In this issue of Blood, Kath et al1 report a novel virus-free strategy to redirect cell specificity via in-frame integration of CD3ζ-deficient chimeric antigen receptors (CARs) within the CD3ζ gene, thereby producing functional CAR fusion genes. This knock-in approach (1) results in T-cell receptor (TCR) ablation; (2) allows physiological CAR regulation by the CD3z promoter; and (3) can be applied to different cell types, that is, TCR-αβ and TCR-γδ effector T cells, regulatory T cells, and natural killer (NK) cells (see figure).
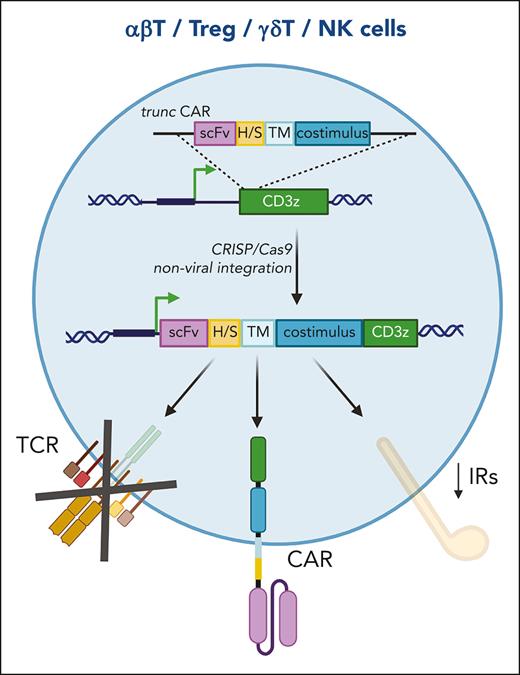
CD3ζ-editing strategy. A CAR missing the activation domain (truncCAR) is integrated in the CD3ζ locus through CRISPR/Cas-9 nonviral technology to obtain a fully functional CAR construct. The proposed strategy brings the CAR under the transcriptional regulation of the CD3ζ promoter, reducing the expression of inhibitory receptors, and ablates the expression of the endogenous TCR. This approach can be applied to all T-cell subsets and NK cells. αβT, T-cell receptor-αβ T lymphocytes; CAR, chimeric antigen receptor; H/S, hinge/spacer sequences; scFv, single-chain fragment variable; TCR, T-cell receptor; TM, transmembrane domain; Treg, regulatory T lymphocytes; γδT, TCRγδ T lymphocytes; NK, natural killer.
CD3ζ-editing strategy. A CAR missing the activation domain (truncCAR) is integrated in the CD3ζ locus through CRISPR/Cas-9 nonviral technology to obtain a fully functional CAR construct. The proposed strategy brings the CAR under the transcriptional regulation of the CD3ζ promoter, reducing the expression of inhibitory receptors, and ablates the expression of the endogenous TCR. This approach can be applied to all T-cell subsets and NK cells. αβT, T-cell receptor-αβ T lymphocytes; CAR, chimeric antigen receptor; H/S, hinge/spacer sequences; scFv, single-chain fragment variable; TCR, T-cell receptor; TM, transmembrane domain; Treg, regulatory T lymphocytes; γδT, TCRγδ T lymphocytes; NK, natural killer.
Most current approaches to generate CAR T cells rely on viral vector–mediated delivery of the CAR transgene into T cells, which results in semirandom integration into the genome and drives CAR expression through strong exogeneous promoters. This approach carries the intrinsic risk of insertional mutagenesis that may promote malignant transformation. Although T cells are considered relatively resistant to genotoxicity, sporadic cases of CAR+ T-cell malignancies have been recently reported using viral vectors and transposons.2,3 Existing data from follow-up studies suggest very low risk with CAR T-cell therapies compared with other cancer treatments, but longer monitoring periods of treated patients and development of safer strategies for CAR redirection are desirable, especially for application in nonlethal diseases such as autoimmune conditions. Kath et al developed a virus-free gene transfer strategy to precisely integrate the CAR gene into the CD3ζ locus, thus reducing the risk of insertional mutagenesis. This approach exploits cell transfection with precomplexed CRISPR-Cas9 ribonucleoproteins and double-stranded DNA, which creates a targeted double-strand break in the CD3ζ locus and serves as a template for homology-directed DNA repair. The resulting fusion gene is composed of the truncated CAR linked to the endogenous CD3ζ, which serves as the activation domain, and is placed under the transcriptional control of the CD3ζ promoter for physiological CAR regulation. The authors report encouraging data regarding off-target assessment, suggesting high-precision CD3ζ targeting. However, caution is called for, because in silico methods can fail to predict experimentally determined off-target sites.
