In this retrospective study, chimeric antigen receptor T cells remained effective in patients with relapsed/refractory large B-cell lymphoma after prior exposure to bispecific antibodies (BsAbs) targeting different antigens. These results are relevant to clinical practice, particularly given the increasing use of BsAbs in earlier treatment lines.
TO THE EDITOR:
The development of T-cell–engaging therapies, particularly chimeric antigen receptor (CAR) T cells and bispecific antibodies (BsAbs), has revolutionized the treatment of patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL). Recently, BsAbs have shown impressive efficacy results in heavily pretreated patients, leading to regulatory approval of glofitamab and epcoritamab as single agents for R/R LBCL exposed to at least 2 prior lines of systemic therapy.1-5 Ongoing trials are exploring their use in combination with other agents in earlier treatment lines, increasing the number of patients exposed to BsAbs before CAR T cells.6-8 However, the relatively similar mechanism of action between both strategies has raised the concern of potential resistance to immune killing after progressing to BsAbs, together with T-cell exhaustion, which could affect subsequent CAR T-cell outcomes. This study evaluates efficacy and toxicity of anti-CD19 CAR T cells in patients with R/R LBCL previously exposed to BsAbs, addressing a key clinical question and aiding treatment sequencing in this setting.9-11
In the first part of the study, we conducted a retrospective analysis of 47 patients with R/R LBCL treated with CD19-targeted CAR T cells after prior BsAb exposure at 11 French and 4 Spanish centers between 2018 and January 2023; patients exposed to CD19/CD3 BsAbs were excluded. All patients provided informed consent; the study was approved by DESCAR-T's ethics committee (French Data Protection Agency number 2208143; Health Data Hub publication number 20221220174727). Baseline characteristics of the study cohort are summarized in Table 1. The best overall response rate (ORR) and complete response rate (CRR) achieved with prior BsAb treatment were 46% and 19%, respectively. The median progression-free survival (PFS) was 3.1 months (95% confidence interval [CI], 2.7-4.4 months), and 6-month PFS was 21% (95% CI, 11%-34%). Cytokine release syndrome (CRS) after BsAbs occurred in 27 (57%) patients, mostly grade 1 to 2 (only 1 grade 3 event) with no reported immune effector cell–associated neurotoxicity syndrome (ICANS). In 26 (55%) patients, BsAb therapy was the last regimen before CAR T cells.
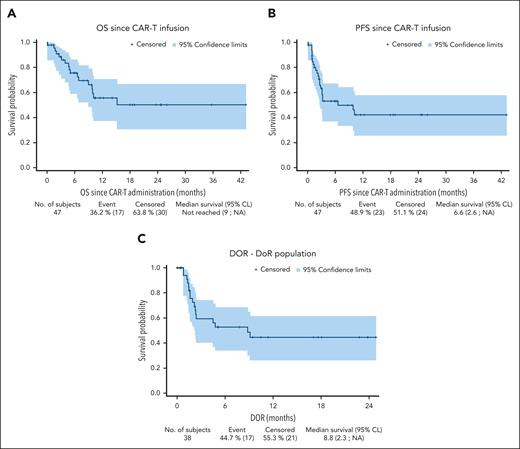
In terms of the subsequent CAR T-cell therapy, 22 (47%) patients received axicabtagene ciloleucel (axi-cel), 20 (42%) received tisagenlecleucel, and 5 (11%) received lisocabtagene maraleucel. The best overall (complete) response rate to CAR T cells was 85% (43%) (supplemental Table 1, available on the Blood website), without significant differences between patients who had previously responded (partial response or complete response [CR]) or not (stable disease or progressive disease) to BsAb treatment (86% [41%] vs 84% [44%]; P = 1.0) (supplemental Table 2). At a median follow-up of 10.5 months, median PFS was 6.6 months (95% CI, 2.6 months-not reached) and median overall survival (OS) was not reached (95% CI, 9.0 months-not reached). The estimated 1-year PFS and OS were 42% (95% CI, 25.9%-57.7%) and 55% (95% CI, 37.5%-70.6%), respectively (Figure 1).
Outcomes after CAR T-cell therapy in patients previously exposed to BsAb treatment. (A) OS after CAR T-cell therapy (n = 47). (B) PFS after CAR T-cell therapy (n = 47). (C) Duration of response (DoR) after CAR T-cell therapy (n = 38). CL, confidence limit; NA, not applicable.
Outcomes after CAR T-cell therapy in patients previously exposed to BsAb treatment. (A) OS after CAR T-cell therapy (n = 47). (B) PFS after CAR T-cell therapy (n = 47). (C) Duration of response (DoR) after CAR T-cell therapy (n = 38). CL, confidence limit; NA, not applicable.
The median time from the last dose of BsAb therapy to leukapheresis was 51 days (range, 13-512 days), whereas the median time from leukapheresis to CAR T infusion was 43 days. The ORR (CR) of patients previously exposed to BsAb within 50 days of leukapheresis was similar to patients who had a longer washout (82% [32%] vs 84% [52%]; P = .36). The same comparable outcomes were observed for PFS and OS (supplemental Figure 1). Efficacy results did not differ significantly when the analysis was restricted to patients previously exposed to single-agent CD20/CD3 BsAbs and when it focused exclusively on axi-cel recipients (data not shown).
Incidence of any grade CRS and ICANS after CAR T cells was 79% (grade ≥3 in 6%) and 23% (grade ≥3 in 2%), respectively. There were no differences in the rate of CRS after CAR T cells according to previous CRS occurrence with BsAbs (78% vs 79%; P = 1.0) (supplemental Table 3). Within the first month post-CAR T, 66% of patients experienced grade ≥3 neutropenia and 45% experienced grade ≥3 thrombocytopenia. During follow-up, 18 (38%) patients died because of disease progression (n = 12), infections (n = 5; 2 septic shock, 1 pneumonia, 1 COVID-19 infection, and 1 multiple organ failure after cytomegalovirus infection), or unknown cause (n = 1).
In the second part of the study, we generated a BsAb-naïve control group with 42 patients treated with axi-cel or tisagenlecleucel among 980 patients from the DESCAR-T registry, via a 1:1 propensity score matching (PSM) analysis including 13 baseline covariates of clinical and prognostic relevance, to compare their outcomes with those of the BsAb-exposed group.12 The 5 patients who received lisocabtagene maraleucel were not included in this analysis because of the lack of an available control partner. Key characteristics of the PSM cohorts are shown in supplemental Table 4; after matching, standardized mean difference remained >0.1 for 6 covariates. The BsAb-exposed group achieved a higher ORR compared with the control group (86% vs 55%; P = .02) but CRR, 1-year PFS, and 1-year OS were not statistically different between BsAb-exposed and naïve patients (43% vs 38% [P = .5], 43% vs 29% [P = .1], and 55% vs 37% [P = .08], respectively) (supplemental Figure S). Concerning the safety profile, there was a comparable rate of CRS and ICANS (any grade and grade ≥2) in both groups (supplemental Table 5).
In this retrospective, multicenter study evaluating efficacy and toxicity of CAR T-cell therapy in patients exposed to BsAbs before leukapheresis, key efficacy outcomes, including response rate and survival, were consistent with those of a matched control group, the pivotal trials, and real-world data studies.13-19 We found no evidence of intrinsic cross-resistance between CAR T cells and BsAbs when both T-cell–engaging approaches did not target the same antigen. Our results strongly suggest (1) a lack of correlation between primary resistance to BsAbs and resistance to CAR T cells in a given patient, in view of the similar ORR and CRR to CAR T irrespective of prior response to BsAbs; and (2) a similar efficacy of CAR T cells in patients previously exposed to BsAb compared with a population of BsAb-naïve patients. Also, the interval between the last dose of BsAbs and leukapheresis did not seem to influence CAR T-cell efficacy. Taking into account that the half-life of BsAbs is relatively short (10-20 days),20,21 the washout period in our study seemed to provide sufficient BsAb clearance to allow T-cell fitness recovery before leukapheresis. However, further studies with larger cohorts including shorter washout periods are warranted to confirm these findings.
Preliminary data on the use of CD20/CD3 BsAbs in patients relapsing after CAR T cells appear promising, with CR rates at ≈30% in R/R LBCL, suggesting that BsAbs are a potential salvage option after CAR T failure.1-5,22 In this study, CAR T cells also appear to be effective in patients who progress after exposure to BsAbs, highlighting that both sequencing modalities seem effective. Importantly, no new safety signals or increased CRS/ICANS incidence were observed with CAR T therapy in BsAb-exposed patients.
Beyond its retrospective nature, our study has some limitations. The small number of patients and the heterogeneity of treatments require larger prospective studies to confirm these results, but the overall findings appear to be consistent in the different subgroup analysis. In addition, despite the inclusion of numerous covariates with prognostic relevance in our PSM analysis, unidentified confounding factors could potentially persist.
