although the patient numbers are low, we found no significant association, suggesting that MDR-1 is not a major cause of drug resistance in this patient population.
The results of the present study are consistent with the hypothesis that normal p53 is important for the cytotoxic effects of chemotherapy in NHL and, conversely, that loss of p53 function (ie, mutations) leads to drug resistance. In contrast to p53, bcl-2 inhibits apoptosis, and, both experimentally and clinically, bcl-2 overexpression has been associated with drug resistance.6,8 However, compared with p53, bcl-2 has less effect in vitro on chemotherapy-induced apoptosis, and its activity may be modulated by heterodimerization with the BAX protein.35 Although we found a trend between bcl-2 expression and drug resistance, a more significant finding was the correlation of tumor proliferation and drug resistance. These findings suggest that tumor proliferation plays a role in the sensitivity (or, conversely, the resistance) of lymphomas to chemotherapy. One possible mechanism for this effect is that the threshold for apoptosis is influenced by tumor proliferation. In tumor samples, for example, high proliferation has been correlated with high rates of spontaneous apoptosis.36 Furthermore, preliminary experimental evidence suggests that resting cells may have lower levels of p53, as compared with dividing cells, and this may affect the threshold for chemotherapy-induced apoptosis. If correct, this could partially explain why slowly proliferating lymphomas such as low-grade NHL are incurable with chemotherapy. In this regard, an intriguing observation is that aggressive clones that develop in patients with low-grade lymphomas may be eradicated with combination chemotherapy.37 We have not infrequently observed that, compared with the low-grade component of the lymphoma, the aggressive clones have higher proliferation rates and lower bcl-2 expression. This observation is consistent with our finding of an inverse correlation between bcl-2 expression and tumor proliferation.
T.F. is supported in part by a grant from Philippe Foundation, Paris and New York.
Address reprint requests to Wyndham H. Wilson, MD, PhD, Medicine Branch, National Cancer Institute, Building 10, Room 12N-226, 9000 Rockville Pike, Bethesda, MD 20892.