Abstract
Hemoglobin (Hb) S Antilles is a naturally occurring form of sickling human Hb but causes a more severe phenotype than Hb S. Two homozygous viable Hb S Antilles transgene insertions from Tg58Ru and Tg98Ru mice were bred into MHOAH mice that express high oxygen affinity (P50 ∼24.5 mm Hg) rather than normal (P50 ∼40 mm Hg) mouse Hbs. The rationale was that the high oxygen affinity MHOAH Hb, the lower oxygen affinity of Hb S Antilles than Hb S (P50 ∼40 v 26.5 mm Hg), and the lower solubility of deoxygenated Hb S Antilles than Hb S (∼11 v 18 g/dL) would favor deoxygenation and polymerization of human Hb S Antilles in MHOAH mouse red blood cells (RBCs). The Tg58 × Tg98 mice produced have a high and balanced expression (∼50% each) of hα and hβS Antilles globins, 25% to 35% of their RBCs are misshapen in vivo, and in vitro deoxygenation of their blood induces 30% to 50% of the RBCs to form classical looking, elongated sickle cells with pointed ends. Tg58 × Tg98 mice exhibit reticulocytosis, an elevated white blood cell count and lung and kidney pathology commonly found in sickle cell patients, which should make these mice useful for experimental studies on possible therapeutic intervention of sickle cell disease.
HEMOGLOBIN (Hb) S Antilles is a natural occurring and less soluble form of Hb S.1 Hb S Antilles has a β23Val → Ile substitution in addition to the β6Glu → Val substitution present in Hb S. The additional amino acid substitution changes the minimum gelling concentration (Csat ) from ∼18 g/dL for deoxygenated Hb S to ∼11 g/dL for deoxygenated Hb S Antilles, and it also shifts the P50 of ∼26.5 mm Hg for Hb S to a P50 of ∼40 mm Hg for Hb S Antilles.
Humans heterozygous for Hb S have red blood cells (RBCs) that contain ∼40% Hb S, but these individuals do not exhibit clinical symptoms of sickle cell disease. In comparison, humans heterozygous for Hb S Antilles have RBCs that contain ∼40% Hb S Antilles, but these individuals exhibit clinical symptoms of sickle cell disease that are similar in severity to those in persons who are homozygous for Hb S.1 This is because Hb S Antilles is less soluble and has a right shift in its oxygen association-dissociation property, which favor deoxygenation and polymerization of Hb S Antilles.
RBCs from transgenic sickle cell mice that synthesize normal-mouse Hb and express high levels of human α (hα) and human βS (hβS) or human βS Antilles (hβS Antilles) will sickle in vitro at a low partial pressure of oxygen.2-6 Although deoxygenated RBCs from these transgenic mice become holly leafed or multispiculated, they do not form classical looking, elongated sickle cells with pointed ends similar to those observed in deoxygenated blood of patients with sickle cell disease. In these transgenic mice, the difference between the oxygen association-dissociation properties of normal mouse Hb (P50 ∼40 mm Hg) and Hb S (P50 ∼26.5 mm Hg)7 8 favors deoxygenation of normal mouse Hb over deoxygenation of Hb S. This reduces the amount of deoxygenated Hb S formed in vivo, which decreases the polymerization of Hb S and the formation of sickled erythrocytes.
Our objective was to develop a line of mice that would exhibit the symptoms of sickle cell disease found in humans who are heterozygous for Hb S Antilles.1 A priori, the requirements seemed to be to produce a mouse whose Hb had the oxygen association-dissociation property of Hb A and then to place Hb S Antilles transgenes into that mouse's genome so the transgenic mice would produce blood RBCs that contained about 40% Hb S Antilles.
We recently developed a strain of mice, called MHOAH, which produce Hbs that have a higher affinity for oxygen (P50 ∼24.5 mm Hg) than normal mouse Hb (P50 ∼40 mm Hg).9 We then bred the hα and hβS Antilles transgenes from Rubin's Tg58/β-thal and Tg98/β-thal lines of mice4 into the genome of MHOAH mice. We expected that the higher oxygen affinity of MHOAH versus normal mouse Hb and the lower oxygen affinity and lower solubility of deoxygenated Hb S Antilles versus Hb S would be a favorable condition for the preferential deoxygenation and polymerization of Hb S Antilles in mouse RBCs.10 In this report we show that Tg58 × Tg98 transgenic sickle cell mice exhibit a high and balanced synthesis (∼50% each) of hα and hβS Antilles globins. These globin chains are assembled to produce ∼38% Hb S Antilles, their RBCs become elongated and form pointed ends when the blood is deoxygenated in vitro, and these mice develop lung and kidney pathology similar to that observed in patients with sickle cell disease.