In conclusion, our data suggest that CAR T-cell therapy remains effective in patients with R/R LBCL after prior exposure to BsAbs when the target antigen is different. Also, lack of response to previous BsAbs does not predict for lower response rates after CAR T cells. These results are relevant to clinical practice to inform on treatment sequencing, and reassuring in view of the increasing use of BsAbs in earlier treatment lines.
Acknowledgments
The authors thank the patients, the Mont-Godinne and Salus Sanguinis foundations, and the Belgian Hematology Society.
Authorship
Contribution: G. Crochet, G.I., P.B., F.M., and R.H. conceived and designed the study; G. Crochet, G.I., A. Couturier, E.B., T.G., G. Cartron, M.K., R.G., N.M.-C., C.C.-L., P.A., M.G., C.S., V.C., S.G., A. Chauchet, E.D., K.B., P.B., F.M., and R.H. provided study materials or patients; G. Crochet, G.I., J.I.-T., J.A.-C., P.B., F.M., and R.H. collected and assembled data; G. Crochet, G.I., T.F., B.L., F.B., P.B., F.M., and R.H. performed data analysis and interpretation; all authors wrote the manuscript; and all authors gave final approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: G. Crochet received honorary/travel grant from Gilead, Roche, Sobi, and AbbVie. G.I. provided consultancy and received honoraria from Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, AbbVie, Janssen, Sandoz, Miltenyi, and AstraZeneca. E.B. received research funding from Amgen and Bristol Myers Squibb; received honoraria from Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, and Sanofi; and was a member on the board of directors or advisory committees of Roche, Gilead, ADC Therapeutics, Takeda, Novartis, and Incyte. T.G. received honoraria, was a member on the board of directors or advisory committees, and received support for attending meetings/travel and participation in a data safety monitoring board or advisory board for Gilead/Kite and Takeda. G. Cartron was a member on the board of directors or advisory committees of MabQi, Ownards Therapeutics, AbbVie, Roche, and Bristol Myers Squibb; and received honoraria from Gilead, Novartis, Miltenyi, Sanofi, AbbVie, Takeda, Roche, Janssen, Celgene, Novartis, and Bristol Myers Squibb. R.G. was on a congress for Gilead, Sandoz, and Sanofi; and performed teaching for Sanofi. C.C.-L. provided consultancy for Ixaka Ltd; and received honoraria from Gilead Kite. P.A. provided consulting/advisory for Roche, Genmab, Janssen, Bristol Myers Squibb, AbbVie, AstraZeneca, and BeiGene; and received honoraria from Roche, Genmab, Janssen, Bristol Myers Squibb, AbbVie, AstraZeneca, Gilead, and Incyte. V.C. received honoraria from Kite, a Gilead Company, Bristol Myers Squibb, Novartis, Incyte, Kyowa Kirin, AbbVie, Ideogen, and Takeda; received travel fees from Pfizer, Kite, a Gilead Company, Bristol-Myers Squibb, and Novartis; and received research funding paid to the institution from AstraZeneca, Bristol Myers Squibb, Novartis, and Ideogen. S.G. received honoraria from Gilead. E.D. provided consultancy and received honoraria from Stemline Therapeutics and ImmunoGen; and received research funding from Chugai. K.B. provided consultancy and received honoraria from Kite/Gilead, Takeda, and Roche. P.B. received honoraria from Allogene, Amgen, Bristol Myers Squibb, Kite/Gilead, Janssen, Jazz Pharmaceuticals, Miltenyi, Novartis, and Nektar. F.M. provided consultancy and was a member on the board of directors or advisory committees for Roche, Gilead, and AbbVie; and was a member on the board of directors or advisory committees for Novartis, Bristol Myers Squibb, Genmab, AstraZeneca, Janssen, Allogene Therapeutics, and Miltenyi. R.H. received honoraria from Bristol Myers Squibb, Celgene, Gilead Sciences, Incyte, Janssen, Kite, Merck Sharp & Dohme, Novartis, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Pere Barba, Department of Hematology, Hospital Universitari Vall d’Hebron. Universitat Autònoma de Barcelona, Pg Vall Hebron 119, 08035 Barcelona, Spain; email: pbarba@vhio.net.
References
Author notes
G. Crochet and G.I. are joint first authors.
F.M. and R.H. are joint senior authors.
Data will be made available on reasonable request to the corresponding author, Pere Barba (pbarba@vhio.net).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.