Kath et al show that CD3ζ editing shares some advantages with CAR insertion into the T-cell receptor (TCR) α-chain constant region (TRAC) locus, which is considered the gold standard for gene-edited CAR T cells.4 Importantly, both strategies restrict transgene expression to specific cell lineages, preventing unwanted CAR expression in B-cell leukemic blasts that may cause relapse in the autologous setting.5 Moreover, in both cases, TCR/CD3 complex assembly and expression on the cell surface is impeded, reducing the risk of graft-versus-host disease in the allogeneic setting but still requiring association with strategies to avoid CAR T-cell rejection. Finally, both TRAC and CD3ζ integration allow physiological TCR-like CAR downregulation after antigen engagement, which may prevent T-cell exhaustion and terminal differentiation. The enrichment of less differentiated and poorly exhausted T-cell subsets in the infusion product has been associated with improved antitumor activity and reduced inflammation-related toxicities in preclinical models and patients.6,7 Although excessive signal strength and duration induced by constitutively active promoters may compromise CAR T-cell fitness, calibrated activation and/or transient rest have been reported to prevent terminal differentiation and exhaustion.4,8,9 Despite CAR downmodulation observed with both TRAC and CD3ζ editing upon antigen engagement, the authors reported lower basal expression levels when the CAR is expressed in the CD3ζ with respect to the TCR locus. Further studies are required to identify whether these differences are promoter-related, construct-related (full CAR vs truncated CAR), or procedure-related (more efficient access to one site over the other). Interestingly, the authors show that CAR expression in the CD3ζ locus can be increased by inserting a GSG linker before the 2A self-cleavage peptide. Functionally speaking, although CD3ζ-edited CAR T cells performed similarly overall to their TRAC-edited counterpart, CD3ζ-edited CD19 CAR T cells carrying the GSG linker showed small but significant superiority in vivo. Further investigation of this design using other CAR targets, such as human epidermal growth factor 2, and the relative impact of the selected costimulatory domain over others will be instrumental to highlight specific advantages.
As opposed to TRAC, CD3ζ editing allows a reduction in required transgene size and permits targeted integration in all T-cell subsets and NK cells, which have shown great promise for CAR-based therapy of cancer.10 Kath et al show that CD3ζ disruption in primary human NK cells did not impair canonical NK functions, such as antibody-dependent cellular cytotoxicity and degranulation, and report for the first time nonviral knock-in to redirect NK specificity with CARs. However, these results are still in the proof-of-concept stage and strategies to increase the efficiency of reprogramming are required before testing in suitable in vivo models. Also, the functional edge of having regulated CAR expression in NK cells remain to be proved, because CAR-NK functionality seems more closely linked to immediate cytotoxic activity than to functional persistence.
CD3ζ editing has great potential for use in the allogeneic setting, using either T cells or NK cells. From this perspective, because electroporation can negatively affect cell survival, expansion, and phenotype, a careful setup of the manufacturing procedure is warranted to generate adequate numbers of high-quality CD3ζ-edited CAR products. Allogeneic platforms carry the great advantage of using healthy donors for starting material, overcoming functional defects imprinted in patients by the underlying disease or previous treatments. Moreover, these strategies would make the treatment available to patients with lymphopenia and speed up drug administration for rapidly progressing diseases. Overall, this would result in simpler manufacturing procedures and reduced costs, allowing for full exploitation of CAR-based approaches.
Kath and colleagues’ strategy provides important improvements for engineered T-cell therapies. Regulated expression through the CD3ζ promoter may improve antitumor efficacy by reducing excessive chronic activation. Nonviral integration of the CAR gene into a specific locus can reduce the risk of insertional mutagenesis. The expression of the CD3ζ gene in different cell types permits editing of all T-cell subsets and NK cells. Finally, their approach is suitable for application in the allogeneic setting, expanding the use of CAR-based therapies to a wider cohort of patients.
Conflict-of-interest disclosure: M.C. is inventor on different patents on cancer immunotherapy, has received research support from Kite/Gilead, and is a consultant for Alia Therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal