Abstract
In the current study, we investigated the role of interleukin-2 (IL-2) and IL-4 as autocrine growth factors responsible for autonomous growth of four murine tumor cell lines: LSA, a radiation leukemia virus-induced T-cell lymphoma; EL-4, a chemically triggered T-cell lymphoma; PE-3T, a T-cell line that underwent spontaneous transformation ex vivo; and P815, a mastocytoma. All tumor cell lines screened constitutively expressed IL-2 receptor (IL-2R) and IL-4R genes. However, only LSA and PE-3T cells expressed IL-2 and IL-4 genes constitutively, whereas EL-4 and P815 tumor cells expressed only IL-4 but not IL-2. Monoclonal antibodies (MoAbs) against IL-2, IL-4, or a combination of these, as well as MoAbs against IL-2R significantly inhibited the proliferation of LSA but not that of other tumor cell lines ex vivo. To exclude the possibility that, in other tumor cell lines, the autocrine growth factor may interact with its receptor within the cell, the ability of antisense phosphorothioate oligonucleotides to inhibit the growth of the tumor cells was tested. The antisense phosphorothioate oligonucleotides specific for IL-2, IL-4, IL-2Rβ, or IL-2Rγ chains, added in culture, could markedly inhibit the growth of LSA but not that of the other tumor cell lines screened. Inasmuch as IL-2Rβ and IL-2Rγ subunits also serve as a component of the receptors for IL-4, IL-7, IL-9, and IL-15, the above data suggested that such cytokine redundancy was not responsible for autonomous growth of the other tumor cell lines. Addition of exogenous IL-2 or IL-4 to the tumor cell cultures caused significant enhancement in the proliferation of PE-3T cells, whereas other cell lines were either not significantly affected or slightly inhibited from growing. Interestingly, the LSA tumor growth in nude mice was significantly inhibited after treatment of these mice with a combination of MoAbs against IL-2 and IL-4. Together, our studies show for the first time that IL-2 and IL-4 may serve as autocrine growth factors in the autonomous proliferation of tumor cells, particularly those that are retrovirally induced. Second, some tumor cell lines, despite expressing certain cytokines and their receptors constitutively, may not depend exclusively on such factors for autocrine growth.
THERE IS INCREASING evidence to suggest that tumorigenic transformation of cells may result from aberrant regulation of autocrine growth factor production.1 In this autocrine mechanism, a cell may constitutively secrete and respond to the growth factor, resulting in tumorigenic transformation. Such a mechanism was first described for cells transformed by infection with transforming viruses.2,3 Recently, several factors were shown to contribute to the tumorigenicity of cells; these factors include: platelet-derived growth factor,4 epidermal growth factor/transforming growth factor-α,5,6,7 fibroblast growth factor family,8 interleukin-11 (IL-11),9 IL-6,10 tumor necrosis factor (TNF ),11 and so on. In addition to the above, IL-2, a major T-cell growth factor, has also been incriminated in the tumorigenic transformation of T cells.12 In particular, the IL-2 autocrine loop may play an important role in the development of T-cell lymphomas or adult T-cell leukemias by infection with human T-cell lymphotrophic viruses (HTLVs).13-15 Moreover, transfection of the IL-2 gene into T cells has also been shown to result in autonomous growth and tumorigenesis in vivo.16,17 In an earlier study, we reported that some normal T-cell lines, on long-term culture, underwent spontaneous transformation ex vivo and started growing autonomously.18 Such cells constitutively expressed IL-2 and IL-2 receptor (IL-2R) genes and could be inhibited from growing in vivo and ex vivo by agents that blocked IL-2 production or IL-2R expression, thereby suggesting that dysregulation in IL-2 production was sufficient to trigger transformation and tumorigenesis in vivo.18
IL-2R has been shown to be expressed not only by lymphoid cells but also by nonlymphoid cells.19-22 In fact, in a recent study that screened a variety of human solid tumor cell lines, it was reported that, in 36 of the 38 malignant tumors examined, IL-2R was constitutively expressed.23 These data suggested that IL-2R expression may be characteristic of most human malignant cells and that IL-2 may play a role in malignant cell proliferation through IL-2R.23 Although, IL-2R seems to be expressed on a wide variety of tumors, whether such tumor cells also constitutively express IL-2 or other cytokines that can act through the IL-2R, such as IL-4, IL-7, IL-9, and IL-15, and can be responsible for autocrine stimulation and growth, is not clear. In the current study, we systematically examined four murine transformed cell lines of both T- and non–T-cell origin that constitutively expressed IL-2R and delineated whether these cell lines expressed IL-2 and IL-4 genes constitutively and whether such cells were dependent on IL-2 or IL-4 for autocrine growth. Our data show that all four cell lines screened expressed IL-2R and IL-4R genes. However, only one of the in vivo originated tumor cell lines of T-cell origin was dependent on IL-2 and IL-4 for ex vivo autocrine growth as well as for in vivo tumorigenicity. These studies show that dysregulation in T-cell growth factor production and responsiveness can lead to cell transformation and tumorigenesis.
MATERIALS AND METHODS
Mice.Adult male athymic Nu/Nu nude mice were purchased from the National Institutes of Health (NIH; Bethesda, MD).
Tumor cell lines.LSA, is a radiation leukemia virus-induced T-cell lymphoma syngeneic to C57BL/6 mouse.24 This cell line has been extensively characterized and used in our previous studies.25 EL-4 is a chemically induced lymphoma also syngeneic to C57BL/6 mouse.26 P815 is a mastocytoma syngeneic to DBA/2 mouse.27 PE-3 is a CD8+ αβ T-cell receptor–positive cytotoxic T-cell line that was established from LSA tumor-bearing mice.28 This cytotoxic T-cell line was specific to LSA tumor and was originally maintained in culture by addition of exogenous IL-2 (50 U/mL) and occasional stimulation with X-irradiated LSA tumor cells.28 About 6 months after its establishment, this cell line underwent spontaneous transformation, started to grow autonomously in the absence of exogenous IL-2, and was designated PE-3T. The cell line was tumorigenic in vivo when injected into nude mice. All tumor cell lines were maintained by culturing them in tissue-culture flasks in RPMI-1640 medium with additional supplements and 10% fetal bovine serum as described.18 It should be noted that LSA, EL-4, and P815 tumor cell lines readily grow in normal syngeneic hosts, whereas the PE-3T cell line is tumorigenic only in immunodeficient but not in the normal syngeneic host.
Antibodies (Abs).The monoclonal antibodies (MoAbs) used were in culture supernatants as described in detail elsewhere.18,29,30 The MoAbs against IL-2 were obtained from hybridoma S4B6; anti–IL-2R was obtained from PC61.5.3 and 7D4; and anti–IL-4 was obtained from 11B11. The MoAb against IL-4R was purchased from Genzyme (Cambridge, MA). The 11B11 hybridoma was kindly provided by Dr W. Paul (NIH). Because S4B6, PC61.5.3, and 11B11 are rat-IgG hybridomas, normal rat-IgG (Jackson Immunoresearch Laboratories Inc, Baltimore, MD) was used as a control. All other hybridomas were purchased from American Type Culture Collection (ATCC; Rockville, MD). All MoAbs were concentrated using Amicon filtration (Amicon, Danvers, MA) as described.18 For the in vivo treatment, the concentrated hybridoma supernatants were precipitated using ammonium sulfate followed by extensive dialysis in phosphate-buffered saline.
ILs.Recombinant human IL-2 (rIL-2) was kindly provided by Dr M. Gately (Hoffmann-LaRoche, Nutley, NJ). Recombinant murine IL-4 (rIL-4) was generously provided by Dr C.W. Reynolds (NIH).
Flow cytometric analysis of IL-2R or IL-4R expression.The expression of IL-2R or IL-4R by tumor cells was determined by immunofluorescence.28 Briefly, for IL-2R, 1 × 106 tumor cells were washed in phosphate-buffered saline containing 0.1% sodium azide and incubated at 4°C for 30 minutes in a test tube with Abs directed against IL-2R (7D4). After washing the cells twice, fluorescein isothiocyanate (FITC)-conjugated secondary Ab consisting of F(ab′ )2 fragments of antirat IgM (Cappel, Durham, NC) was added, and the cells were incubated on ice for 30 minutes. The cells were washed twice and analyzed using a flow cytometer (Epics V, model 752; Coulter, Hialeah, FL). The negative controls consisted of cells incubated with normal rat IgM followed by FITC-conjugated secondary Abs. The same procedure was used for IL-4R expression except for the following: in the primary incubation, we used MoAbs against IL-4R followed by FITC-conjugated F(ab′ )2 fragments of antirat IgG (Cappel).
Cell proliferation.The proliferation of the cells in culture was studied by enumerating viable cells using trypan-blue dye exclusion assay.18 The cells were cultured in 96-well tissue-culture plates at a concentration of 5 × 104 cells/well in 0.2 mL complete RPMI-1640 medium supplemented with 10% fetal calf serum. To these cultures, various concentrations of MoAbs against the growth factors or their receptors or antisense oligonucleotides were added. The anti–IL-2, anti–IL-4, and anti–IL-2R MoAbs were tested at 1:2, 1:4, and 1:10 dilutions of concentrated culture supernatants, and antisense oligonucleotides were tested at 25, 50, and 100 μmol/L concentrations. These concentrations were based on their ability to inhibit the IL-2– and IL-4–induced proliferation of HT-2 cells and autonomous growth of IL-2 autocrine-dependent proliferation of AutoD1.4T cells.18 25 The cultures were incubated at 37°C for 1 to 3 days, and, every 24 hours, the cells were enumerated for viability and growth. To accommodate for the growth, the cultures were split every 24 hours, and all cultures received fresh medium and additional growth factor antagonists.
In addition, in some experiments, the cell proliferation was also measured by 3H-thymidine incorporation assay as described.18 Briefly, the cells were cultured in 96-well tissue-culture plates as described above and were pulsed with 0.1 μC of 3H-thymidine followed by cell harvesting 8 hours later. The radioactivity was measured using a liquid scintillation counter. In experiments studying the effect of cyclosporin (CsA; kindly provided by Sandoz Pharmaceutical, Sandoz Research Institute, East Hanover, NJ) on cell proliferation, CsA was prepared by dissolving 1 mg of CsA in 0.1 mL ethanol and 0.02 mL of Tween-80, followed by the addition of 1 mL of RPMI-1640. The medium used for dissolving the highest concentration of CsA was used as a vehicle control.18
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of cytokine and cytokine receptor gene expression.The RT-PCR method was used to study whether the transformed cells constitutively express IL-2, IL-4, IL-2R, and IL-4R genes as described in detail elsewhere.18 29 Briefly, total RNA was extracted from the cells at various time intervals of cell culture and was reverse-transcribed. The cDNA samples were subjected to PCR amplification using synthetic oligonucleotide primers for various cytokine and their receptor genes, with β-actin serving as an internal control. The following oligonucleotide primer sequences were used to amplify: for the IL-2 gene, 5′-TTCAAGCTCCACTTCAAGCTCTACAGCGGAAG and 3′-GACAGAAGGCTATCCATCTCCTCAGAAAGTCC; for the IL-4 gene, 5′-CCAGCTAGTTGTCATCCTGCTCTTCTTTCTCG and the 3′-CAGTGATGTGGACTTGGACTCATTCATGGTGC; for the IL-4R gene, 5′-GCTTCTCTGACTACATCCGCACTTCCACG and the 3′-TTGACTCCTGGCTTCGGGTCTGCTTATCC; for the β-actin gene, 5′-TATCCTGACCCTGAAGTACCCCATT and 3′-AGCACAGCTTCTCTTTGATGTCACG; and for the IL-2R β-chain p75, 5′-TGGAGAACAGGATGACTACTGTGC and 3′-GACCAGGAAGTAGCAAATGGAGTTC. The primers for IL-2R and β-actin were synthesized by Oligos Etc (Wilsonville, OR), and the primers for IL-2, IL-4, and IL-4R were purchased from Clontech (Palo Alto, CA). The PCR product was electrophoresed through a 1.5% agarose gel containing ethidium bromide. The demonstration of a single 464-, 413-, 384-, 545-, 792-bp band was considered to be indicative of the expression of β-actin, IL-2, IL-4, IL-2Rβ-chain p75, and IL-4R genes, respectively.
Antisense oligonucleotides.The antisense oligonucleotides specific for IL-2, IL-4, IL-2Rβ-chain p75 and IL-2Rγ-chain p64 genes were designed to hybridize at sequences immediately downstream from the initiation codon of the mRNA of different genes. Because different cell lines may have different exonuclease or endonuclease activities, the selected oligonucleotides were modified to be nuclease-resistant by making them 100% phosphorothioated.31,32 The antisense oligonucleotide sequences were as follows: for IL-2 gene, 5′ GAGCTGCATGCTGTA 3′; for IL-4 gene, 5′ CTGGGGGTTGAGACC 3′; for IL-2Rβ gene, 5′ AAGAGCTATGGTAGC 3′; and for IL-2Rγ gene, 5′ CAATAATAGTTTCAA 3′. The oligonucleotides were dissolved in Tris-EDTA buffer at pH 7.4 and added directly at different concentrations to cultures of tumor cells. Inasmuch as controls, such as oligonucleotides that have the same base composition but a randomized sequence, often produce biological effects that are indistinguishable from the antisense oligonucleotides,33 we used nuclease-resistant oligonucleotides and cell lines that were independent of IL-2– or IL-4–induced autocrine growth as controls for any nonspecific action of the phosphorothioate oligonucleotides.
In vivo tumor induction.The LSA or P815 tumor cells (0.5 × 106) were injected into nude mice subcutaneously. These mice received 650 μg of anti–IL-2 and 650 μg of anti–IL-4 MoAbs once every other day intraperitoneally for 16 days. The control mice received similar concentrations of normal rat-IgG. The tumor growth was monitored daily, and, after 17 days, the mice were killed and the tumor growth was assessed by surgically removing the tumors and measuring the weight. In all experiments, groups of four mice were used.
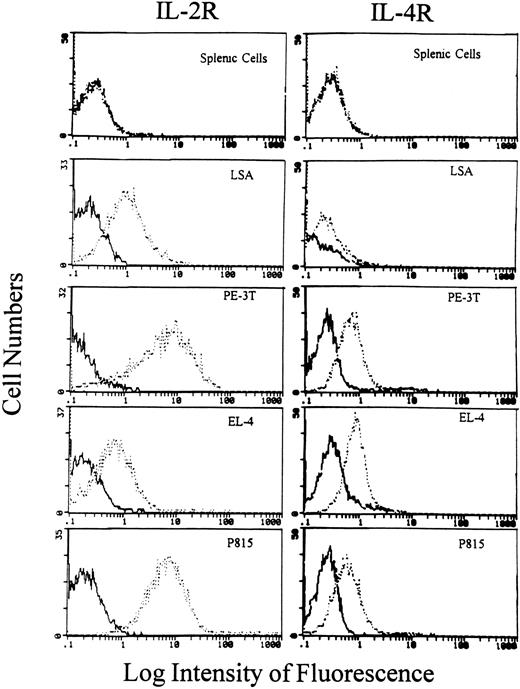
Flow cytometric analysis of IL-2R and IL-4R expression on tumor cells. Various tumor cells as well as normal C57BL/6 splenic cells were incubated with anti–IL-2R MoAbs (7D4) or anti–IL-4R MoAbs followed by FITC-conjugated secondary Abs as described in Materials and Methods. The cells were analyzed using a flow cytometer. The bold histogram represents the negative control, and the broken histogram shows cells stained for IL-2R or IL-4R expression.
Flow cytometric analysis of IL-2R and IL-4R expression on tumor cells. Various tumor cells as well as normal C57BL/6 splenic cells were incubated with anti–IL-2R MoAbs (7D4) or anti–IL-4R MoAbs followed by FITC-conjugated secondary Abs as described in Materials and Methods. The cells were analyzed using a flow cytometer. The bold histogram represents the negative control, and the broken histogram shows cells stained for IL-2R or IL-4R expression.
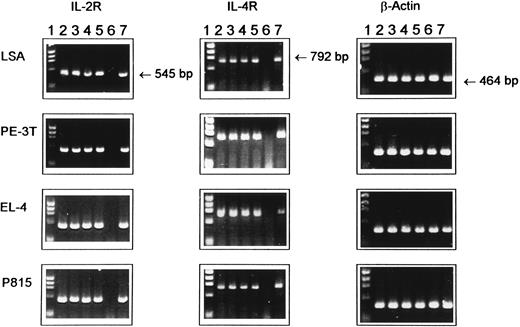
Expression of IL-2R and IL-4R mRNA in tumor cell lines as detected by RT-PCR. Total RNA was extracted from various cell lines at 12, 24, 36, or 48 hours of ex vivo culture and was reverse-transcribed, and cDNA samples were subjected to PCR amplification using synthetic oligonucleotide primers for IL-2R, IL-4R, and β-actin as an internal control. The PCR product was electrophoresed through 1.5% agarose gel containing ethidium bromide. Lane 1, molecular size standard; lanes 2 through 5, cytokine gene expression at 12, 24, 36, and 48 hours of culture, respectively; lane 6, negative control consisting of normal unstimulated spleen cells from C57BL/6 mice; and lane 7, spleen cells cultured with ConA for 8 hours, used as a positive control.
Expression of IL-2R and IL-4R mRNA in tumor cell lines as detected by RT-PCR. Total RNA was extracted from various cell lines at 12, 24, 36, or 48 hours of ex vivo culture and was reverse-transcribed, and cDNA samples were subjected to PCR amplification using synthetic oligonucleotide primers for IL-2R, IL-4R, and β-actin as an internal control. The PCR product was electrophoresed through 1.5% agarose gel containing ethidium bromide. Lane 1, molecular size standard; lanes 2 through 5, cytokine gene expression at 12, 24, 36, and 48 hours of culture, respectively; lane 6, negative control consisting of normal unstimulated spleen cells from C57BL/6 mice; and lane 7, spleen cells cultured with ConA for 8 hours, used as a positive control.
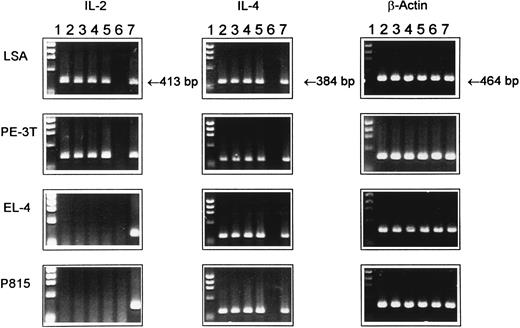
Expression of IL-2 and IL-4 mRNA in tumor cell lines as detected by RT-PCR. Total RNA was extracted from various cell lines at 12, 24, 36, or 48 hours of ex vivo culture and was reverse-transcribed, and cDNA samples were subjected to PCR amplification using synthetic oligonucleotides primers for IL-2, IL-4, and β-actin as an internal control. The PCR product was electrophoresed through 1.5% agarose gel containing ethidium bromide. Lane 1, molecular size standard; lanes 2 through 5, cytokine gene expression at 12, 24, 36, and 48 hours of culture, respectively; lane 6, negative control consisting of normal unstimulated spleen cells from C57BL/6 mice; and lane 7, spleen cells cultured with ConA for 8 hours, used as a positive control.
Expression of IL-2 and IL-4 mRNA in tumor cell lines as detected by RT-PCR. Total RNA was extracted from various cell lines at 12, 24, 36, or 48 hours of ex vivo culture and was reverse-transcribed, and cDNA samples were subjected to PCR amplification using synthetic oligonucleotides primers for IL-2, IL-4, and β-actin as an internal control. The PCR product was electrophoresed through 1.5% agarose gel containing ethidium bromide. Lane 1, molecular size standard; lanes 2 through 5, cytokine gene expression at 12, 24, 36, and 48 hours of culture, respectively; lane 6, negative control consisting of normal unstimulated spleen cells from C57BL/6 mice; and lane 7, spleen cells cultured with ConA for 8 hours, used as a positive control.
Statistical analysis.The experimental groups were compared with the controls using Student's t-test, and P < .05 values were considered to be statistically significant.
RESULTS
Detection of IL-2R and IL-4R on the tumor cell lines.In the current study, we used four tumor cell lines, two of which were previously characterized as T-cell lymphoma lines originated in vivo (LSA and EL-4); a third cell line (PE-3T) was also of T-cell origin but was transformed ex vivo, and a fourth cell line was characterized as a mastocytoma (P815) that originated in vivo. Initially, we screened these cell lines for phenotypic expression of IL-2R and IL-4R, as shown in Fig 1. All the four cell lines screened expressed the IL-2R and IL-4R; therefore, these cell lines were further used in our study for the analysis of the constitutive expression of IL-2, IL-4, and their receptor gene expression. In these experiments, normal spleen cells served as a negative control.
RT-PCR analysis of the expression of IL-2R and IL-4R genes.Using PCR analysis, it was observed (Fig 2) that the LSA, PE-3T, EL-4, and P815 tumor cell lines expressed IL-2R and IL-4R at all time intervals tested such as at 12, 24, 36, and 48 hours of culture, thereby showing that these cell lines constitutively expressed the IL-2R and IL-4R genes. In this experiment, β-actin served as a positive internal control. In addition, unstimulated spleen cells from C57BL/6 mice served as a negative control for the growth factor receptors and spleen cells stimulated with concanavalin A (ConA) served as a positive control for the expression of the growth factor receptors.
Constitutive expression of IL-2 and IL-4 genes in tumor cells.Using RT-PCR analysis we further addressed whether the tumor cell lines under investigation constitutively expressed IL-2 and IL-4 mRNA. The data shown in Fig 3, indicated that LSA and PE-3T tumor cells expressed IL-2 and IL-4 mRNA, whereas EL-4 and P815 tumor cells expressed only the IL-4 but not IL-2 gene. These data suggested that tumor cell lines, including those of T-cell origin, despite expressing IL-2R, do not always express IL-2 gene. Also, it was interesting to note that all tumors cell lines screened constitutively expressed IL-4 gene, thereby suggesting the possibility that IL-4 may serve as an autocrine growth factor.
It should be noted that we were unable to detect significant levels of IL-2 or IL-4 in the culture supernatants of these tumor cell lines. This may result from a number of factors discussed subsequently.
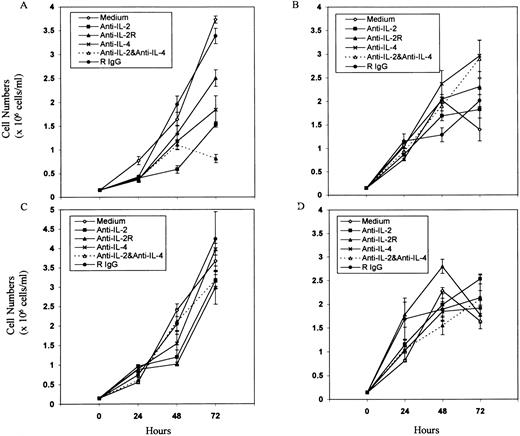
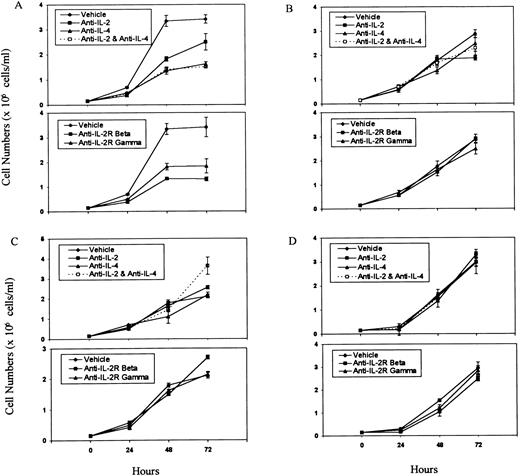
Effect of the addition of MoAbs against growth factors or their receptors on tumor cell growth ex vivo.Having established that some of the tumor cell lines screened constitutively expressed IL-2, IL-4, and their receptors, we next screened whether addition of MoAbs against these growth factors or their receptors would inhibit the autonomous growth ex vivo. To this effect, various concentrations of anti–IL-2, anti–IL-2R, or anti–IL-4 MoAbs or a combination of Abs against IL-2 and IL-4 were used in cultures, and a representative experiment using one of the concentrations of these Abs is shown in Fig 4. As a control, in these studies, normal rat IgG was used in addition to the medium control. The concentrations of Abs against IL-2, IL-2R, and IL-4 were previously shown to inhibit the IL-2– or IL-4–induced growth of HT-2 cells. The MoAbs added did not have a statistically significant inhibitory effect on the growth of tumor cells when screened for a period of 72 hours on P815, EL-4, and the PE-3T (Figs 4B through D). Interestingly, however, Abs against IL-2, IL-2R, and IL-4 or a combination of Abs against IL-2 and IL-4 dramatically inhibited the ex vivo growth of LSA tumor cell line, particularly by 72 hours of culture (P < .005; Fig 4A). Similar results were obtained when cell proliferation was measured by 3H-thymidine uptake assay (data not shown). These data showed that IL-2 and IL-4 may serve as autocrine growth factors regulating LSA tumor cell proliferation but not that of other tumor cell lines screened, despite the fact that other tumors constitutively expressed certain growth factors or growth factor receptors. The fact that combination of anti–IL-2 and anti–IL-4 Abs could inhibit the LSA tumor cell proliferation better than either of the Abs alone suggested that both IL-2 and IL-4 were involved in growth regulation.
Effect of addition of Abs against T-cell growth factors or their receptors on tumor cell proliferation. Tumor cell lines LSA (A), PE-3T (B), EL-4 (C), and P815 (D) were cultured in the presence of MoAbs against IL-2, IL-2R, IL-4, or a combination of anti–IL-2 and anti–IL-4 at 1:4 dilution of purified Abs for 0 to 72 hours. Cells incubated with media alone or with normal rat IgG were used as controls. The cell proliferation was measured by counting the viable cells using trypan-blue dye exclusion. Means of cell numbers from experimental groups and the control ± SEM were plotted.
Effect of addition of Abs against T-cell growth factors or their receptors on tumor cell proliferation. Tumor cell lines LSA (A), PE-3T (B), EL-4 (C), and P815 (D) were cultured in the presence of MoAbs against IL-2, IL-2R, IL-4, or a combination of anti–IL-2 and anti–IL-4 at 1:4 dilution of purified Abs for 0 to 72 hours. Cells incubated with media alone or with normal rat IgG were used as controls. The cell proliferation was measured by counting the viable cells using trypan-blue dye exclusion. Means of cell numbers from experimental groups and the control ± SEM were plotted.
The effect of addition of antisense phosphorothioate oligonucleotides (APOs) specific for the growth factors or their receptors on cell proliferation ex vivo.It was possible that the reason why MoAbs against the growth factors or their receptor may not have inhibited the proliferation of PE-3T, EL-4, and P815 cells was that, in these cell lines, the growth factor may not be actively secreted outside the cell; thus, the growth factor may interact with its receptor within the cell, which may then lead to autocrine stimulation and proliferation.1 To address this possibility, we used various concentrations of APOs, specific to various growth factors and their receptors, which would specifically inhibit the endogenous gene activation. The representative data using 20 μmol/L of APOs, shown in Fig 5, showed that the APOs specific for IL-2, IL-4, and the combination of IL-2 and IL-4 or against the IL-2Rβ or IL-2Rγ chains failed to inhibit the proliferation of the PE-3T, EL-4, and P815 cells. However, when used against LSA cells, the APOs specific for IL-2, IL-4, or the combination of these as well as those against IL-2Rβ or IL-2Rγ chains could markedly inhibit the growth of the LSA tumor cell line (P < .05; Fig 5A). These data corroborated the results obtained using MoAbs against the growth factors or their receptors and showed that, of the four cell lines screened, only the LSA tumor cells were using both IL-2 and IL-4 as autocrine growth factors for the ex vivo cell proliferation.
Effect of APOs on tumor cell proliferation. Tumor cell lines LSA (A), PE-3T (B), EL-4 (C), and P815 (D) were incubated with 20 μmol/L concentration of APOs for 0 to 72 hours. The viable cell number was calculated as described in Fig 4. Means of cell numbers from experimental groups and the control ± SEM were plotted.
Effect of APOs on tumor cell proliferation. Tumor cell lines LSA (A), PE-3T (B), EL-4 (C), and P815 (D) were incubated with 20 μmol/L concentration of APOs for 0 to 72 hours. The viable cell number was calculated as described in Fig 4. Means of cell numbers from experimental groups and the control ± SEM were plotted.
Effect of CsA on tumor cell growth.To address the possibility that PE-3T, EL-4, and P815 tumor cells used some other cytokines for autonomous growth, we tested the effect of CsA on these cell lines because CsA is known to block the synthesis of a variety of cytokines.34 The results of these studies showed that CsA caused a dose-dependent inhibition of the proliferation of all the tumor cell lines tested (data not shown).
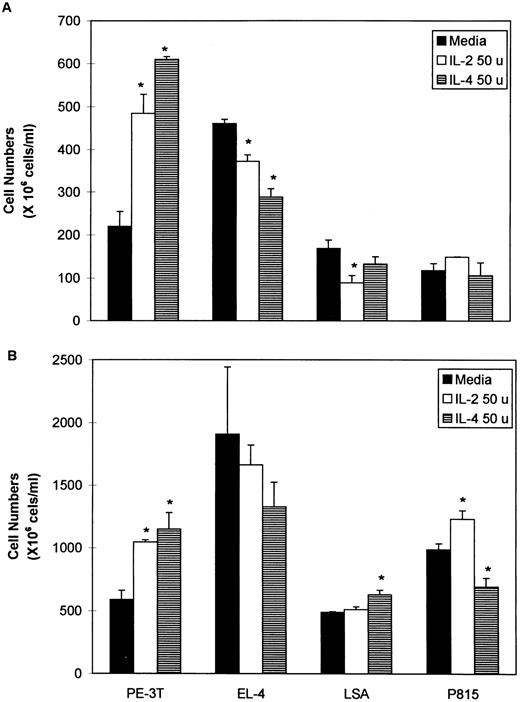
Effect of addition of exogenous IL-2 and IL-4 on tumor cell proliferation.Because the tumor cell lines screened expressed IL-2R and IL-4R, we next addressed whether addition of exogenous IL-2 or IL-4 would influence the growth of the tumor cells in culture. To this effect, 50 or 100 U of rIL-2 or rIL-4 were added to tumor cell cultures that were next studied for growth for 24 to 48 hours. The representative data using 50 U of ILs is shown in Fig 6. These data showed that exogenous IL-2 or IL-4 have varying effects on the growth of the cell lines. Interestingly, IL-2 and IL-4 caused a dramatic increase in the proliferation of the ex vivo transformed cell line, PE-3T. On EL-4 and LSA, the ILs caused a significant inhibition at 24 hours, but the cells recovered by 48 hours. With P815, the ILs failed to cause significant alterations in growth at 24 hours, whereas, at 48 hours, IL-2 caused an increase and IL-4 caused a decrease in cell proliferation. Together, these data suggested that addition of exogenous growth factors can have varying effects on tumor cell lines that express the receptors for such growth factors.
Effect of addition of rIL-2 or rIL-4 on tumor cell proliferation. Tumor cell lines, LSA, PE-3T, EL-4, and P815 were cultured in the presence of 50 U (50 u) of rIL-2 or rIL-4 for 24 (A) or 48 (B) hours. Cells incubated with media alone were used as a control. The cell proliferation was measured by counting the viable cells using trypan-blue dye exclusion. Means of cell numbers from experimental groups and the control SEM were plotted. Statistically significant differences (P < .05) from the control values have been indicated with an asterisk.
Effect of addition of rIL-2 or rIL-4 on tumor cell proliferation. Tumor cell lines, LSA, PE-3T, EL-4, and P815 were cultured in the presence of 50 U (50 u) of rIL-2 or rIL-4 for 24 (A) or 48 (B) hours. Cells incubated with media alone were used as a control. The cell proliferation was measured by counting the viable cells using trypan-blue dye exclusion. Means of cell numbers from experimental groups and the control SEM were plotted. Statistically significant differences (P < .05) from the control values have been indicated with an asterisk.
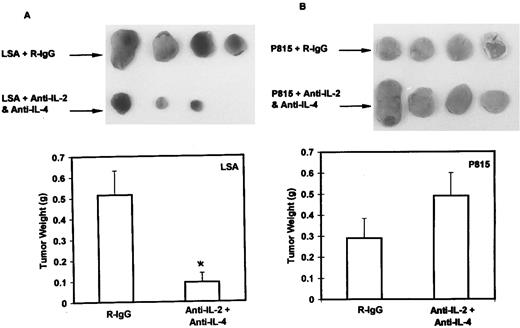
Inhibition of in vivo tumorigenicity of LSA using MoAbs against IL-2 and IL-4.After showing that the ex vivo growth of LSA is mediated by the autocrine stimulation of both IL-2 and IL-4, we investigated whether the in vivo tumorigenicity of LSA was also mediated by production of autocrine growth factors. Because treatments that antagonize IL-2 and IL-4 would interfere with antitumor immunity of the immunocompetent host, this possibility was tested in immunodeficient, nude mice. To accomplish this, we induced subcutaneous LSA tumors in nude mice, and, on the same day as tumor challenge, these mice were injected with normal rat-IgG as a control or with a combination of equal amounts of MoAbs against IL-2 and IL-4 as described in Materials and Methods. The subcutaneously injected tumors grew as localized solid tumors without undergoing metastasis. As shown in Fig 7A, a dramatic inhibition of LSA tumor growth in the nude mice treated with the combination of MoAbs against the growth factors was observed. Interestingly, one of the mice treated with Abs against IL-2 and IL-4 did not show any tumor growth. These experiments were repeated with consistent results in which control mice showed a mean tumor weight of 0.33 ± 0.10 g, whereas the experimental group treated with MoAbs against growth factor showed a mean tumor weight of 0.16 ± 0.06 g. It should be noted that MoAbs against IL-2 or IL-4 alone were not effective in inhibiting the LSA tumor growth in vivo (data not shown). Also, similar treatment of P815 tumor cell in nude mice failed to cause significant inhibition of in vivo growth (Fig 7B). These results show that LSA tumor cells depended on IL-2 and IL-4 for tumorigenesis even in vivo.
Abs to IL-2 and IL-4 can inhibit the in vivo growth of LSA but not that of P815 in nude mice. Groups of four nude mice injected with LSA (A) or P815 (B) subcutaneously were treated on the same day and were treated thereafter for 16 days with intraperitoneal injections of normal rat IgG (R-IgG; control) or with a combination of MoAbs against IL-2 and IL-4, on alternate days. On the seventeenth day, tumors were surgically removed and weighed. The vertical bars represent the mean ± SE values of tumor weight. Statistically significant differences (P < .05) from the control values have been indicated with an asterisk. It should be noted that, in one LSA-bearing nude mouse treated with anti–IL-2 and anti–IL-4 Abs, the tumor completely failed to grow, and the mouse survived indefinitely.
Abs to IL-2 and IL-4 can inhibit the in vivo growth of LSA but not that of P815 in nude mice. Groups of four nude mice injected with LSA (A) or P815 (B) subcutaneously were treated on the same day and were treated thereafter for 16 days with intraperitoneal injections of normal rat IgG (R-IgG; control) or with a combination of MoAbs against IL-2 and IL-4, on alternate days. On the seventeenth day, tumors were surgically removed and weighed. The vertical bars represent the mean ± SE values of tumor weight. Statistically significant differences (P < .05) from the control values have been indicated with an asterisk. It should be noted that, in one LSA-bearing nude mouse treated with anti–IL-2 and anti–IL-4 Abs, the tumor completely failed to grow, and the mouse survived indefinitely.
DISCUSSION
In the current study, we investigated four murine cell lines of different origins for their ability to grow ex vivo using IL-2 and IL-4 as autocrine growth factors. It was interesting to note that all four cell lines screened constitutively expressed both IL-2R and IL-4R genes. However, when expression for the growth factors was screened, it was observed that LSA and PE-3T, but not EL-4 and P815, cells constitutively expressed the IL-2 gene. In contrast, IL-4 gene was expressed constitutively by all four cell lines screened. Despite this, it was noted that the cell lines markedly differed in their ability to show IL-2– or IL-4–dependent autocrine growth. For example, of the four cell lines screened, only LSA was dependent on IL-2 and IL-4 for autonomous growth ex vivo, whereas none of the other cell lines screened could be inhibited by MoAbs against either the growth factors, IL-2 and IL-4, or their receptors.
It should be noted that, in the current study, we used RT-PCR to show constitutive expression of growth factor genes, because we were unable to show detectable levels of these cytokines secreted in culture using enzyme-linked immunosorbent assay. The detection of growth factors may be difficult if the cells use these factors for autonomous growth, as previously observed with other cell lines in our laboratory.30 Alternatively, the cytokine may not be secreted and may be localized to the cytoplasm, nucleus, or the cell membrane. However, the constitutive expression of IL-2 and IL-4 genes as shown using RT-PCR and the ability of anti–IL-2 and anti–IL-4 MoAbs or antisense to inhibit the LSA tumor growth ex vivo or in vivo, show that this cell line was using IL-2 and IL-4 for autonomous growth.
The reason why some tumor cells, despite expressing the growth factor or their receptor genes constitutively, fail to show an autocrine pattern of growth is not clear. It can be speculated that there may be other growth factors and their receptors that were not screened in this study that may be involved in the autonomous growth of these cells. Second, it is possible that these cell lines are independent of autocrine growth factors for their proliferation. Third, multiple cytokines such as IL-1, IL-6, TNF-α, and TNF-β have been shown earlier to act synergistically to maintain the growth of transformed cell lines.35 Thus, blocking a few cytokines may not be sufficient to inhibit the tumor cell growth. In this context, it is interesting to note that, in an earlier study, we observed that a spontaneously ex vivo transformed T-cell clone, similar to PE-3T cells, expressed constitutively IL-2 and IL-2R genes and was completely inhibited from growing ex vivo, as well as inducing tumors in vivo, in the presence of IL-2 or IL-2R antagonists.18
In the current study, we also tested whether addition of exogenous IL-2 or IL-4 to the tumor cells would affect their growth ex vivo. These data showed that IL-2 and IL-4 enhanced the proliferation of PE-3T cells and had varied effects on other cell lines, particularly inhibiting the growth of LSA and EL-4 cells slightly. These data suggested that PE-3T cells produce low levels of IL-2 and IL-4; therefore, the addition of exogenous growth factors may facilitate their growth. As regards other cell lines, previous studies have shown that exogenous IL-2 may in fact inhibit the growth of IL-2R+ tumor cells.50 The mechanism involved in such differential effects of growth factors needs further elucidation.
Recently, the results of a number of studies have shown that IL-2R is expressed on a wide variety of human lymphoid as well as nonlymphoid tumor cells.23 Furthermore, secretion of IL-2 by such malignant cells has also been used to suggest that IL-2 may play an important role as an autocrine growth factor in the proliferation of malignant cells in vivo.23 However, in addition to IL-2, a variety of other cytokines may share a common receptor, thereby making it difficult to treat such tumors in vivo if the tumor cell growth is dependent on multiple cytokines. For example, IL-2R consists of three subunits the α, β, and γ chains.36 IL-2Rβ is a subunit of IL-15R and IL-2Rγ is a subunit for IL-4R, IL-7R, IL-9R, and IL-15R.37-40 Thus, such redundancy in the cytokine function may make it difficult to identify and treat tumors that are dependent on multiple cytokines for autonomous growth in vivo. However, in the current study, we observed that APOs against IL-2Rβ or IL-2Rγ subunits blocked LSA proliferation but not that of other tumor cell lines. Because these subunits are also a component of receptors for IL-4, IL-7, IL-9, and IL-15,41 we can rule out the possible involvement of these cytokines. However, the ability of CsA to inhibit the growth of all such cell lines supports the notion that cytokines other than those mentioned above may participate in the autocrine regulation.
It is becoming increasingly clear that IL-2 may serve as an autocrine growth factor responsible for maintenance and proliferation of human T cells in vivo. The possibility that IL-2 may be involved in the autocrine growth factor-dependent cell transformation was suggested in a human T-cell lymphoma line derived from patient with adult T-cell leukemia.12 Further studies in adult T-cell leukemia patients as well as in T-cell lines infected with HTLV have suggested that autocrine IL-2–induced self-stimulation may play an important role in T-cell transformation.13-15 Furthermore, IL-2 transcript and protein were detected at the single cell level in most cells of HTLV-1 infected T-cell lines.42 HTLV-1 contains a region called pX that encodes for at least two regulatory proteins, tax and rex.43,44 Tax is a potent transcriptional transactivator of the provirus LTR; furthermore, it can transactivate and induce the expression of a number heterologous cellular genes, some of which are also involved in T-cell growth proliferation and possibly transformation.45 The cellular genes that are known to be transactivated by tax include IL-2, IL-2Rα, IL-3, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF ), and TNF-β. Tax has also been shown to transactivate the promoters of a number of oncogenes. Thus, the transactivation of IL-2 and IL-2R has led to the hypothesis that constitutive production and responsiveness to autocrine IL-2 may be responsible for HTLV-1 induced T-cell transformation and the development of adult T-cell leukemia. In this context, it is interesting to note that the LSA tumor cell line that originated in vivo after low-dose radiation treatment is a murine retrovirus-induced tumor and, therefore, may share similar characteristics with HTLV in being able to activate cytokine genes and trigger autonomous growth.
Despite examples in which IL-2 or other growth factors have been shown to be involved in cell transformation, it is not proven that such growth factor production is totally responsible for the maintenance of neoplastic transformation in vivo.1 However, in a previous study we showed that the induction of tumors in vivo caused by an ex vivo transformed tumor cell line, AutoD1.4T, was completely inhibited by injection of MoAbs against IL-2 and IL-2R, thereby showing that IL-2 can serve as an exclusive growth factor responsible for the maintenance of neoplasia in vivo.18 The present study extends our earlier observation and shows for the first time that an in vivo originated lymphoma cell line is also dependent on T-cell growth factors IL-2 and IL-4 for in vivo growth. The fact that in one of the four mice studied the LSA tumor cells failed to grow suggests that IL-2 and IL-4 may be the sole factors responsible for maintenance of tumor growth in vivo.
The data from the current and previous studies18 have significant implications in immunotherapy of cancer and viral infections. It has been suggested that administration of tumor- or virus-specific T cells may offer an effective way to treat cancer and certain viral infections.46,47 However, studies from our laboratory have shown that, during ex vivo culture, mutants may originate that use autocrine growth factors for cell transformation capable of inducing tumors in the immunodeficient host.18 In a recent study by Treisman et al,48 the investigators showed that a T-cell line retrovirally transduced with cDNA for IL-2 was able to grow in an autocrine fashion, and this cell line was able to maintain antigen-specific responsiveness and failed to induce tumors in normal syngeneic mice. The investigators suggested that such cell lines can be used for immunotherapy, thereby obviating the need to administer IL-2 for the maintenance of the T-cell lines in vivo. In these studies, however, the investigators did not address whether the IL-2–transduced lymphocytes would induce tumors in vivo in immunodeficient mice. For example, we noted that the ex vivo transformed T-cell lines namely, the AutoD1.4T and PE-3T, were not able to grow in normal mice but were able to induce tumors in immunodeficient, nude mice.18 Thus, careful consideration should be given to immunotheraputic approaches that use T cells transfected with growth factor gene, particularly in the immunodeficient host.
The reason why ex vivo transformed T-cell lines (such as AutoD1.4T and PE-3T) that produce IL-2 and IL-4 constitutively fail to induce tumors in normal syngeneic host but do so in immunodeficient host is not clear. However, the results of preliminary studies showed that the ex vivo transformed cell lines can trigger a strong immune response mediated both by natural killer cells and cytotoxic T cells, thereby preventing them from growing in vivo.18 The strong antitumor immunity induced by the ex vivo transformed cell lines may have resulted from the active secretion of IL-2, IL-4, or other cytokines by these cells when injected into normal mice. Such an observation has also been made by other investigators who have tried to transfect tumor cell lines with IL-2 and other growth factor genes.49 These findings raise the question of why tumors originated in vivo, such as LSA or HTLV-induced T-cell tumors, known to depend on IL-2 autocrine stimulation, fail to trigger a strong immune response in the patient and succeed in inducing malignancies. There could be multiple explanations for this phenomenon, and one important factor could be that the in vivo transformed T-cell lines that constitutively express IL-2 and depend on IL-2 for malignancy may also produce certain immunosuppressive cytokines that may suppress the antitumor immunity and facilitate the growth of these tumor cells in vivo. Preliminary studies performed in our laboratory have shown that, whereas the in vivo transformed cell line LSA produces constitutively transforming growth factor-β and IL-10, the ex vivo transformed cell lines AutoD1.4T and PE-3T do not produce transforming growth factor-β and express low levels of IL-10. This difference may provide one possible explanation as to how a cell may use IL-2 for autocrine growth and transformation and simultaneously use immunosuppressive cytokines to shield from the anticancer immunity, thus providing the cell with the ablity to induce malignancy. Further studies on the nature of cytokines produced by transformed cells in regulating autocrine growth and the antitumor immunity of the host should provide useful information on tumorigenesis and possible approaches to treat cancer.
Supported in part by a grant from the American Cancer Society.
Address reprint requests to Mitzi Nagarkatti, PhD, Department of Biomedical Sciences and Pathobiology, Virginia-Maryland Regional College of Veterinary Medicine, Virginia Tech, Blacksburg VA 24061.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal