In this issue of Blood, Kogler et al present novel findings on the in vivo function of 14-day cold-stored platelets (CSPs) in plasma.1 There has been a resurgence in CSP use for therapeutic transfusion over the past decade. Unlike room temperature–stored platelets (RSPs), which have increased transfusion-transmitted infection (TTI) risk, require constant agitation, and are short-lived (5-7 days), CSPs carry a reduced TTI risk, require no agitation, and offer extended shelf life (currently 14 days with potential for 21 days). US Food and Drug Administration (FDA) guidance on platelets2 (June 2023) allows CSPs for treatment of active bleeding “when conventional platelets are not available or their use is not practical.” Conversely, recent FDA guidance (December 2020) requiring enhanced testing or pathogen reduction of RSP underscores their TTI risks.2
The transfusion model evaluated by Kogler et al consisted of endogenous platelet cyclooxygenase-1 inhibition by acetylsalicylic acid (ASA) ingestion with evaluation of posttransfusion performance of CSPs determined at 1, 4, and 24 hours. Interestingly, and in contrast with 7-day RSPs, 14-day CSPs were unable to restore platelet function at any time point. The authors acknowledge that this contradicts prior CSP studies in ASA-treated volunteers, although those were performed with whole blood–derived platelets rather than apheresis products. Because of, at least in part, cold-induced receptor clustering and alterations to glycoprotein expression,3 CSPs are cleared rapidly from circulation in noninjured, healthy subjects on transfusion, a trait demonstrated by Murphy and Gardner4 in 1969, which ultimately resulted in abandonment of their use for decades. Several laboratories, including our own,5 have demonstrated enhanced hemostatic function in CSPs by several metrics; Kogler et al report that CSPs are more procoagulant than RSPs, primarily through the enhanced thrombin generation capacity of CSPs. It is plausible that rapid clearance of hemostatically functional CSPs leaves behind phosphatidylserine-expressing platelets whose primary hemostatic competency is to generate thrombin, akin to those described by Vulliamy et al.6 Indeed, Kogler et al demonstrated a declining corrected count increment over time for CSPs, consistent with prior work showing more rapid clearance than RSPs.
Despite the lack of a nucleus, platelets are complex cells that participate in a host of functions, including hemostasis, thrombosis, wound healing, and immune responses to injury. Aggregometry (typically by light transmission or electrical impedance methods) has historically been considered the “gold standard” of platelet functional evaluations, but Kogler et al acknowledge the limitations of this in vitro method: it is time-consuming, requires large sample volumes, and does not adequately model the complex interactions in which platelets engage. Assays, such as aggregometry and viscoelastometry, thus far, have been poor predictors of posttransfusion platelet clinical function. Although Kogler et al present an innovative strategy to predict aggregation responses in vivo, ultimately their conclusions are based on thrombin generation and aggregation assays as surrogate functional markers, relying on ex vivo benchtop testing rather than patient outcomes. They acknowledge the limitation of using healthy recipients who do not demonstrate a clinical need for platelet transfusion.
Although novel approaches to understanding functional mechanisms will undoubtedly reveal more insights into the biology/pathophysiology of platelets, when it comes to patient care, the research community must answer 2 primary questions for a transfusion product: (1) does it do more good than harm, and (2) can it be efficiently supplied? CSPs already help fulfill the latter criterion through their inherently improved bacterial safety and shelf life, and clinical trials in bleeding patients should answer the former. Although Kogler et al present the first posttransfusion comparison of CSPs and RSPs stored in plasma to the end of their shelf lives, the authors note that their work should be evaluated with caution until clinical trial data are generated. Fortunately, randomized clinical trials are underway and being reported. Strandenes et al7 reported hemostatic efficacy of (up to) 14-day CSPs, as measured by chest drain output, in a pilot trial involving complex cardiothoracic patients. The ongoing Chilled Platelet Study (CHIPS; NCT04834414), a phase 3, randomized, multicenter, international study evaluating blood product use, chest tube output, and additional functional outcome measures posttransfusion of platelets in adult and pediatric cardiac surgery patients, should be even more informative, as transfused platelet units are derived from a variety of collection platforms and storage temperatures/durations (up to 21 days), with or without pathogen reduction. Validation data generated from the CHIPS in vitro companion study have already revealed functional differences between common collection platforms on the day of collection.8 Several other studies are being performed in trauma patients (Cold Stored Platelet in Hemorrhagic Shock (CriSP-HS), NCT04667468; Cold-Stored Platelet Early Intervention in Traumatic Brain Injury [CriSP-TBI], NCT04726410).
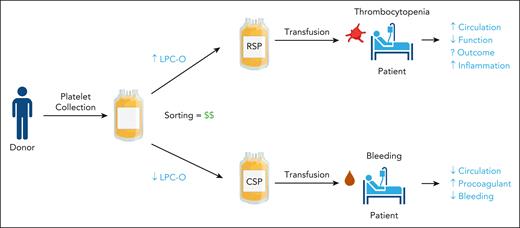
Kogler et al introduce the intriguing concept of using lipid content (lysophosphatidylcholine-O species specifically) in platelet products as a metric to predetermine ideal storage temperature (see figure). It is possible that platelets from some donors may be better suited for cold storage than others; certainly, donor characteristics and blood chemistry play a significant role in platelet properties.9 However, like many personalized medicine opportunities, the implications an undertaking of this nature would have—particularly on blood banks—must be considered. At present, whole blood and platelet donations have reached an all-time low; in early 2024, the American Red Cross declared an emergency blood shortage and issued a call for donors. But maintaining separate “ideal temperature” platelet stores could create a significant inventory imbalance and reduce the availability of products in either group. Even if shortages are overcome, testing and sorting platelet units would increase donor center costs. Historically, and with good reason, blood banks have been reluctant to adopt platelet production changes, including use of CSPs. Would adoption of predetermined storage temperature for platelet units be met with the same hesitancy? Only clinical data will determine if the shelf-life extension and functional improvements offered by CSPs are sufficiently significant to warrant their widespread adoption. The fact that currently ongoing clinical trials in acutely bleeding patients have trended toward positive outcomes with CSPs, and more importantly have not been stopped for futility, seems promising.
Kogler et al suggest that lipid metabolite content, particularly deacetylated lysophosphatidylcholine (LPC-O), could be used as a screening and sorting test for platelets to be stored at either room temperature storage (22°C; RSPs) or cold storage (4°C; CSPs), paving the way for a personalized medicine approach to platelet use. Potential concerns with this approach include increased cost to blood centers and increased inflammatory response with higher LPC-O. The debate over circulation time (RSPs) vs hemostatic function (CSPs) remains unresolved until clinical trial data are available.
Kogler et al suggest that lipid metabolite content, particularly deacetylated lysophosphatidylcholine (LPC-O), could be used as a screening and sorting test for platelets to be stored at either room temperature storage (22°C; RSPs) or cold storage (4°C; CSPs), paving the way for a personalized medicine approach to platelet use. Potential concerns with this approach include increased cost to blood centers and increased inflammatory response with higher LPC-O. The debate over circulation time (RSPs) vs hemostatic function (CSPs) remains unresolved until clinical trial data are available.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal