Key Points
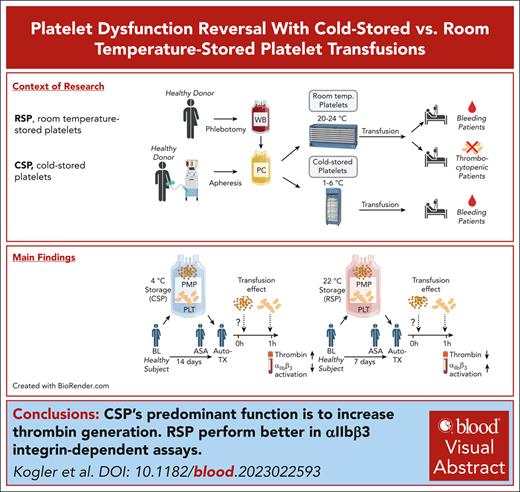
CSPs increase thrombin generation in recipients but fail to show integrin activation.
Procoagulant function of CSPs can be predicted by lyso-platelet activating factor species, even before storage.
Visual Abstract
Platelets are stored at room temperature for 5 to 7 days (room temperature–stored platelets [RSPs]). Because of frequent and severe shortages, the US Food and Drug Administration recently approved up to 14-day cold-stored platelets (CSPs) in plasma. However, the posttransfusion function of CSPs is unknown and it is unclear which donors are best suited to provide either RSPs or CSPs. In this study, we sought to evaluate the posttransfusion platelet function and its predictors for platelets stored for the maximum approved storage times (7-day RSPs and 14-day CSPs) in healthy volunteers on acetylsalicylic acid (ASA). We conducted a randomized crossover study in 10 healthy humans. Individuals donated 1 platelet unit, stored at either 22°C or 4°C based on randomization. Before transfusion, participants ingested ASA to inhibit endogenous platelets. Transfusion recipients were tested for platelet function and lipid mediators. Platelet units were tested for lipid mediators only. A second round of transfusion with the alternative product was followed by an identical testing sequence. RSPs reversed platelet inhibition significantly better in αIIbβ3 integrin activation–dependent assays. In contrast, CSPs in recipients led to significantly more thrombin generation, which was independent of platelet microparticles. Lysophosphatidylcholine-O species levels predicted the procoagulant capacity of CSPs. In contrast, polyunsaturated fatty acid concentrations predicted the aggregation response of RSPs. In summary, we provide, to our knowledge, the first efficacy data of extended-stored CSPs in plasma. Our results suggest that identifying ideal RSP and CSP donors is possible, and pave the way for larger studies in the future. This trial is registered at www.ClinicalTrials.gov as #NCT0511102.
Introduction
The factors that determine platelet function upon transfusion are unknown. Although some platelet storage markers correlate with circulation time and recovery, stored platelet posttransfusion function is currently impossible to predict by pretransfusion in vitro tests. One parameter that recently regained attention is storage temperature. Room temperature storage of platelets for up to 5 to 7 days is the current gold standard because it maximizes circulation time. However, maintaining room temperature–stored platelets (RSPs) is difficult and expensive; they are the most common cause of transfusion-transmitted infection, and recent clinical trials cast doubt on their efficacy and safety.1,2 Cold platelet storage could alleviate these shortcomings.3 However, the most promising data on the efficacy of extended cold-stored platelets (CSPs) in plasma comes from in vitro testing showing a procoagulant phenotype combined with superior or similar integrin function as RSPs.3-11 Recently, the US Food and Drug Administration (FDA) effectively allowed all blood banks in the United States to manufacture and transfuse up to 14-day-stored CSPs to patients who are actively bleeding (“if RSPs are unavailable or not practical”) despite a paucity of posttransfusion data.12 A pilot trial compared extended-stored CSPs with a historic RSP control group in patients receiving cardiac surgery. In that study, RSPs and CSPs were stored in additive solution (platelet additive solution-E, SSP+; Macopharma, Tourcoing, France), a platelet preparation not licensed in the United States.13 A recent retrospective study found delayed CSPs (ie, units that were first room temperature (RT)-stored then cold-stored just before expiration) were associated with increased postoperative transfusion requirements.14 Historic reports on the posttransfusion function of CSPs yielded contradictory results.15-20 Although the body of literature supporting the advantageous in vitro function of extended-stored CSPs is expansive, it is currently unknown to what extent, if any, the in vitro function of CSPs translates into in vivo or posttransfusion function. Adding urgency to the subject is the increasing difficulty of maintaining an adequate platelet inventory for blood centers and hospitals.21-23
Bioactive lipids, such as lysoglycerophospholipids (LPLs), oxylipins and their precursors, and polyunsaturated fatty acids (PUFAs), are critical mediators of platelet function and membrane stability.24-26 However, their evaluation thus far has been limited to the pretransfusion settings, that is, fresh or stored platelets.27,28 Previously described mechanisms include the activation of the prostacyclin receptor and the inhibition of the peroxisome proliferator activator receptor α.29,30 Lysophosphatidylcholine-O (LPC-O) species increase cyclic adenosine monophosphate concentrations in platelets and inhibit thrombin-induced platelet aggregation.31 In the platelet transfusion setting, certain oxylipins correlate with platelet recovery and survival.32 Therefore, in this study, we sought to address 2 critical knowledge gaps: (1) we compared the posttransfusion hemostatic function of autologous 7-day RSPs (the current clinical maximum) with that of 14-day plasma-stored CSPs (the maximum allowable storage time recently approved by the FDA) in a randomized crossover trial with healthy humans treated with acetylsalicylic acid (ASA), and (2) we tested for LPLs, oxylipins, and PUFAs in storage bags and recipients to identify predictors for posttransfusion function in recipients of CSPs and RSPs. The prespecified primary end point was platelet aggregation in whole blood (VerifyNow ASA, Accumetrics Corp, San Diego, CA) at 1 hour posttransfusion to measure the reversal of ASA antiplatelet therapy by platelet transfusion. Secondary and exploratory end points included platelet function tests, hemostasis tests, microparticle analyses, and lipidomic analyses.
Methods
An extended version of “Methods” is available in the supplemental Material, available on the Blood website.
Healthy human individuals research (including CONSORT criteria)
Volunteers were recruited via paper and Internet advertisements from December 2020 to September 2021 and enrolled at the Bloodworks Northwest Research Institute in Seattle, WA. Inclusion criteria were self-reported good health, age between 18 and 59 years, temperature of ≤99.5°F, systolic blood pressure of ≤180 mm Hg; diastolic blood pressure of ≤100 mm Hg, and heart rate of 40 to 100 beats per minute. Exclusion criteria were personal or family bleeding history; a history of thrombosis; and a history of, or currently prescribed, antiplatelet or anticoagulant agents, or other drugs known to significantly affect platelet function (full list of criteria is in the Study Protocol, available in the supplemental Material).
Participants were randomly assigned to receive CSPs or RSPs first. No formal blinding was performed. In each period, participants underwent plateletpheresis with storage at either room temperature or 4°C. ASA (325 mg) was administered 24 hours before transfusion. Testing was done at baseline (pre-ASA); before transfusion (post-ASA); and 1 hour, 4 hours, and 24 hours after transfusion. The study design is shown in Figure 1A. Adverse events were assessed at all study visits and by participant reports. A protocol change to include a no-transfusion control group was planned but not implemented. The Western IRB Copernicus Group institutional review board approved the study. Written informed consent was obtained. We conducted the study in accordance with the Declaration of Helsinki. All authors had access to the reported data.
Effect of RSP and extended-stored CSP transfusion in healthy humans on ASA. (A) In each of the 2 periods, healthy humans underwent plateletpheresis with platelet storage (randomized to RSPs or CSPs). The healthy humans then received an ASA loading dose, 24 hours before transfusion, and autologous platelet transfusion with blood assessments at baseline (BL; ie, without ASA), before transfusion (ASA), and at multiple time points after transfusion (1 hour, 4 hours, and 24 hours). Participants completed an RSP period (receiving 7-day RT-stored platelets) and a CSP period (receiving 14-day 4°C-stored platelets) as outlined in this overview schematic. (B) The number of RT (red circles) and 4°C (blue circles) total platelets transfused. The platelet count (C) and corrected count increments (CCI) (D) during each period (red circles: RSP period; blue squares: CSP period; 4 hours, ∗P = .0204; 24 hours, ∗∗P = .0047). (E) Platelet AA–stimulated whole-blood aggregation was measured by VerifyNow ASA (1 hour, ∗∗P = .0055; 24 hours, ∗P = .0198). Maximum light transmission aggregation (defined as maximum aggregation recorded [in percent] within 10 minutes after agonist stimulation) in response to 0.5 mM AA (1 hour, ∗P = .0347; 4 hours, ∗∗P = .0081) (F), and collagen (2.5 μg/mL; 24 hours, ∗P = .0493) (G). The thrombin generation potential of the participant’s whole blood–derived PRP was measured immediately before and 1 hour after transfusion with a commercially available fluorogenic thrombin generation assay. Results are reported as the change from the pretransfusion value to the 1-hour posttransfusion value for thrombin generation peak (defined as maximum concentration during recording; ∗P = .0429) (H), start tail time (time until end of thrombin generation; ∗P = .0266) (I), time to thrombin peak (time between begin and peak of thrombin generation) (J), and lag time (time from assay start to begin of thrombin generation) (K). Data are shown as mean ± standard error of the mean (SEM), panels H-K shown with individual values. Unpaired data, n = 7 to 9, individual P values are shown in the text above. RT, room temperature.
Effect of RSP and extended-stored CSP transfusion in healthy humans on ASA. (A) In each of the 2 periods, healthy humans underwent plateletpheresis with platelet storage (randomized to RSPs or CSPs). The healthy humans then received an ASA loading dose, 24 hours before transfusion, and autologous platelet transfusion with blood assessments at baseline (BL; ie, without ASA), before transfusion (ASA), and at multiple time points after transfusion (1 hour, 4 hours, and 24 hours). Participants completed an RSP period (receiving 7-day RT-stored platelets) and a CSP period (receiving 14-day 4°C-stored platelets) as outlined in this overview schematic. (B) The number of RT (red circles) and 4°C (blue circles) total platelets transfused. The platelet count (C) and corrected count increments (CCI) (D) during each period (red circles: RSP period; blue squares: CSP period; 4 hours, ∗P = .0204; 24 hours, ∗∗P = .0047). (E) Platelet AA–stimulated whole-blood aggregation was measured by VerifyNow ASA (1 hour, ∗∗P = .0055; 24 hours, ∗P = .0198). Maximum light transmission aggregation (defined as maximum aggregation recorded [in percent] within 10 minutes after agonist stimulation) in response to 0.5 mM AA (1 hour, ∗P = .0347; 4 hours, ∗∗P = .0081) (F), and collagen (2.5 μg/mL; 24 hours, ∗P = .0493) (G). The thrombin generation potential of the participant’s whole blood–derived PRP was measured immediately before and 1 hour after transfusion with a commercially available fluorogenic thrombin generation assay. Results are reported as the change from the pretransfusion value to the 1-hour posttransfusion value for thrombin generation peak (defined as maximum concentration during recording; ∗P = .0429) (H), start tail time (time until end of thrombin generation; ∗P = .0266) (I), time to thrombin peak (time between begin and peak of thrombin generation) (J), and lag time (time from assay start to begin of thrombin generation) (K). Data are shown as mean ± standard error of the mean (SEM), panels H-K shown with individual values. Unpaired data, n = 7 to 9, individual P values are shown in the text above. RT, room temperature.
VerifyNow ASA
VerifyNow ASA was a test sent out to the University of Washington clinical laboratory at Harborview (Seattle, WA). Briefly, whole blood was collected in a 3.2% sodium citrate tube, transported to the laboratory, and inserted into the VerifyNow instrument with an ASA test cartridge. The platelet aggregation induced by arachidonic acid (AA) in whole blood was measured as aspirin reaction units (ARUs), a readout of light transmission through the sample. An ARU of <550 indicates platelet dysfunction by cyclooxygenase-1 (COX-1) inhibition with, for example, ASA.
Thrombin generation assay
Platelets were obtained from transfusion recipients and sampled from storage bags. After platelets were removed from the bag, they were diluted with separately stored, autologous fresh frozen plasma (stored at −80°C on the day of collection) to a target concentration of 1 × 105/μL. The diluted platelets were tested in a commercially available fluorogenic Stago Thrombinoscope assay (Stago, Parsippany, NJ, USA).
Light transmission aggregometry
Platelets were isolated from 3.2% citrated whole blood as platelet-rich plasma (PRP) by centrifugation at 200g for 5 minutes and adjusted to 3 × 105/μL with autologous platelet-poor plasma (PPP). We performed the experiment at 37°C under stirring conditions (1200 rpm). After the addition of agonists (0.5 mM AA, or 2.5 μg/mL collagen), light transmission was recorded over 10 minutes on a Chrono-log 4-channel optical aggregation system (Chrono-log, Havertown, PA).
PMP characterization
We followed previously described methods to initially prepare and freeze samples.33 In brief, whole blood in 3.2% sodium citrate was collected from transfusion recipients, centrifuged for 20 minutes at 1550g, and the cell-depleted supernatant was frozen. For stored platelet samples, PRP was spun and stored identically. Samples for flow cytometry were tested with a CytoFLEX LX Flow Cytometer (Brea, CA). Microparticles (MPs) were identified by size and defined as large (0.5-μm to 1-μm equivalent) and small (0.1-μm to 0.5-μm equivalent) with the help of nanobeads, as outlined in Figure 2A-C. Platelet MPs (PMPs) were identified in the MP gates by size, defined as CD41a-positive events and as phosphatidyl serine (PS)-positive events with fluorescein isothiocyanate–labeled lactadherin (Haematologic Technologies Prolytix, Essex Junction, VT), P-selectin positive with anti-CD62P-PE, and isotype control (all BD Pharmingen, Franklin Lakes, NJ; Figure 2D-F).
Increased small PMPs and thrombin generation with CSPs in vitro. (A) After platelet storage at both RT and 4°C conditions, PPP was prepared by centrifugation with 1 freeze-thaw cycle before testing. (B) Using the CytoFLEX flow cytometer, and nanobeads, we identified MPs with a large MP size gate (1-μm equivalent to 0.5-μm equivalent) and a small MP size gate (between 0.5-μm and 0.1-μm equivalent beads). (C) The gates were applied to MPs in plasma. (D) Within the respective large and small MP size gates, PMPs were defined as all CD41a+ events. Both large and small PMPs were further gated for lactadherin (E) or CD62P (gray trace indicates isotype control) (F) positivity within the CD41+ gate. (C-F) Example data from stored platelet samples and gating for large-sized events. (G) PMPs of both RSPs and CSPs were identified as events within small and large size gates that were CD41a+ and the concentration of each reported (large, ∗P = .0369; small, ∗∗∗∗P < .0001; small vs large, RSPs ∗∗P = .002; CSPs ∗∗∗∗P < .0001). The concentration of large and small PMPs that were also positive for lactadherin (small, ∗P = .0113; small vs large, CSPs ∗∗P = .005, RSPs ∗∗P = .002) (H) or CD62P (small, ∗∗∗P = .0002; small vs large, RSPs ∗P = .04; CSPs ∗∗∗∗P < .0001) (I). The MP-mediated thrombin generation potential of PPP from storage bags was measured and reported as thrombin generation peak (∗P = .0202) (J), start tail time (∗P = .0479), (K), time to thrombin peak (L), and lag time (M). Data are shown as mean ± SEM and individual values. Unpaired data, n = 9, individual P values are shown in the text above. RT, room temperature.
Increased small PMPs and thrombin generation with CSPs in vitro. (A) After platelet storage at both RT and 4°C conditions, PPP was prepared by centrifugation with 1 freeze-thaw cycle before testing. (B) Using the CytoFLEX flow cytometer, and nanobeads, we identified MPs with a large MP size gate (1-μm equivalent to 0.5-μm equivalent) and a small MP size gate (between 0.5-μm and 0.1-μm equivalent beads). (C) The gates were applied to MPs in plasma. (D) Within the respective large and small MP size gates, PMPs were defined as all CD41a+ events. Both large and small PMPs were further gated for lactadherin (E) or CD62P (gray trace indicates isotype control) (F) positivity within the CD41+ gate. (C-F) Example data from stored platelet samples and gating for large-sized events. (G) PMPs of both RSPs and CSPs were identified as events within small and large size gates that were CD41a+ and the concentration of each reported (large, ∗P = .0369; small, ∗∗∗∗P < .0001; small vs large, RSPs ∗∗P = .002; CSPs ∗∗∗∗P < .0001). The concentration of large and small PMPs that were also positive for lactadherin (small, ∗P = .0113; small vs large, CSPs ∗∗P = .005, RSPs ∗∗P = .002) (H) or CD62P (small, ∗∗∗P = .0002; small vs large, RSPs ∗P = .04; CSPs ∗∗∗∗P < .0001) (I). The MP-mediated thrombin generation potential of PPP from storage bags was measured and reported as thrombin generation peak (∗P = .0202) (J), start tail time (∗P = .0479), (K), time to thrombin peak (L), and lag time (M). Data are shown as mean ± SEM and individual values. Unpaired data, n = 9, individual P values are shown in the text above. RT, room temperature.
Analysis of free LPLs, oxylipins, and PUFAs
As described previously, free LPLs, oxylipins, and PUFAs in platelets were analyzed by liquid chromatography–tandem mass spectrometry.34-36 Briefly, analytes were extracted by 80% methanol (vol/vol) with an internal standard mixture containing deuterium-labeled analogs (Cayman Chemical) and 17:1 lysophospholipids (Avanti Polar Lipids). Analytes were detected using multiple reaction monitoring in the negative ion mode and were quantified by peak area relative to each corresponding internal standard.