MATERIALS AND METHODS
Mice.Mice express two adult α-globin and two adult β-globin genes. During studies on the induction of mutations by ethylnitrosourea (ENU) in mice, two heritable Hb variants were found. An Hb mutation, Hbag2, was found in a progeny of a male DBA/2 mouse treated with ENU. In mice that are homozygous for Hbag2, 55% of the mαg2 globin encoded by the 5′ adult α-globin gene contains an α89His-Leu substitution and 45% of the mαa globin encoded by the 3′ adult α-globin gene is normal. The P50 of a mixture of 55% mαg22mβs2 and 45% mαa2mβs2 Hbs in RBCs of Hbag2/Hbag2; Hbbs/Hbbs mice is 31.5 ± 0.2 mm Hg. Another Hb mutation, Hbbs2, was found in a progeny of a female C57BL/6 mouse treated with ENU. In mice that are homozygous for Hbbs2, 70% of the βs2 globin encoded by the 5′ adult β-globin gene contains a β59Lys-Ile substitution and 30% of the βs globin encoded by the 3′ adult β-globin gene is normal. The P50 of a mixture of 70% mαa2mβs22 and 30% mαa2mβs2 Hbs in RBCs of Hbaa/Hbaa; Hbbs2/Hbbs2 mice is 31.2 ± 0.2 mm Hg. Mice that carried each of these mutations were mated to produce a line of mice that were homozygous for both mutations. The line was called MHOAH because RBCs of these mice contain high oxygen affinity Hbs. Overall, the mixture of ∼39% mαg22mβs22 (P50 ∼17 mm Hg), and ∼16% mαg22mβs2 (P50 ∼24.5 mm Hg), ∼32% mαa2mβs22 (P50 ∼27.5 mm Hg), and ∼13% maa2mβs2 (P50 ∼40 mm Hg) Hbs in RBCs of MHOAH mice (Hbag2/Hbag2; Hbbs2/Hbbs2) has a significantly higher affinity for oxygen (P50 ∼24.5 mm Hg) than normal mouse Hb (P50 ∼40 mm Hg).9 In MHOAH mice, ∼87% of the Hb has an affinity for oxygen comparable with or greater than that of human Hb A.
Constructs of the hα and hβS Antilles genes used to produce Tg58/β-thal and Tg98/β-thal mice have been described.4 The Hb S Antilles transgene insertions from Tg58/β-thal and Tg98/β-thal mice were bred into the genome of MHOAH mice; these transgenic mice will be called M-Tg58Ru and M-Tg98Ru, respectively. Finally, the transgene insertion from M-Tg98Ru mice was bred into the genome of M-Tg58Ru mice to produce the doubly homozygous Tg58 × Tg98 line of Hb S Antilles transgenic mice.
Hematology and erythrocyte characteristics.Hematologic data on peripheral blood were obtained by an Ortho Diagnostics Systems, Inc, ELT-15 Hematology Analyzer11 (Westwood, MA) and by manual methods.12 Lysates of blood from M-Tg58Ru and Tg58 × Tg98 transgenic sickle cell mice are turbid. After adding blood to Drabkin's reagent, the samples were clarified by centrifugation at 10,000g for 10 minutes to obtain an accurate optical density reading of the Hb concentration. The incidence of reticulocytes was determined from blood films stained with new methylene blue and also from blood stained with thiazole orange (Retic Count; Becton Dickinson, San Jose, CA) and analyzed by flow cytometry.13 Blood films of peripheral blood were made on glass coverslips and stained with Wright's stain, and the morphology of 500 or more erythrocytes per animal was examined and classified as normal or misshapen by light microscopy.
Stepwise Percoll gradients14 were used to examine the density distribution of erythrocytes from 6 MHOAH, 9 M-Tg58Ru, 6 M-Tg98Ru, and 7 Tg58 × Tg98 transgenic sickle cell mice. A 100-μL aliquot of freshly drawn blood was washed and suspended in 0.4 mL of phosphate-buffered saline (PBS), layered on top of a stepwise gradient of Percoll diluted with PBS at the specific gravities shown, and centrifuged at 400g for 20 minutes.
Sodium metabisulfite-induced RBC sickling.To examine the morphology of erythrocytes in vivo, blood was collected from the orbital sinus and transferred directly into 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.3. To induce deoxygenation and RBC sickling, blood was transferred directly into 1% phosphate-buffered Na2S2O5 or was allowed to stand in sealed microhematocrit tubes. Aliquots of induced sickle cells were removed from buffered 1% Na2S2O5 after 1 to 6 hours or from sealed hematocrit tubes after 24 hours, fixed in buffered 2.5% glutaraldehyde, and stained with Wright's stain for examination by light microscopy. Deoxygenated blood was also fixed in 2.5% glutaraldehyde, coated with OsO4 , dehydrated in a series of increasing concentration of ethanol, gold shadowed, and photographed by scanning electron microscopy.
Hb P50 values.Hb oxygen association-dissociation analyses were performed by a TCS Hemox-Analyzer (TCS Medical Products Division, Southhampton, PA).15 The P50 values were read from oxygen association curves as deoxygenated blood samples were slowly and fully oxygenated at 37°C over a period of 10 to 15 minutes.
Analysis of Hb and globin.High performance liquid chromatography (HPLC) was used to separate and quantify the multiple mouse, mouse-human hybrid, and human Hb tetramers.16 The globin chains were separated by electrophoresis in 12% polyacrylamide gels (PAGE) containing 6 mol/L urea, 2% Triton X-100, and 5% acetic acid.17 The relative amount of each globin was determined by quantitative scanning transmission densitometry of Coomassie blue stained gels. Two or more concentrations of each sample were separated on the same gel to establish that densitometric readings of all bands were on a linear scale.
Necroscopy and histology.Age- and sex-matched MHOAH controls and transgenic sickle cell mice were weighed and necropsied. Tissues from 10 controls, 10 M-Tg98Ru, 42 M-Tg58Ru, and 23 Tg58 × Tg98 mice were fixed in 10% buffered formalin. Histological sections were stained with hematoxylin and eosin for routine histological examination or stained with Pearl's Prussian blue to identify deposits of stainable iron.
RESULTS
MHOAH-transgenic sickle cell mice.The hα and hβS Antilles transgenes from Tg58/β-thal and Tg98/β-thal mice were bred into the genetic background of MHOAH mice. Transgenic mice homozygous for either the Tg58Ru or Tg98Ru insertion (M-Tg58Ru and M-Tg98Ru, respectively) and mice doubly homozygous for both transgene insertions (Tg58 × Tg98) were viable and most of them were fertile. The MHOAH controls and the three lines of transgenic sickle cell mice have the same major histocompatibility genotype (H-2d) and by pedigree they are alike at ∼90% of the genome. Studies in progress indicate that the mean age at death for M-Tg58Ru and Tg58 × Tg98 mice will be ∼16 months compared with ∼24 months for MHOAH and M-Tg98Ru mice.
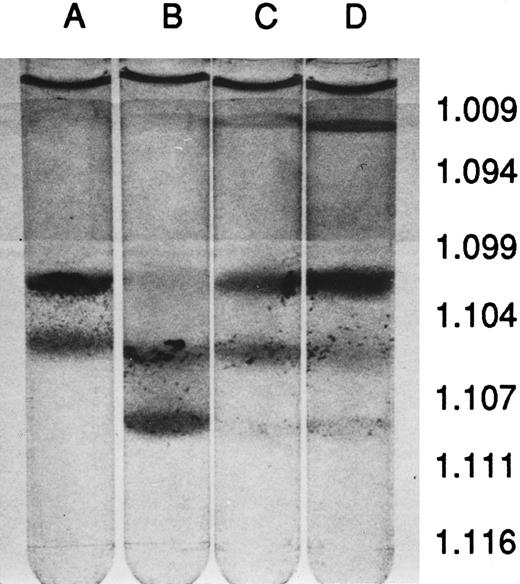
Hematology and erythrocyte characteristics.The peripheral blood of MHOAH mice has been shown to have normal hematologic values9 so the hematologic indices of blood from transgenic sickle cell mice were compared with MHOAH controls in Table 1. Except for the small increase in the reticulocyte count, the hematology values for M-Tg98Ru mice were not significantly different from the control. However, blood from the M-Tg58Ru and Tg58 × Tg98 transgenic sickle cell mice had significantly elevated white blood cells (WBC) and reticulocyte counts. The serum from MHOAH and M-Tg98Ru mice had a normal straw color, but the serum from M-Tg58Ru and Tg58 × Tg98 mice had a reddish tinge, indicating that hemolysis was occurring in vivo in M-Tg58Ru and Tg58 × Tg98 mice. Although the RBCs, blood Hb concentration (HGB), means corpuscular volume (MCV), mean corpuscular Hb (MCH), and mean corpuscular Hb concentration (MCHC) values for M-Tg58Ru and Tg58 × Tg98 mice were significantly lower than for MHOAH controls, these transgenic mice were not anemic. RBCs from MHOAH and M-Tg98Ru mice were normal in size and shape, but anisocytosis, poikilocytosis, polychromatic RBCs, and intercellular debris (fragments of RBCs and microvesicles) were observed in Wright's-stained films of freshly drawn blood from M-Tg58Ru and Tg58 × Tg98 mice. The RBC fragments and microvesicles imparted a turbidity that resulted in erroneously high values for Hb if the intercellular debris was not removed by centrifugation. In fully oxygenated blood, 1% to 3% of the RBCs in blood from M-Tg58Ru and Tg58 × Tg98 mice did not regain normal morphology and may be irreversibly sickled cells. Centrifugation of blood in Percoll stepwise gradients showed that blood from all three lines of transgenic sickle cell mice contained some RBCs that were denser than RBCs from MHOAH mice (Fig 1). Somewhat surprising, blood from M-Tg98Ru mice, which did not exhibit other signs of sickle cell disease, contained a large number of dense RBCs.
Percoll density-gradient centrifugation of blood. (A) MHOAH, (B) M-Tg98Ru, (C) M-Tg58Ru, and (D) Tg58 × Tg98. Blood from M-Tg58Ru and Tg58 × Tg98 mice contained 13% to 15% reticulocytes that sedimented largely to the interface of PBS and the 1.094 specific gravity Percoll. Blood from each line of transgenic sickle cell mice had denser than normal RBCs that sedimented to the interface of the 1.107 and 1.111 specific gravity Percoll.
Percoll density-gradient centrifugation of blood. (A) MHOAH, (B) M-Tg98Ru, (C) M-Tg58Ru, and (D) Tg58 × Tg98. Blood from M-Tg58Ru and Tg58 × Tg98 mice contained 13% to 15% reticulocytes that sedimented largely to the interface of PBS and the 1.094 specific gravity Percoll. Blood from each line of transgenic sickle cell mice had denser than normal RBCs that sedimented to the interface of the 1.107 and 1.111 specific gravity Percoll.
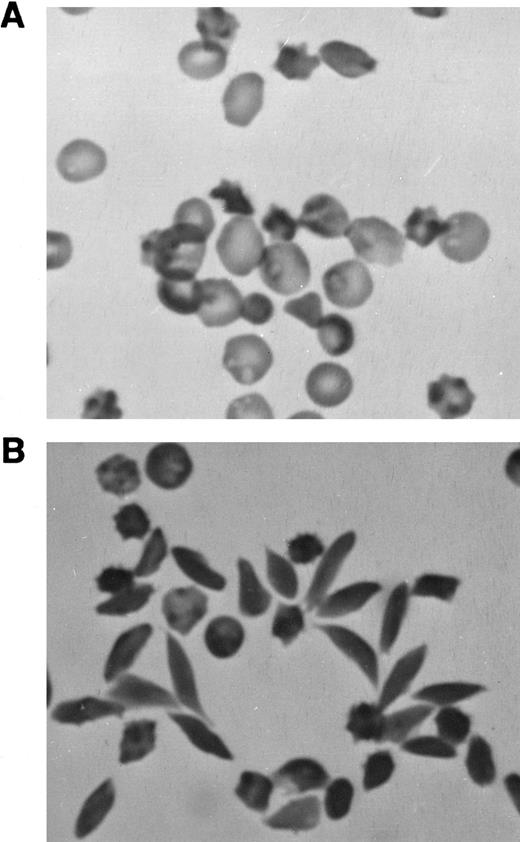
Sodium metabisulfite-induced RBC sickling.A few (1% to 3%) abnormally shaped erythrocytes were seen in freshly drawn, glutaraldehyde-fixed peripheral blood from M-Tg98Ru mice (Table 1). However, many more misshapen erythrocytes were observed in the peripheral blood from M-Tg58Ru (10% to 15%) and Tg58 × Tg98 (25% to 35%) mice (Table 1 and Fig 2A). Very few elongated sickle cells were induced in samples of deoxygenated blood from M-Tg98Ru (0% to 1%) and M-Tg58Ru (1% to 5%) mice (Table 1), but 30% to 50% of the RBCs in deoxygenated blood from Tg58 × Tg98 mice formed elongated sickle cells with pointed ends (Table 1, Fig 2B, and Fig 3).
Photomicrographs of blood from Tg58 × Tg98 mice. (A) Freshly drawn blood was transferred directly into 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer. In the peripheral blood, many RBCs (25% to 35%) were misshapen but very few had the classical looking shape of sickle cells. (B) Many elongated sickle cells with pointed ends (30% to 50%) developed when blood was deoxygenated in phosphate-buffered 1% Na2S2O5 . (Original magnification × 400.)
Photomicrographs of blood from Tg58 × Tg98 mice. (A) Freshly drawn blood was transferred directly into 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer. In the peripheral blood, many RBCs (25% to 35%) were misshapen but very few had the classical looking shape of sickle cells. (B) Many elongated sickle cells with pointed ends (30% to 50%) developed when blood was deoxygenated in phosphate-buffered 1% Na2S2O5 . (Original magnification × 400.)
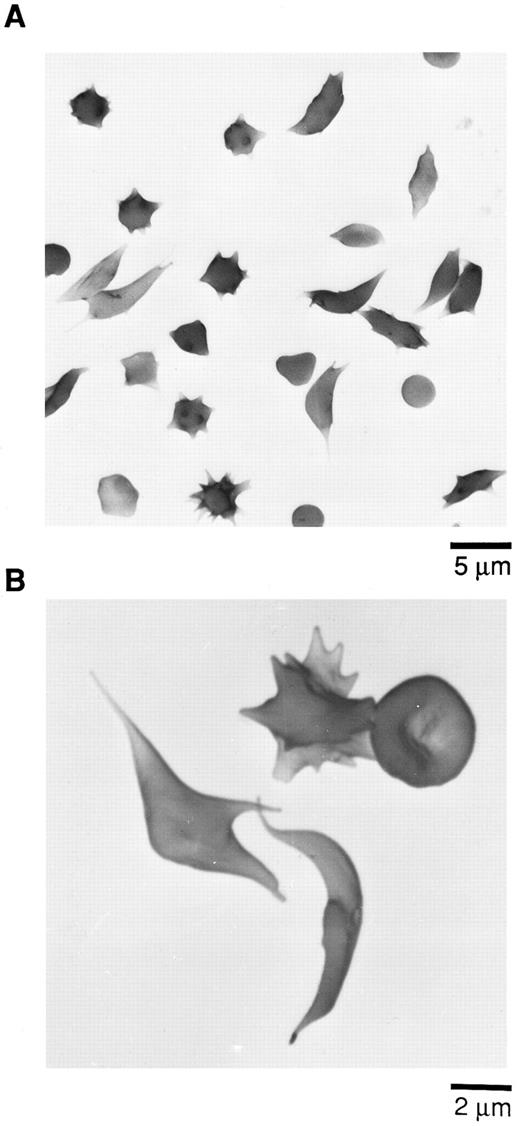
Scanning electron micrographs (SEM) of sickle cells in blood from Tg58 × Tg98 mice after deoxygenation in 1% Na2S2O5 . Deoxygenated blood was fixed in buffered 2.5% glutaraldehyde and processed for SEM as described in the Materials and Methods. (A) Lower magnification to show the incidence and variety of sickle cells in deoxygenated blood. (B) Higher magnification to show more detail of the various shapes of sickled, multispiculated, and normal shaped RBCs in deoxygenated blood.
Scanning electron micrographs (SEM) of sickle cells in blood from Tg58 × Tg98 mice after deoxygenation in 1% Na2S2O5 . Deoxygenated blood was fixed in buffered 2.5% glutaraldehyde and processed for SEM as described in the Materials and Methods. (A) Lower magnification to show the incidence and variety of sickle cells in deoxygenated blood. (B) Higher magnification to show more detail of the various shapes of sickled, multispiculated, and normal shaped RBCs in deoxygenated blood.
Hb P50 values.The oxygen association P50 values of Hbs from transgenic sickle cell mice were intermediate between those of MHOAH Hb and Hb S Antilles (Table 1). The values recorded represent the P50 values for the mixtures of Hb tetramers (Table 2) in RBCs of these mice.
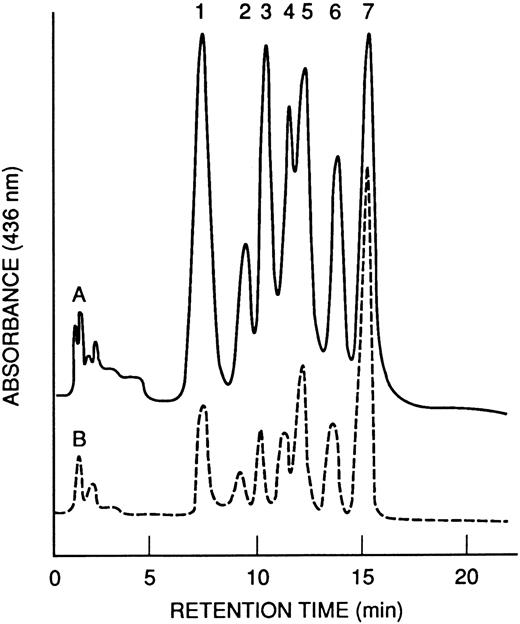
Hb and globin analyses.The HPLC profiles of Hb from M-Tg58Ru and Tg58 × Tg98 mice are shown in Fig 4. The nine kinds and quantities of symmetrical tetramers of Hb produced in these transgenic sickle mice are shown in Table 2. Increased amounts of Hb S Antilles (∼13%, 16%, and 38%) were found in M-Tg98Ru, M-Tg58Ru, and Tg58 × Tg98 mice, respectively. The globin chains were separated by PAGE (Fig 5), and the relative amount of each globin was quantified by scanning densitometry. The average percentage of each α globin/total α globins and of each β globin/total β globins were comparable for PAGE and HPLC analyses and are shown in Table 3. Analysis of the PAGE electrophoreograms of five samples of M-Tg98Ru and seven samples each of M-Tg58Ru and Tg58 × Tg98 RBCs showed that the relative amounts of hα globin/total globins and of hβS Antilles/total globins were 19.64% ± 0.66% and 11.93% ± 0.75% in M-Tg98Ru RBCs, 13.85% ± 0.90% and 15.96% ± 1.44% in M-Tg58Ru RBCs, and 24.06% ± 0.76% and 23.10% ± 0.94% in Tg58 × Tg98 RBCs. Moreover, the total α globin/total β globin ratios were 1.00, 0.98, and 0.97 in M-Tg98Ru, M-Tg58Ru, and Tg58 × Tg98 RBCs, respectively. Although there was significantly more hα than hβS Antilles globin in M-Tg98Ru RBCs and slightly but not significantly more hβS Antilles than hα globin in M-Tg58Ru RBCs, equivalent amounts of hα and hβS Antilles globins were present in the RBCs of Tg58 × Tg98 mice.
HPLC profiles of Hb from transgenic mice. (A) M-Tg58Ru; (B) Tg58 × Tg98. Hb tetramers in each of the 7 peaks are listed in Table 2. Hb S Antilles (peak 7) was more abundant in blood from Tg58 × Tg98 than M-Tg58Ru mice.
HPLC profiles of Hb from transgenic mice. (A) M-Tg58Ru; (B) Tg58 × Tg98. Hb tetramers in each of the 7 peaks are listed in Table 2. Hb S Antilles (peak 7) was more abundant in blood from Tg58 × Tg98 than M-Tg58Ru mice.
PAGE of Hbs in Triton X-100 and acid-urea. The strain of mice from which the Hb was obtained and the kinds of globin chains present in each Hb are as follows: Lane 1, strain C57BL/6, mαa, and mβs; lane 2, HBB/S2, mαa, mβs, and mβs2; lane 3, HBA/G2, mαa, mαg2, and mβs; lane 4, M-Tg58Ru, mαa, mαg2, hα, mβs, mβs2, and hβS Antilles; lane 5, M-Tg98Ru, mαa, mαg2, hα, mβs, mβs2, and hβS Antilles; and lane 6, Tg58 × Tg98, mαa, mαg2, hα, mβs, mβs2, and hβS Antilles.
PAGE of Hbs in Triton X-100 and acid-urea. The strain of mice from which the Hb was obtained and the kinds of globin chains present in each Hb are as follows: Lane 1, strain C57BL/6, mαa, and mβs; lane 2, HBB/S2, mαa, mβs, and mβs2; lane 3, HBA/G2, mαa, mαg2, and mβs; lane 4, M-Tg58Ru, mαa, mαg2, hα, mβs, mβs2, and hβS Antilles; lane 5, M-Tg98Ru, mαa, mαg2, hα, mβs, mβs2, and hβS Antilles; and lane 6, Tg58 × Tg98, mαa, mαg2, hα, mβs, mβs2, and hβS Antilles.
In each line of transgenic sickle cell mice, the quantities of mα2mβ2 and hα2hβ2S Antilles tetramers were significantly more, and the quantities of mα2hβ2S Antilles and hα2mβ2 were significantly less than would be expected if assembly of the Hb tetramers was random and proportional to the concentration of the globin chains in the RBCs (Table 4). Analysis of the Hb tetramers and globin chains in RBCs of Tg58 × Tg98 mice indicated that assembly of mα2mβ2 and hα2hβ2S Antilles was favored (1.20 and 1.61 times expected, respectively) over assembly of mα2hβ2S Antilles and hα2mβ2 (0.59 and 0.58 times expected, respectively). The data also indicated that the preferential assembly of homologous tetramers over assembly of mouse-human hybrid tetramers was increased when the amount of the human globins synthesized was increased.
Necropsy and histology.No pathology was observed in MHOAH mice, and no pathology was observed in M-Tg98Ru mice. In contrast, gross and histologic pathology was observed in M-Tg58Ru and Tg58 × Tg98 mice. The spleens of 10 MHOAH mice averaged 80 ± 5 mg, and the spleens of 42 M-Tg58Ru and 23 Tg58 × Tg98 mice averaged 168 ± 22 and 177 ± 28 mg, respectively. The lungs of M-Tg58Ru and Tg58 × Tg98 mice were often retracted in the pleural cavity, petechiae were observed on the surfaces of some lungs and kidneys, some lungs had a reddish color indicative of interstitial hemorrhage, the kidneys had a dark brownish color indicative of hemosiderin deposits, and extensive sequestration of blood was observed in the liver of two M-Tg58Ru mice.
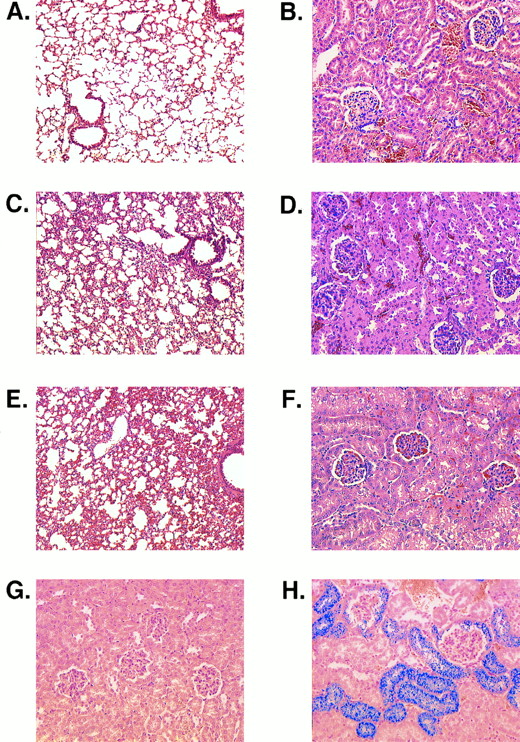
In histologic sections, marked congestion was observed in the spleens, kidneys, lungs, and livers of M-Tg58Ru and Tg58 × Tg98 mice. Their spleens exhibited expanded erythropoiesis, capsular and trabecular fibrosis, and large deposits of stainable iron. The alveolar septa in the lungs of M-Tg58Ru and Tg58 × Tg98 mice were thickened (Fig 6A, C, and E). Their lungs also showed fibrosis of interstitial tissue, hemorrhage, and an accumulation of numerous iron-laden macrophages. In the kidneys of M-Tg58Ru and Tg58 × Tg98 mice, the glomerular tufts were often shrunken and many of the capillary loops were filled with erythrocytes (Fig 6B, D, and F ). Some glomeruli had completely atrophied. Proteinaceous deposits were observed along the collecting tubules in a few kidneys of M-Tg58Ru and Tg58 × Tg98 mice. The epithelial cells of Bowman's capsule and of the proximal tubules contained large amounts of stainable iron (Fig 6G and H), indicating that plasma Hb had leaked from the glomerulus into Bowman's capsule. The liver of M-Tg58Ru and Tg58 × Tg98 mice contained foci of extramedullary hematopoiesis and areas of ischemic necrosis. Sickled RBCs and fibrin thrombi were also observed in small venules of the liver. Liver macrophages and Kupffer cells were laden with stainable iron but iron deposits were not observed in liver parenchymal cells.
Histologic sections. (A) Lung of an MHOAH mouse showing thin alveolar septa. (B) Kidney of an MHOAH mouse. (C) Lung of an M-Tg58Ru mouse showing congestion and thickening of the alveolar septa. (D) Kidney of an M-Tg58Ru mouse showing RBC congestion in the glomerular tufts and swelling of the capsular epithelium. (E) Lung of a Tg58 × Tg98 mouse showing congestion and thickening of the alveolar septa. (F ) Kidney of a Tg58 × Tg98 mouse showing RBC congestion in the glomerular tufts and swelling of the capsular epithelium. (G) Kidney of an MHOAH mouse stained with Pearl's Prussian blue and counterstained with eosin showing no deposit of stainable iron. (H) Kidney of a Tg58 × Tg98 mouse stained with Pearl's Prussian blue and counterstained with eosin showing that deposits of stainable iron are present in epithelial cells of Bowman's capsule and proximal tubules. (Original magnification × 100 for lung and × 200 for kidney.)
Histologic sections. (A) Lung of an MHOAH mouse showing thin alveolar septa. (B) Kidney of an MHOAH mouse. (C) Lung of an M-Tg58Ru mouse showing congestion and thickening of the alveolar septa. (D) Kidney of an M-Tg58Ru mouse showing RBC congestion in the glomerular tufts and swelling of the capsular epithelium. (E) Lung of a Tg58 × Tg98 mouse showing congestion and thickening of the alveolar septa. (F ) Kidney of a Tg58 × Tg98 mouse showing RBC congestion in the glomerular tufts and swelling of the capsular epithelium. (G) Kidney of an MHOAH mouse stained with Pearl's Prussian blue and counterstained with eosin showing no deposit of stainable iron. (H) Kidney of a Tg58 × Tg98 mouse stained with Pearl's Prussian blue and counterstained with eosin showing that deposits of stainable iron are present in epithelial cells of Bowman's capsule and proximal tubules. (Original magnification × 100 for lung and × 200 for kidney.)
DISCUSSION
The principal aim of these studies was to produce transgenic mice that would exhibit symptoms of sickle cell disease similar to those found in humans who are heterozygous for Hb S Antilles.1 To accomplish this, the hα and hβS Antilles transgene insertions from Tg58/β-thal and Tg98/β-thal mice4 were bred into the genome of MHOAH mice10 that produce mouse Hbs having an overall oxygen affinity property similar to that of human Hb A. Three lines of Hb S Antilles transgenic mice were produced. The M-Tg98Ru, M-Tg58Ru, and Tg58 × Tg98 lines of transgenic sickle cell mice expressed ∼13%, 16%, and 38% Hb S Antilles, respectively (Table 1). M-Tg98Ru mice that produced the lowest amount of Hb S Antilles did not exhibit signs of sickle cell disease. M-Tg58Ru and Tg58 × Tg98 mice that produced larger amounts of Hb S Antilles had reduced RBC, HGB, MCV, and MCHC values but were not anemic; however, they did exhibit reticulocytosis, elevated WBC, and kidney and lung pathology (Table 1 and Fig 6). These clinical and pathological symptoms are similar to those commonly found in patients with sickle cell disease.
The number of misshapen RBCs found in vivo and the number of elongated sickle cells induced by deoxygenation of blood in vitro correlated well with the quantities of hα and hβS Antilles globins synthesized and the quantities of Hb S Antilles assembled in the RBCs (Tables 1-4). RBCs of M-Tg98Ru mice synthesized significantly more hα than hβS Antilles, and RBCs of M-Tg58Ru mice synthesized slightly more hβS Antilles than hα (Table 3). High and balanced amounts of hα and hβS Antilles were synthesized in RBCs of Tg58 × Tg98 mice that express both transgene insertions.
In all lines of Hb S Antilles sickle cell mice, some of the Hb precipitated and adhered to the inner surface of the RBC membrane and many of the RBCs become denser than normal (Fig 1). This was particularly evident in the Percoll gradients of all six samples of RBCs from M-Tg98Ru mice that express more hα than hβS Antilles globin. In M-Tg98Ru mice the concentration of Hb S Antilles (3.94 g/dL) plus other Hbs that contain hβS Antilles (3.57 g/dL) was too low (∼7.5 g/dL) to form polymers when the blood was deoxygenated in vitro. Although anisocytosis, poikilocytosis, and polychromatic RBCs were commonly observed (10% to 15%) in peripheral blood of M-Tg58Ru mice, only 1% to 5% of the RBCs formed elongated sickle cells, although 25% to 30% of the RBCs became multispiculated when blood from M-Tg58Ru mice (∼16% Hb S Antilles) was deoxygenated in vitro (Table 1). The concentration of Hb S Antilles (4.36 g/dL) plus other Hbs that contain hβS Antilles (5.27 g/dL) was ∼9.6 g/dL in RBCs of M-Tg58Ru mice, which was also too low to form long polymers of deoxygenated Hb S Antilles. However, in erythrocytes of Tg58 × Tg98 mice the concentration of Hb S Antilles (10.99 g/dL) plus other Hbs that contain hβS Antilles (4.41 g/dL) was high enough (∼15.4 g/dL) to exceed the Csat of ∼11 g/dL for deoxygenated Hb S Antilles. In Tg58 × Tg98 mice, 25% to 35% of the erythrocytes became deformed in vivo (Table 1 and Fig 2); furthermore, when blood from Tg58 × Tg98 mice was deoxygenated in vitro, 30% to 50% of the RBCs formed classical looking, elongated sickle cells with pointed ends (Table 1 and Figs 2 and 3).
How does our Tg58 × Tg98 mouse model of Hb S Antilles compare with other transgenic sickle cell mouse models? Greaves et al2 reported on a transgenic mouse that expressed 83% Hb S. The mouse had a sightly elevated reticulocyte count (4.4%) but the mouse was not anemic. RBCs in the peripheral blood had normal morphology, although most of the blood RBCs sickled when the blood was deoxygenated in vitro. In another transgenic mouse that expressed 35% Hb S, the RBCs did not sickle when deoxygenated in vitro.
Ryan et al3 develop two lines of transgenic Hb S mice that expressed ∼50% Hb S in mice of normal genotype and also in mice heterozygous for murine β-thalassemia. The latter group of mice had enlarged spleens, an elevated reticulocyte count (7.4%), and slightly lower than normal RBC, HGB, and hematocrit (HCT) values. Less than 1% of the RBCs from the former group of mice sickled, but more than 90% of the RBCs from the latter group of mice sickled when their blood was deoxygenated in vitro. In mice homozygous for murine β-thalassemia, 77% of the β-globin was hβS and large amounts of denatured Hb accumulated on the inner surface of the RBC membrane.18
In the Tg58/β-thal line of mice developed by Rubin et al,4 the hemizygous insertion of hα and hβS Antilles transgenes expressed 17.3% hα and 49.3% hβS Antilles globins that assembled to form 8.3% Hb S Antilles plus 40.4% of another Hb (mα2hβ2S Antilles). About 30% of these RBCs exhibited varying degrees of abnormal shapes and sickling when deoxygenated in vitro, and similar abnormal RBC shapes and sickling were induced in vivo when these mice were exposed to hypoxia (8.4% oxygen) for 10 days. Rubin et al4 did not describe the characteristics of Tg98/β-thal mice; however, we found that Tg98/β-thal mice, which were bred homozygous for both murine β-thalassemia and the Tg98Ru transgene insertion, expressed 27% Hb S Antilles. Their hematology values were normal and their RBCs did not sickle when the blood was deoxygenated in vitro.
Trudel et al19,20 used a recombinant hβ-globin gene construct, hβSAD, to produce Hb SAD mice that exhibit a very severe form of sickle cell disease. The hβSAD globin chain has a third, β121Glu-Gln, amino acid substitution in addition to the two amino acid substitutions in the hβS Antilles globin chain compared with the amino acids in the hβA globin chain. Coexpression of the hα and hβSAD transgenes produced 19% Hb SAD in normal mice and 26% Hb SAD in heterozygous β-thalassemic mice. Hb SAD has a low oxygen affinity similar to that of Hb S Antilles; in addition, deoxygenated Hb SAD has a lower solubility than Hb S Antilles.19 Neonatal Hb SAD mice were anemic but adults had normal hematocrits, although they exhibited chronic hemolysis and elevated (6.2%) reticulocyte counts.19 Sickled RBCs were visible in fixed sections of bone marrow and spleen, and microvascular occlusions and secondary end-organ pathology were observed in the spleen, lung, kidney, and liver of Hb SAD mice.20 The reported pathological findings were congestive splenomegaly, pulmonary congestion, hemorrhage, thrombosis and fibrosis, renal congestion, glomerulopathy and fibrosis, extramedulary hematopoiesis in the liver and lung, systemic hemosiderosis, priapism, and penile hemorrhage.
The Tg58/β-thal line of transgenic Hb S Antilles mice from Rubin et al4 was bred to a line of transgenic Hb S mice developed by F. Costantini5 to produce a second generation of transgenic mice that expressed both Hb S and Hb S Antilles.6 The latter line of mice showed a more severe phenotype than the former. The doubly transgenic mice expressed 58% hα, 34% hβs, and 28% hβS Antilles globins in heterozygous β-thalassemic mice, and 58% hα, 42% hβS, and 36% hβS Antilles globins in homozygous β-thalassemic mice. The quantities of Hb S and Hb S Antilles were not reported. Blood from these mice contained 10% reticulocytes and a large number of high-density RBCs, and most of the RBCs sickled in a conventional sickle test or when allowed to deoxygenate slowly in sealed microhematocrit tubes. The mice had a reduced ability to concentrate urine, indicative of a reduced renal function. In addition, the mice had elevated levels of aspartate amino transferase and alanine amino transferase, indicative of a reduced liver function. The mice also exhibited hepatosplenomegaly, ischemic infarcts in the liver, splenic congestion and fibrosis, glomerular and peritubular vessel congestion, septal thickening in the lung, and pyknotic neurons in the brain.
From these published reports, we conclude that the symptoms of sickle cell disease are more severe in Hb SAD mice20 than in our Tg58 × Tg98 mouse model of Hb S Antilles disease described in this report. The pathobiology of sickle cell disease in Tg58 × Tg98 mice is comparable with that in doubly transgenic Hb S and Hb S Antilles mice6 and is more severe than in other transgenic sickle cell mice.2-5 Although Hb SAD mice express lower amounts of a sickling Hb than Tg58 × Tg98 mice, the low oxygen affinity of Hb SAD and the lower solubility of deoxygenated Hb SAD compared with Hb S Antilles favors the deoxygenation and polymerization of Hb SAD even in the presence of large amounts (∼75%) of mouse and mouse-human hybrid Hbs. Similarly, although Tg58 × Tg98 mice express lower amounts of a sickling Hb than some transgenic Hb S mice3,5 and doubly transgenic Hb S and Hb S Antilles mice,6 the lower oxygen affinity and lower solubility of deoxygenated Hb S Antilles compared with Hb S favors deoxygenation and polymerization of Hb S Antilles in the presence of appreciable amounts (∼60%) of mouse and mouse-human hybrid Hbs, which contain mutant globins and have a higher affinity for oxygen than normal mouse and mouse-human hybrid Hbs.9
Other factors also contributed to the expression of sickle cell disease in Tg58 × Tg98 mice. The combined expression of the Tg58Ru and Tg98Ru transgene insertions in Tg58 × Tg98 mice resulted in a high and balanced synthesis of hα and hβS Antilles globins (Table 3). Furthermore, the hα and hβS Antilles globins assembled preferentially so that more than the expected amount of Hb S Antilles was present (Table 4). The presence of 38% Hb S Antilles in RBCs of Tg58 × Tg98 mice is comparable with the 40% Hb S Antilles present in RBCs of humans who are heterozygous for Hb S Antilles.1 Because these Tg58 × Tg98 mice exhibit erythrocyte sickling, reticulocytosis, elevated WBC, and the secondary end-organ pathology commonly found in the lung, liver, kidney, and spleen of patients with sickle cell disease, these mice should be an excellent experimental animal model to study the long-term effects of current drug therapies, including hydroxyurea,21 for the treatment of sickle cell disease, and to evaluate future experimental methods, including gene therapy22 and bone marrow transplantation,23 for possible intervention of sickle cell disease.
Supported by Grants No. HL-43375 and DE-AC05-96OR22464 with Lockheed Martin Research; Grants No. P60 HL-38737 and K14 HL 03141 with Meharry Medical College; and Grants No. HL-20985 and DE-AC03-76SF00098 with the University of California.
Address reprint requests to R.A. Popp, PhD, Biology Division, PO Box 2009, Oak Ridge National Laboratory, Oak Ridge, TN 37831.