In this issue of Blood, Gulla et al identify GABA type A receptor-associated protein, GABARAP, as a key player in mediating resistance to immunogenic chemotherapy in multiple myeloma (MM), by halting calreticulin translocation to the external leaflet of the cell membrane. By shedding light on immunogenic cell death (ICD), the authors provide a framework for potential novel combination treatments including ICD inducers to restore anti-MM immunity.1
The recognition and removal of stressed, ageing, and dying cells by phagocytes is vital for maintaining health. These homeostatic processes also play a crucial role in the immune response against cancer. During ICD, deteriorating tumor cells shed specific signals conducive to phagocytosis, including the exposure of calreticulin, an endoplasmic reticulum protein, on the cell surface. This presentation of an “eat me” signal facilitates the engulfment of cancer cell by antigen-presenting cells (APCs) such as dendritic cells and macrophages. These APCs subsequently process and present tumor antigens to T cells, thereby initiating an adaptive antitumor immune response.2,3 Cancer cells can exploit various pathways to overcome the induction of ICD. Thus, a deeper understanding of the biologic mechanisms involved by these cancer cells and devising strategies to counteract them is an important strategy to improving cancer treatment.4
Gulla et al reported in 2021 that bortezomib induces calreticulin-dependent ICD in MM cells.5 The induction of ICD is associated with a strong antitumor immune response in immunocompetent mice, with the simultaneous induction of immunological memory. Thus, a low dose of bortezomib produces tumor regression in mice bearing syngeneic tumors.5
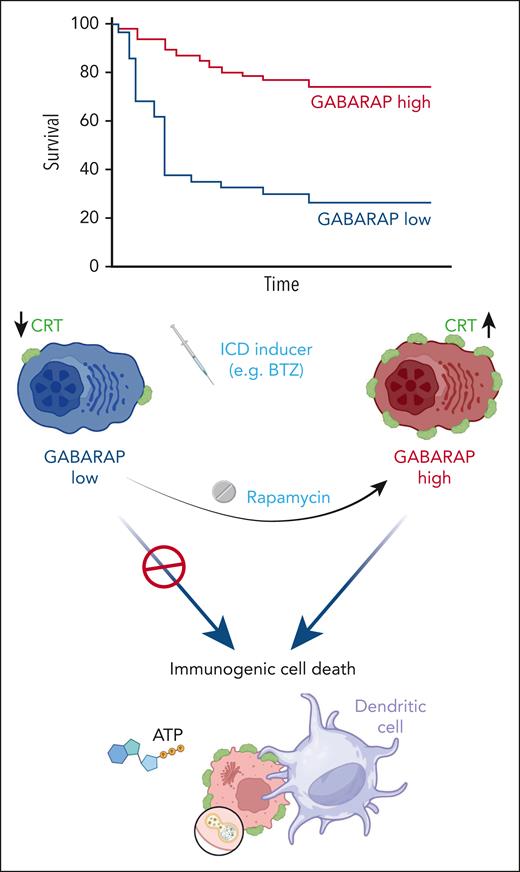
Here, Gulla et al investigated the mechanisms involved in resistance to the immunogenic effects of bortezomib in MM. They analyzed a public data set to identify the prognostic role of GABARAP, a well-described vesicular trafficking and autophagy regulator.6 Next, by using MM cell lines, animal models and single-cell RNA sequencing, the authors identified GABARAP as a mediator of bortezomib resistance, by modulating phagocytosis, T cell–mediated cell lysis, and autophagy. Furthermore, the authors found that GABARAP impacts the fitness of organelles such as the Golgi apparatus and endoplasmic reticulum, suggesting its involvement in cellular homeostasis and immune evasion.
Conventionally, cytotoxic sensitivity loss has been linked to extrinsic and intrinsic mechanisms.7 Gulla et al figured out a novel mechanism of drug resistance via GABARAP-calreticulin-dependent ICD. In vitro, a lower level of GABARAP impaired calreticulin exposure and T-cell responses. This finding reveals a mechanism of primary resistance to proteasome inhibition due to a lack of both spontaneous and ICD-mediated anti-MM immunity. Based on the proposed model, a multimodal approach to quantify the effects beyond direct cytotoxicity is needed to fully characterize the clinical impact of these findings.5
Gulla et al also found that rapamycin restored signaling lost with GABARAP knockout during bortezomib treatment, identifying autophagy modulation as a potential clinical strategy. However, ICD is also triggered by other anti-MM therapeutics.7 Therefore, boosting immunogenicity deserves broader exploration, such as the impact on BCMA-, CD38-, SLAMF7-, GPRC5D-, and FcRH5-directed therapies.7 Thus, the GABARAP level might impact other clinical scenarios besides bortezomib-induced ICD. A deeper understanding of the immune response and its impact on drug resistance could pave the way to revive antitumor immunity with combination approaches.
Gulla et al’s research also was based on the hypothesis that MM immune evasion in present in some patients with high-risk disease, as the GABARAP locus is on the short arm of chromosome 17 that is deleted in this subgroup of patients. Indeed, GABARAP expression is broadly downregulated in MM plasma cells compared with normal plasma cells from healthy individuals, and its expression is particularly low in patients with del17p. Consequently, subjects with low GABARAP levels, particularly those with del(17p), may not undergo ICD during bortezomib treatment. Del(17p) in MM correlates with an increased immunosuppressive tumor microenvironment, representing a high-risk signature that may impair calreticulin pathways and diminish tumor recognition by the immune system. Comprehensively characterizing the relationship between underlying tumor genetics, immune status, and “eat me” signal modulation could guide more effective personalized interventions, paving the way for new combination therapy in challenging cases, to overcome aggressive and refractory MM (see figure).8
The absence of GABARAP inhibits the presentation of calreticulin on the cell surface during ICD. Enhancing autophagy alongside immunogenic cell death can reinstate immunogenicity. ATP, adenosine triphosphate; BTZ, bortezomib; CRT, calreticulin.
The absence of GABARAP inhibits the presentation of calreticulin on the cell surface during ICD. Enhancing autophagy alongside immunogenic cell death can reinstate immunogenicity. ATP, adenosine triphosphate; BTZ, bortezomib; CRT, calreticulin.
Sklavenitis-Pistofidis et al showed that aggressive disease behavior biology, as determined by the presence of del(17p), is a significant predictor of shorter PFS in patients with high-risk smoldering MM harboring dysfunctional immuno-microenvironment.9 Therefore, these patients may also benefit from ICD-directed approaches. Collectively, risk stratification may be impacted by the immune status identified in this article.
Finally, immunogenic chemotherapy has been generally associated with long-term therapeutic success.10 Myeloma behaves in much the same way, as demonstrated by an ICD signature obtained interrogating the gene expression data from the newly diagnosed cases enrolled in the Intergroupe Francophone Du Myelome/Dana-Farber Cancer Institute 2009, NCT01191060 trial.5 It is therefore tempting to speculate that the clinical benefit of ICD induction could be explored by prospective studies targeting high-risk ICD signatures. Overall, this study highlights the intricate interplay between autophagy regulators like GABARAP and the immune response to cancer therapies, identifying potential targets for enhancing the efficacy of immunogenic treatments in MM and for overcoming drug resistance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.