Statistics
Based on previous study results, a minimum sample size of 6 was required to provide >98% power to detect a difference of 130 ARUs for the primary end point between CSPs and RSPs. The protocol specified a statistical analysis plan between RSPs and CSPs with a 2-tailed, paired t test. Because 2 volunteers only completed 1 treatment period (1 CSP and 1 RSP), an unpaired post hoc analysis was also performed. A P value of ≤ .05 was considered significant, except for multiple comparisons in lipidomic experiments, in which a false discovery rate of 1% was used (GraphPad Software, Prism 6.05). Pearson correlation coefficient was calculated to test for linear correlation. Liquid chromatography–tandem mass spectrometry multiple reaction monitoring data were analyzed with MetaboAnalyst Software 5.0.37
Results
Healthy human participant demographics and recruitment
Over 10 months, 11 healthy participants were enrolled. One participant was excluded because of abnormal baseline platelet function. One participant did not complete the RSP period because of the low pH of the stored platelets and 1 participant did not complete the CSP period because of a scheduling conflict (supplemental Figure 1), but both completed the corresponding alternative transfusion. In aggregate, 9 data sets are available for both treatment periods, 8 of which represent matched (paired) data, whereas 2 additional participants provided an unpaired data set. For most storage unit data, 9 paired data sets are available. Participant baseline characteristics are summarized in supplemental Table 1.
Platelet unit characteristics and safety assessment
All platelet units passed quality control assessments after storage except for 1 RSP, which had to be discarded because of low pH. All transfused platelets were tolerated well by the recipients, but 1 adverse event occurred (headache) that was considered unrelated to transfusion because it occurred 48 hours after the intervention. The platelet concentration in the storage bag did not differ at baseline (supplemental Figure 2A). Over storage time, cold temperature reduced the platelet concentration significantly compared with RT (supplemental Figure 2A), possibly because of observed aggregates or microaggregates.38 However, some studies described a platelet count decrease mediated by a hitherto unknown mechanism, even in the absence of microaggregates.39,40 The absolute number of transfused platelets and the number of transfused platelets as a percentage of absolute (endogenous and transfused) circulating platelets did not differ significantly between the 2 treatment groups (Figure 1B; supplemental Figure 2B).
Platelet transfusion parameters
There was no significant difference in recipients’ absolute platelet counts before or after transfusion between RSP and CSP groups (Figure 1C), but after accounting for the recipient’s body surface area, the number of transfused platelets, and the platelet count increment (known as corrected count increments), we found significantly lower corrected count increments in the CSP group after 4 hours and 24 hours (Figure 1D). The average time between the 2 transfusion periods was 22 days, and the shortest was 11 days (supplemental Table 1).
Function of transfused platelets
We observed normal platelet function at baseline and appropriate inhibition of platelet function after ASA dosing (Figure 1E). Transfusion of RSPs significantly improved platelet function as measured by VerifyNow ASA at 1 hour (which was the prespecified primary end point of the study) and at 24 hours (prespecified secondary outcome; P = .0055 and P = .0198, respectively) but not at 4 hours after transfusion compared with CSPs (Table 1; Figure 1E). Transfusion of CSPs failed to overcome the effect of ASA at any time. Similarly, platelet aggregation in response to AA showed improved platelet response 1 hour (P = .03) and at 4 hours (P = .008) after transfusion with RSPs than with CSPs (Figure 1F). ASA led to a modest inhibition of collagen-mediated platelet aggregation, and recipients of RSPs were significantly more responsive to collagen 24 hours after transfusion (Figure 1G).
To clarify the potential procoagulant role of CSP transfusion, we measured thrombin generation before and 1 hour after transfusion in PRP derived from recipients’ whole blood. We found a statistically significant increase in peak thrombin generation in recipients after CSP transfusion compared with RSPs (P = .04; Figure 1H). In accordance, we found a shorter time-to-peak thrombin generation without significance (Figure 1I), and a significantly shorter tail time (Figure 1J). No significant change was seen for lag time (Figure 1K).
PMPs before and after transfusion
To test whether CSPs themselves or CSP-derived microparticles were responsible for the increase in peak thrombin, we first assessed the PPP of stored platelet units for PMPs (Figure 2A). To allow for the detection of small PMPs as small as 100 nm, we used a CytoFLEX flow cytometer and validated small PMP detection using a multicolor approach that allowed for simultaneous assessment of CD41a, lactadherin, and CD62P (Figure 2A-F). Small MPs were significantly more abundant in CSPs than in RSPs with all gating strategies, including pan-platelet marker–positive events (CD41a; Figure 2G), PS-positive events (Figure 2H), and P-selectin–positive events (Figure 2I). Large PMPs were only significantly more abundant in CSPs than in RSPs in the pan-platelet gate (Figure 2G). Similarly, small PMPs were significantly more abundant than large PMPs in both groups (Figure 2G-I). We then tested stored PPP with a thrombin generation assay and observed a significantly increased peak thrombin generation and a reduced tail time in CSP units (Figure 2J-K). We also observed shortened time-to-peak and shortened lag times with pretransfusion PPP, albeit without significance (Figure 2L-M).
To clarify the possible posttransfusion relevance of the additional PMPs in CSPs, we assessed the number of large and small PMPs before and after transfusion in recipients (supplemental Figure 3A). We did not observe any increase in large or small PMPs, not by size, PS, or P-selectin (supplemental Figure 3B-D) after transfusion of either platelet product, suggesting clearance of the PMPs occurred within the first hour. Accordingly, we did not find more thrombin generation in PPP obtained after CSP transfusion (supplemental Figure 3E-H). In fact, PPP derived from whole blood 1 hour after RSP transfusion led to significantly more thrombin generation than after CSP transfusion (supplemental Figure 3E). Together, these findings suggest that our observed increase in thrombin generation 1 hour after CSP transfusion (Figure 1I) was dependent on transfused platelets and independent of PMPs.
Lipid metabolite analyses
We tested platelet storage bags and transfusion recipients for oxylipins, PUFAs, and LPLs, including LPC, lysophosphatidylethanolamine (LPE), lysophosphatidylserine (LPS), and lysophosphatidylinositol (LPI) species. To confirm platelet ASA inhibition, we first measured the changes after a single loading dose. We found significantly lower COX-1–dependent oxylipins, such as thromboxane B2 (TxB2), 12-hydroxyeicosatrienoic acid (12-HHTrE), and 11-hydroxyeicosatetraenoic acid (11-HETE) in volunteers treated with ASA compared with baseline (supplemental Table 2).
Lipid metabolites in stored platelets
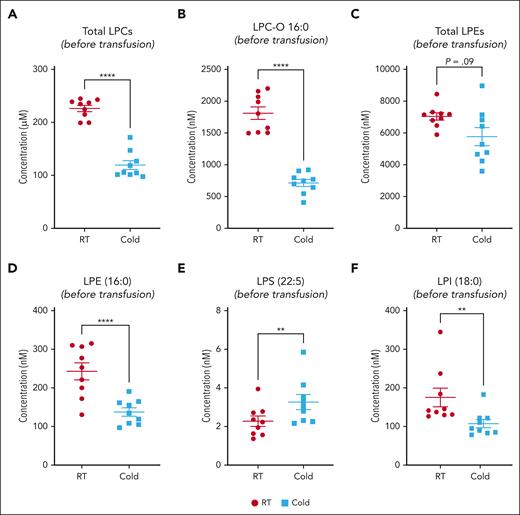
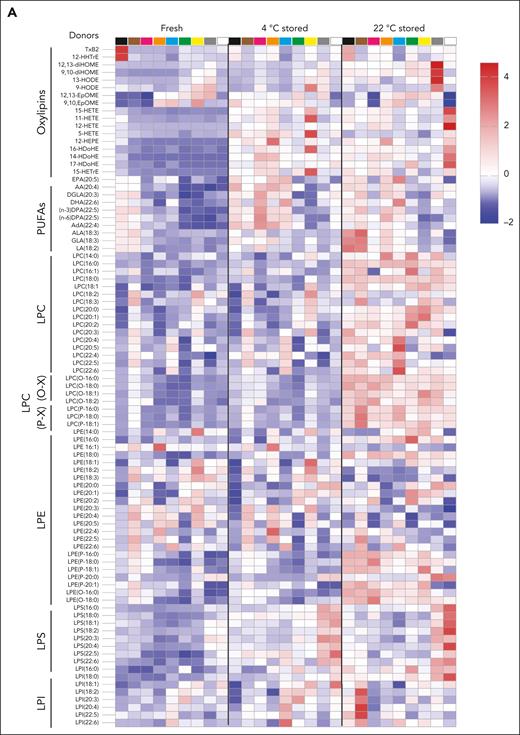
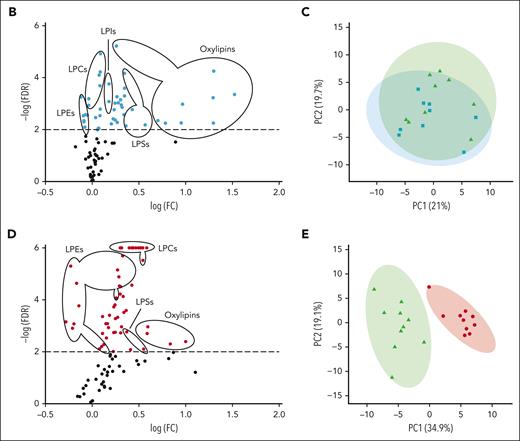
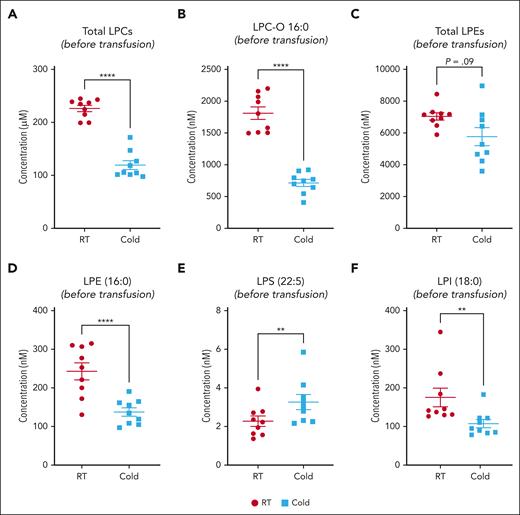
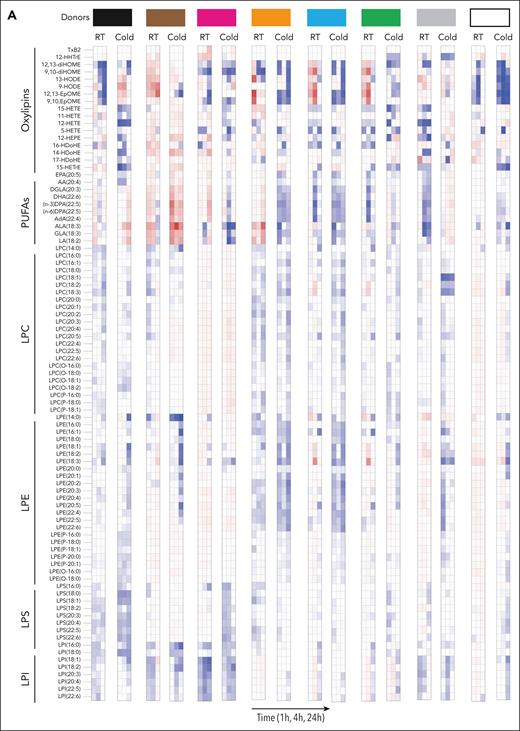
We observed a heterogeneous storage response for oxylipins and PUFAs (Figure 3A); however, LPC species increased more broadly and significantly in RSPs than in CSPs (Figure 3A-B,D). LPS species increased in both storage temperatures but more uniformly in RSPs (Figure 3A-B,D). Changes in LPE species were not uniform, with some species increasing and others decreasing, but the decrease of some LPEs was more pronounced in RSPs than in CSPs (Figure 3B,D). Oxylipins increased to a similar degree at both storage temperatures, but the number of oxylipins with significance and more change from baseline was higher in CSPs (Figure 3B,D). In a principal component (PC) analysis (Figure 3C,E) we found stored CSP samples mixed with baseline values in the 2-dimensional scores plot (PC1 vs PC2), whereas stored RSP samples were clearly separated from baseline (Figure 3C,E). PC1 explained 27% of the variance between baseline and stored CSP and RSP samples (supplemental Figure 4A). The top 15 metabolites from the variable importance in projection plot featured LPC species (including LPC-O and LPC-P species), LPE, and LPI species (supplemental Figure 4B). The concentrations of total LPCs were significantly higher in RSPs than in CSPs, including all LPC-O (16:0, 18:0, 18:1, and 18:2), and LPE species (Figure 4A-C). Certain LPS species were significantly higher in CSPs (Figure 4E), and some LPI species were higher in RSPs (Figure 4F). Because of the high donor-to-donor variability, we also compared the relative increase normalized to baseline (supplemental Figure 5). Total LPC, LPE, and LPI levels increased more in RSPs than in CSPs, whereas total LPS species increased significantly more in CSPs (supplemental Figure 5A-D). Interestingly, all LPC-O species increased significantly more in RSPs than CSPs (supplemental Figure 5E-H). The linoleic acid (LA)-derived oxylipin 12,13-diHOME was the only oxylipin that differed significantly between CSPs and RSPs when normalized to baseline (supplemental Figure 5I-L).
Lipid mediator concentration comparison between RSPs and CSPs. (A-F) Samples at the end of storage were compared between RSPs and CSPs. Data shown as individual values with mean and SEM. n = 9 (paired analysis) with α = 0.05 for total LPL comparisons with Holm-Šídák correction for multiple (n = 4) comparisons. Individual species were tested with FDR of Q = 1% (89 comparisons); ∗∗P < .01 and ∗∗∗∗P < .0001. LPL, lysoglycerophospholipids; RT, room temperature.
Lipid mediator concentration comparison between RSPs and CSPs. (A-F) Samples at the end of storage were compared between RSPs and CSPs. Data shown as individual values with mean and SEM. n = 9 (paired analysis) with α = 0.05 for total LPL comparisons with Holm-Šídák correction for multiple (n = 4) comparisons. Individual species were tested with FDR of Q = 1% (89 comparisons); ∗∗P < .01 and ∗∗∗∗P < .0001. LPL, lysoglycerophospholipids; RT, room temperature.
Lipid metabolites of transfusion recipients
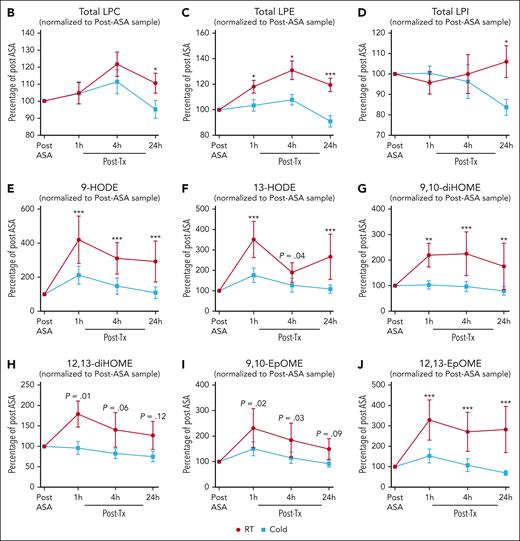
We normalized the recipient lipid mediator data to post-ASA administration to detect specific differences between transfusion of CSPs and RSPs in recipients. In recipients, total LPC, and LPI levels were significantly higher only at 24 hours after transfusion of RSPs, whereas LPE species were significantly higher at 1 hour, 4 hours, and 24 hours after transfusion of RSPs than after transfusion of CSPs (Figure 5A-D). LA-derived oxylipins, derived from cytochrome (CYP) and lipoxygenases, such as dihydroxyoctadecenoic acids (diHOMEs) and hydroxyoctadecadienoic acids (HODEs) were significantly higher in recipients of RSPs than in recipients of CSPs (Figure 5E-J).
Storage and recipient lipid metabolites predictors of posttransfusion platelet function
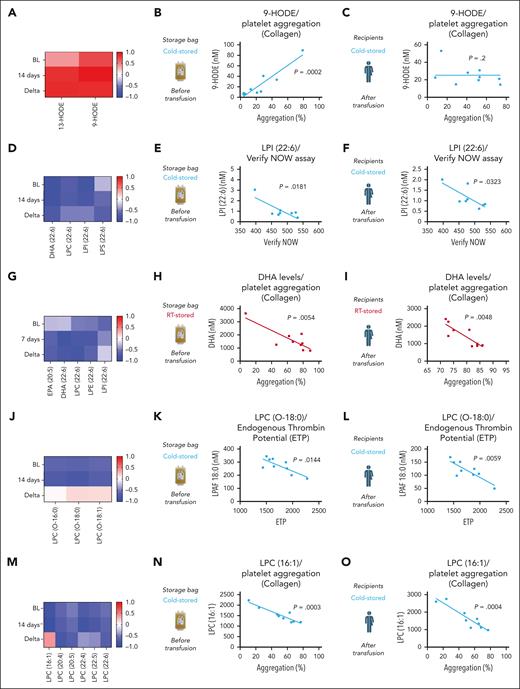
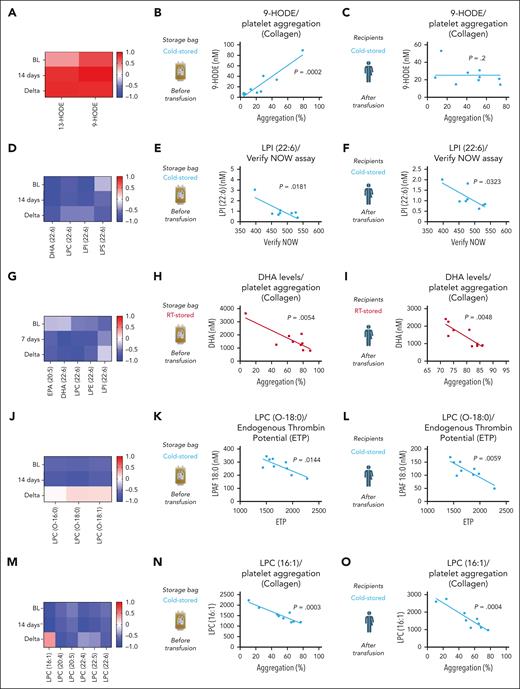
To identify predictors of posttransfusion platelet function we performed correlation analyses of our targeted lipid panel (both storage unit and recipient) and the corresponding platelet function data (from recipients). LA-derived oxylipins (HODES) in storage bags predicted collagen-induced platelet aggregation in CSP recipients (Figure 6A-B). However, the correlation was no longer detectable upon transfusion in the recipient (Figure 6C). LPI species from CSP units and CSP recipients correlated negatively with platelet aggregation in whole blood (Figure 6D-F). PUFAs, including those conjugated to LPL correlated negatively with platelet aggregation in plasma (Figure 6G-I). As shown earlier, CSPs’ main function appears to provide a procoagulant surface after 14 days of storage. Therefore, a predictor of thrombin generation was of high interest. As such, we found LPC-O species to negatively correlate with endogenous thrombin generation potential of stored CSPs and platelets from recipients of CSP transfusion (Figure 6J-L). No correlation was observed upon transfusion between RSPs and LPC-O species (supplemental Figure 6A-C). Because blood banks will have to assign the platelet storage temperature within the first hours after donation, we also tested for prestorage correlation of LPC-O species. Indeed, prestorage levels of LPC-O also predicted recipient endogenous thrombin generation potential (supplemental Figure 6D). For CSPs, we also observed a negative correlation of LPC species with collagen-induced aggregation in both CSP storage unit and CSP transfusion recipient (Figure 6M-O).
Correlation of lipid mediators and platelet function parameters. The left column (panels A,D,G,J,M) shows the correlation coefficient r in heat maps for BL, end of storage (7 and 14 days, respectively), and for the difference between BL and end of storage (Delta). The middle column (panels B,E,H,K,N) shows the correlation between platelet functional parameters and storage bag lipid mediator concentration. The right column (panels C,F,I,L,O) shows the correlation between platelet functional parameters and recipient bioactive lipid mediator concentration. Data are shown as heat maps and linear regression analysis plots; n = 8 to 9 in each group, and individual P values are shown in each figure. DHA, docosahexaenoic acid; ETP, endogenous thrombin potential.
Correlation of lipid mediators and platelet function parameters. The left column (panels A,D,G,J,M) shows the correlation coefficient r in heat maps for BL, end of storage (7 and 14 days, respectively), and for the difference between BL and end of storage (Delta). The middle column (panels B,E,H,K,N) shows the correlation between platelet functional parameters and storage bag lipid mediator concentration. The right column (panels C,F,I,L,O) shows the correlation between platelet functional parameters and recipient bioactive lipid mediator concentration. Data are shown as heat maps and linear regression analysis plots; n = 8 to 9 in each group, and individual P values are shown in each figure. DHA, docosahexaenoic acid; ETP, endogenous thrombin potential.
Discussion
Our study provides, to our knowledge, the first posttransfusion head-to-head comparison of CSPs and RSPs stored in plasma up to the maximum FDA-approved storage times. We assessed transfused platelet quality in a mixed population of endogenous (COX-1 inhibited) platelets and transfused platelets with uninhibited COX-1 function. When our testing indicated none, or reduced ASA effect in the VerifyNow assay, transfused platelets generated enough thromboxane to cause platelet aggregation in the mixed population, thereby normalizing the hemostatic response. This approach has been established before in other studies to test transfused platelet function.41 ASA’s active metabolite, salicylic acid, has a half-life of 3 to 4 hours and causes irreversible COX-1 inhibition, making it highly unlikely that ASA affected the transfused platelet population. In historic studies, 3-day stored CSPs led to a more rapid and potent reversal of the effect of ASA as measured by bleeding time, contradicting our findings.18,20 However, the difference between 3 and 14 days of storage is large enough to suggest that it could be responsible for the functional decrease of CSPs observed in our study. In our previous crossover study using aspirin and clopidogrel in healthy humans and comparing the effect of 5-day RSPs and 5-day CSPs we found equivalence in the VerifyNow ASA assay, and light transmission aggregometry with AA.11 Although we still observed improvement with 7-day RSPs in this study, this effect was essentially nonexistent with 14-day CSPs, contradicting a large body of pretransfusion literature.7,8,38,42-44 Extended-stored CSPs undergo irreversible shape change.45 A possible explanation for our findings is that this irreversible shape change affects their ability to rejuvenate in vivo upon transfusion, but this needs to be investigated further. Surprisingly, we did not observe ASA reversal with RSPs at the VerifyNow 4-hour time point. The VerifyNow test was a send-out test and was likely affected by delayed after-hours testing, in contrast to the in-house aggregometry test, which shows sustained ASA reversal. In our study, CSPs were significantly more procoagulant in transfusion recipients suggesting that the hemostatic effect of extended-stored CSPs may be more akin to cryopreserved (ie, frozen) or lyophilized platelets, albeit with a shorter circulation time.46-48 Yet, although we show a statistically significant increase in thrombin generation parameters with CSP transfusion compared with control, it is unclear whether the difference we observed 1 hour after transfusion is clinically meaningful.
A historical assessment of CSP PMPs observed an increased number of smaller MPs.48 Nash et al published pretransfusion data on CSPs and RSP-derived PMPs using the same high-resolution flow cytometer that we used.49 In accordance with our findings they describe more PMPs in CSPs at 14 days than in RSPs at 7 days. The hemostatic function did not differ between CSPs and RSPs when they adjusted the PMP numbers. In our study, PMPs were not responsible for the increase in thrombin generation in recipients of CSPs. Instead, we found transfused platelets to be responsible for the procoagulant effect. One important limitation is that we investigated autologous CSPs in healthy humans and assessed the platelet function recovery after transfusion. Testing CSPs in healthy volunteers allows us to understand the impact on platelet function and recovery in detail, before testing their use in patients with bleeding or those at risk of bleeding (the current indications of CSPs). In addition, this approach eliminates potentially important variables, such as ABO incompatibility. Using ASA reversal can be considered a surrogate marker for hemostatic function in patient with trauma or undergoing surgery. The earliest testing time point for platelet function was 1 hour after transfusion. Recipients of CSPs may experience a more substantial, immediate but short-lived (<1 hour) procoagulant impact after transfusion due to transfused PMPs. Very limited data are available on the in vivo clearance kinetics of PMPs in transfused platelets. A previous report in patients with thrombocytopenia reports a half-life of large PMPs of 5.8 hours using conventional flow cytometry.33 The clearance likely depends on the phenotype and size of PMPs, as well as recipient factors. In contrast to other published data,33 we did not see a PMP increase, after either transfusion of RSPs, or CSPs. One possible explanation is that clearance of PMPs is accelerated in healthy humans compared with patients with multiple morbidities. Potential mechanisms include splenic sequestration, and cell–PMP adhesion.
The relatively low inclusion number of 10 healthy volunteers is a limitation, but by using a crossover design and thereby using each participant as their own control, we eliminated the between-subject variability. Combining targeted lipid metabolite analysis of stored and transfused platelets before and after ASA dosing allowed us to not only analyze lipid levels but also to correlate them with functional data to identify predictors of RSP and CSP function, respectively. Lipids are important in this context because they have been shown to influence posttransfusion platelet function in mice.26 PC-O–linked species are highly proinflammatory bioactive lipids released by various cells upon stimulation. We found LPC-O species (the deacetylated form of PC-O) to have a negative predictive effect on thrombin generation potential. LPC-O species inhibit platelet aggregation and thereby oppose the effect of PC-O species, but the negative correlation with thrombin generation after cold storage suggests that LPC-O species could also be inhibitors of their procoagulant function, which has not been described before.31 We found thrombin generation to be the main function of extended-stored platelets in plasma. Therefore, in the future, measuring LPC-O levels may help to decide which platelets are most procoagulant and are best suited for cold storage.
Numerous previous studies have described an inhibitory effect of ω-3 PUFAs on platelet aggregation, but the effect on stored platelet function and posttransfusion platelet function was unknown.50-53 In our study, PUFAs in the storage bag and recipients were strong predictors for reduced aggregation after transfusion. Whether this is predictive of decreased or increased protection from hemorrhage in transfusion recipients remains to be investigated. One previous study reported on the metabolomics of CSPs but did not include bioactive lipid mediators or functional data.54
Our data suggest limitations on the predictive value of many common in vitro assays for the posttransfusion function of stored platelets. Although we support our findings with mechanistic insight through targeted lipid mediator analysis and identify markers with possible improved predictive value, caution is warranted until further data from randomized clinical trials in patients with cancer or trauma, or those undergoing surgery are available.
Acknowledgments
The authors thank Renetta Stevens, Derek Nazareth, and Tena Petersen for administrative support. The authors thank Amily Guo and Xiaoping Wu for helping with the platelet microparticle analysis.
M.S. received funding from the American Society of Hematology (ASH Scholar Award), the National Institutes of Health, National Heart, Lung, and Blood Institute (1R01HL153072-01), and the Department of Defense (W81XWH-12-1-0441, EDMS 5570).
Authorship
Contribution: V.J.K. and J.A.M. performed experiments, analyzed data, and wrote the manuscript; T.Ö. analyzed data, wrote the manuscript, and designed and created figures for the manuscript; S.L.B. and D.A.B. performed experiments and analyzed data; M.B.-R. recruited and consented patients, and performed platelet collections; Y.W., H.J.J., and F.R. performed experiments and analyzed data; X.F. designed experiments, analyzed data, and edited the manuscript; M.S. outlined the study, designed experiments, analyzed data, and wrote a first draft of the manuscript; and all authors provided feedback on the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.S. has received research support from Terumo Blood Component Technologies and Cerus. The remaining authors declare no competing financial interests.
Correspondence: Moritz Stolla, Bloodworks Northwest Research Institute, 1551 Eastlake Ave, Suite 100, Seattle, WA 98102; email: mstolla@bloodworksnw.org.
References
Author notes
V.J.K. and J.A.M. contributed equally to this study.
All deidentified data are available on request from the corresponding author, Moritz Stolla (mstolla@bloodworksnw.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Effect of RSP and extended-stored CSP transfusion in healthy humans on ASA. (A) In each of the 2 periods, healthy humans underwent plateletpheresis with platelet storage (randomized to RSPs or CSPs). The healthy humans then received an ASA loading dose, 24 hours before transfusion, and autologous platelet transfusion with blood assessments at baseline (BL; ie, without ASA), before transfusion (ASA), and at multiple time points after transfusion (1 hour, 4 hours, and 24 hours). Participants completed an RSP period (receiving 7-day RT-stored platelets) and a CSP period (receiving 14-day 4°C-stored platelets) as outlined in this overview schematic. (B) The number of RT (red circles) and 4°C (blue circles) total platelets transfused. The platelet count (C) and corrected count increments (CCI) (D) during each period (red circles: RSP period; blue squares: CSP period; 4 hours, ∗P = .0204; 24 hours, ∗∗P = .0047). (E) Platelet AA–stimulated whole-blood aggregation was measured by VerifyNow ASA (1 hour, ∗∗P = .0055; 24 hours, ∗P = .0198). Maximum light transmission aggregation (defined as maximum aggregation recorded [in percent] within 10 minutes after agonist stimulation) in response to 0.5 mM AA (1 hour, ∗P = .0347; 4 hours, ∗∗P = .0081) (F), and collagen (2.5 μg/mL; 24 hours, ∗P = .0493) (G). The thrombin generation potential of the participant’s whole blood–derived PRP was measured immediately before and 1 hour after transfusion with a commercially available fluorogenic thrombin generation assay. Results are reported as the change from the pretransfusion value to the 1-hour posttransfusion value for thrombin generation peak (defined as maximum concentration during recording; ∗P = .0429) (H), start tail time (time until end of thrombin generation; ∗P = .0266) (I), time to thrombin peak (time between begin and peak of thrombin generation) (J), and lag time (time from assay start to begin of thrombin generation) (K). Data are shown as mean ± standard error of the mean (SEM), panels H-K shown with individual values. Unpaired data, n = 7 to 9, individual P values are shown in the text above. RT, room temperature.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/20/10.1182_blood.2023022593/4/m_blood_bld-2023-022593-gr1.jpeg?Expires=1768385480&Signature=LykhcwXh7Jjsa797bCwHFsF4R88BLi7~i62mV-fKquX2FelrBp8d-ShKISukgu-1A2d-yfBJO2g2rtnuQ9O-QFdcem1zDxIMqW1dv7FMLowzzUWq8avmmpkt2OY4T8a4LCAei~pRfMoUQOrRUBkPsvTXpzXN4ktR8rty4Mwu3TWRfSWLRT41dPWWA0NKEl7CUeVuKGmMgSa2jpkqdmbu6JgvUrPQ-ktCDRS65ZkoCrcETU77W12rVZoVEJw7ktkj5T4uaBD2sWckmjMmAQoztbVBHiUnVuiCfhXEZI8iHX6qL-gzvSGNGB1081vZBva3eDLMRP8sbh-NmfBwinsARg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)