REVEAL is a prospective observational study that enrolled 2510 patients with PV, with median follow-up of 44.7 months (range, 2-59 months).
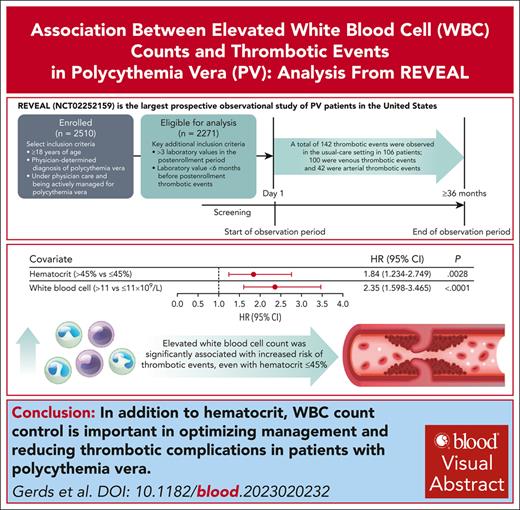
Acute and sustained WBC count elevation was significantly associated with increased thrombotic event risk, even with hematocrit level ≤45%.
Visual Abstract
Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by clonal proliferation of hematopoietic progenitor cells and is associated with an increased risk of thrombotic events (TEs). Established risk factors for TEs in patients with PV include advanced age, TE history, and elevated hematocrit. Although an association of TE with elevated white blood cell (WBC) counts has been suggested by retrospective studies, this relationship needs further validation. The prospective observational study of patients with polycythemia vera in US clinical practices (REVEAL) study collected prospective clinical data from 2510 patients with PV with a median follow-up of 44.7 months (range, 2-59 months) from enrollment. Using time-dependent covariate Cox proportional hazards models, blood counts were individually modeled with sex, age, disease duration, TE history at enrollment (baseline covariates), and treatment (time-dependent covariate). Analysis of 2271 participants identified 142 TEs in 106 patients. Significant associations with initial TE occurrence during the study period were observed for hematocrit level >45% (hazard ratio [HR], 1.84; 95% confidence interval [95% CI], 1.234-2.749; P = .0028) and WBCs >11 × 109/L (HR, 2.35; 95% CI, 1.598-3.465; P < .0001). Elevated WBC count was significantly associated with initial TE occurrence in both low-risk and high-risk PV. When hematocrit was controlled at ≤45%, WBC count >12 × 109/L was significantly associated with TE occurrence (HR, 1.95; 95% CI, 1.066-3.554; P = .0300). The results support incorporation of WBC count into PV risk stratification and studies of treatment strategies, and indicate the importance of controlling both hematocrit and WBC count in disease management. This trial was registered at www.clinicaltrials.gov as #NCT02252159.
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by clonal proliferation of hematopoietic progenitor cells. Almost all (≥95%) cases of PV harbor an acquired mutation in the Janus kinase 2 gene (JAK2; including JAK2 p.V617F and JAK2 exon-12 mutations), which is associated with increased risk of thrombotic events (TEs).1-4 Cardiovascular complications that arise from heightened TE risk contribute to increased morbidity and mortality in patients with PV,4,5 leading to a reduced life expectancy compared with age- and sex-matched healthy controls.6 Advanced age (≥60 years) and a history of TEs have been recognized as important predictors of subsequent TEs and cardiovascular-related events, and together these form the basis of the conventional model for PV risk stratification and treatment.7-9
Between 34% and 41% of patients with PV experience TEs10,11; arterial events, including transient ischemic attack, acute myocardial infarction, stroke, and peripheral arterial occlusions, and venous events, including deep vein thromboses of the extremities, abdominal cavity, and pulmonary emboli, are among the most common clinical complications.4 In a large international study of 1545 patients with PV, 12% and 9% of patients experienced postdiagnosis arterial and venous TEs, respectively.6 Of the 347 deaths that occurred during a median follow-up of 6.9 years (range, 0-39.3 years), 32 (9.8%) resulted from thrombotic complications.6 One of the goals of therapy for PV therefore is to reduce the risk of TEs and control PV-related symptoms; these goals are achieved most commonly through phlebotomy alone or in combination with cytoreductive agents such as hydroxyurea (HU).1,7
The association between elevated hematocrit and TE risk was established via a randomized controlled trial5,7,12; patients with hematocrit level >45% were found to be significantly more at risk of developing TEs than patients whose hematocrit level was <45%. These data formed the basis for the current PV disease management recommendation of maintaining a hematocrit level <45%.5,7,12 Retrospective analyses of the association between other hematologic parameters with TEs have produced conflicting results, and such associations have not been evaluated in a prospective clinical trial.13-18 Although there is real-world evidence to suggest an association between elevated white blood cell (WBC) count and increased TE risk,17,18 associations of WBC count with TE risk are yet to be included as part of a PV risk assessment model.
The REVEAL study (prospective observational study of patients with polycythemia vera in US clinical practices) enrolled 2510 patients with PV treated in community and academic practices and represents the largest prospective study in PV to date. This prespecified subanalysis of data from REVEAL evaluated associations of hematocrit level, WBC count, and platelet count with TE risk in patients with PV.
Patients and methods
Study design and patients
REVEAL (NCT02252159) is a multicenter, noninterventional, prospective observational study of patients with PV enrolled from community practices and academic centers in the United States from 22 July 2014 to 3 August 2016.19 The study design and eligibility criteria for enrollment in REVEAL have been described previously.19 Briefly, eligible patients were aged ≥18 years with a clinical diagnosis of PV per the judgment of their treating physician and should have been under physician supervision for disease management. Patients were excluded if they were participating in an active blinded clinical trial; had a life expectancy <6 months; had a history of, or were planning to undergo, allogeneic hematopoietic stem cell therapy within 3 months of enrollment; or had a splenectomy. Additional exclusion criteria for this analysis included patients with <3 laboratory values (hematocrit level, WBC count, platelet count, and neutrophil count) in the postenrollment period and patients with a postenrollment TE but without a laboratory value <6 months before that TE.
Institutional review board approvals were obtained from central (Sterling institutional review board; Atlanta, GA) and investigation sites. This study was performed in accordance with the ethical principles of the Declaration of Helsinki and local regulatory requirements, and all patients signed written, informed consent.
Data collection
At enrollment, data including duration of PV diagnosis were collected retrospectively for the 6-month period preceding enrollment. Data for demographics, disease diagnosis and course, and medical and family history were obtained from patient charts and entered into an electronic research form; postenrollment, prospective data were collected during the follow-up period (up to 3 years from last patient enrollment). Prospective data collected included disease course, phlebotomy procedures, laboratory results, and medications from the time of enrollment until patient discontinuation from the study, death, or end of study. TEs were categorized by physician documentation. In cases of ambiguous records, free-text descriptions of events were used to characterize TE type.
Model, covariates, and statistical analysis
The association between blood counts and TE occurrence was assessed using a covariate Cox proportional hazards model using both static and time-dependent variables. Time to first TE in the study period was modeled with follow-up time censored at the last-known visit for patients without a TE; additional TEs occurring beyond the first postenrollment TE for an individual patient were excluded from the time-dependent covariate and survival analyses. Laboratory values, HU treatment, and other PV-directed treatments were included as time-dependent covariates that varied throughout the study period. Use of time-dependent covariates allowed assessment of whether time-dependent changes in laboratory value elevations and/or changes in specific treatments were associated with TE occurrence. Treatment (HU or other PV-directed therapy) data recorded at a particular time point were retained until a new laboratory value was recorded at a subsequent time point or if the patient discontinued or had a TE. Time-dependent laboratory values were independently modeled together with static baseline covariates measured at enrollment (age, sex, disease duration, and TE history) and time-dependent treatment covariates. Linear interpolation was used to determine laboratory values between recorded time points, and the last recorded laboratory value was carried through until the patients had a TE or discontinued the study.
The blood count data obtained from the last laboratory test before the first TE on study or end of follow-up for patients with no TEs were included as binary time-dependent covariates using the following initial thresholds: hematocrit level >45%, WBC count >11 × 109/L, and platelet count >400 × 109/L.20,21 The following alternative thresholds were also evaluated: WBC count <7 × 109/L, ≥7 × 109/L to <8.5 × 109/L, ≥8.5 × 109/L to <11 × 109/L, and ≥11 × 109/L and platelet counts >600 × 109/L.20,22,23 An analysis of the associations between 2 WBC count elevations (>11 × 109/L and >12 × 109/L) and TEs with hematocrit levels controlled at ≤45% was also performed using separate interaction models with an interaction term for WBC count and hematocrit level; a contrast coefficient was specified to create a proportional hazard ratio (HR) for the 2 WBC count elevations at a hematocrit level ≤45%. Data analyses were performed with SAS statistical software, version 9.4 (SAS Institute, Cary, NC).
To evaluate whether sustained laboratory value elevations were associated with TE occurrence, Cox proportional hazards modeling, similar to that described earlier, was used. For a TE observed within the first year of the study, a sustained laboratory value elevation was determined to have occurred if the laboratory value was elevated for >60% of the time from enrollment to TE occurrence. For a TE observed after the first year of the study, a sustained elevation was determined to have occurred if the laboratory value was elevated for >60% of the time in the year preceding the TE occurrence.
HRs and P values were calculated from the Cox proportional hazards model; statistical significance was assessed at P < .05. Association data are presented as HR (95% confidence intervals).
Results
Patient characteristics
Of the 2510 patients enrolled in REVEAL, 2271 (90.5%) were included in this analysis; 239 patients (9.5%) were ineligible because they did not have any evaluable laboratory values, had missing data for the regression model, had <3 laboratory values (blood counts) recorded in the postenrollment period, and/or had no laboratory value <6 months before the TE. Median follow-up time was 44.7 months (range, 2-59 months); 2192 patients (96.5%) had a follow-up time of ≥12 months, 1996 (87.9%) had a follow-up time of ≥24 months, 1802 (79.3%) had a follow-up time of ≥36 months, and 751 (33.1%) had a follow-up time of ≥48 months. The median age of patients was 67 years (range, 22-95 years), and 1229 (54.1%) were male (Table 1). The median disease duration from diagnosis to enrollment was 4.1 years (range, 0-56.3 years), and 456 patients (20.1%) had a history of TE (Table 1). Of the 2271 patients in this study, 77.9% and 22.1% were diagnosed with high-risk and low-risk PV at enrollment, respectively. Patients with high-risk PV were most commonly receiving cytoreductive therapy with HU only (31.8%) or HU plus phlebotomy (26.6%) at the time of enrollment. More than half of the patients with low-risk PV were treated with phlebotomy only (54.3%) at enrollment, and a similar percentage of patients with high-risk (66.5%) and low-risk PV (69.4%) were receiving aspirin at enrollment (Table 1). Anticoagulation therapy was used in 11.5% of all patients at enrollment, and in 36.8% of patients with a history of TE (Table 1; supplemental Table 1, available on the Blood website). A hematocrit level >45% was present in approximately half of patients at enrollment, whereas a WBC count >11 × 109/L or platelets >600 × 109/L were present in ∼30% of patients at enrollment (supplemental Table 2).
Postenrollment TEs
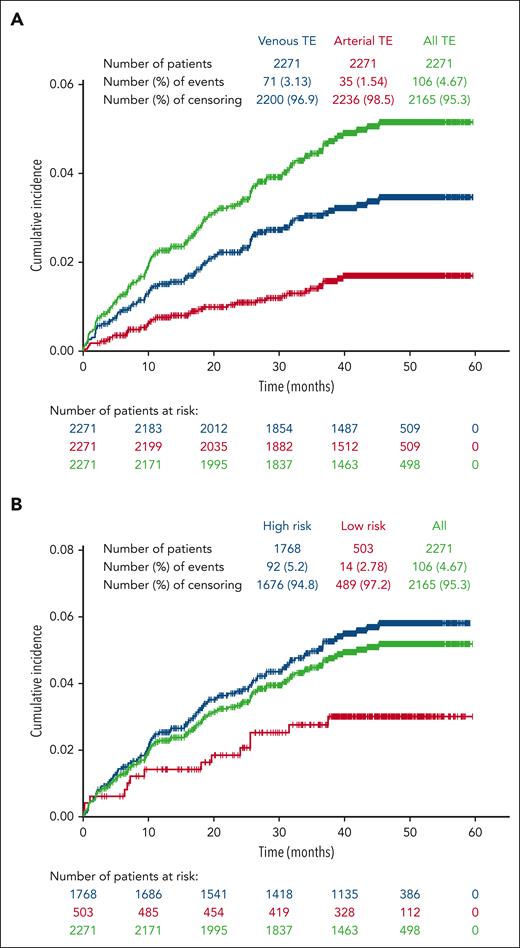
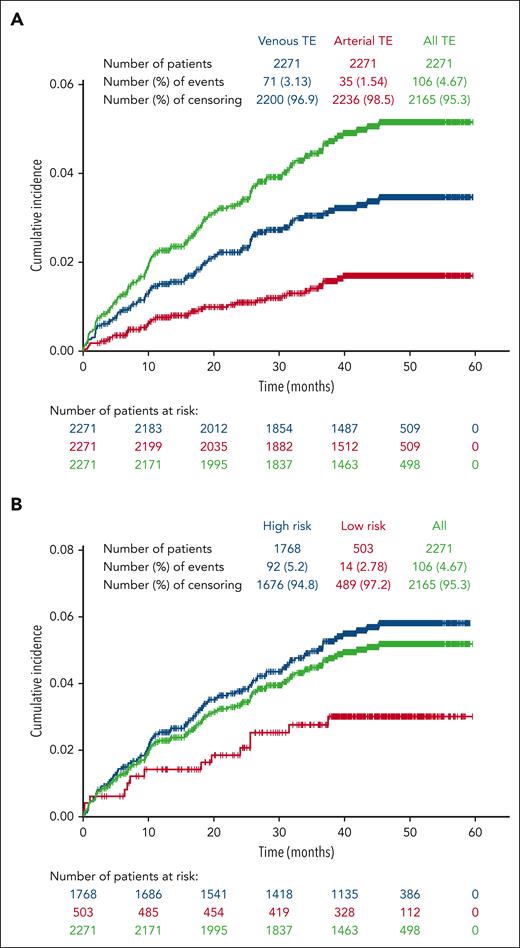
Among the 2271 patients included in this study; 106 patients (4.7%) had a total of 142 TEs with an incidence rate for the entire enrollment period of 1.36 per 100 patient-years. Of the 106 patients with reported TEs during the study period, most had experienced 1 TE (n = 86; 81.1%), with 20 (18.9%) experiencing 2 to 5 TEs (supplemental Table 3). In addition, of the 456 patients who had a history of TE before enrollment, 38 (8.3%) experienced a TE during the study period; 35.8% of patients who experienced a TE during the study had a history of TE before enrollment. Venous TEs were more frequent than arterial TEs, and patients with high-risk PV at enrollment were more likely to experience a TE than those with low-risk PV (Figure 1A-B). Among the total of 142 TEs, 100 were venous events, most commonly deep vein thrombosis (n = 43) and 42 were arterial, most frequently, transient ischemic attack (n = 15; supplemental Table 4). Of the 53 TEs experienced during the study period by patients who had a prior history of TEs, 41 were venous TEs, most commonly deep vein thrombosis (n = 19) and 12 were arterial events, the most common being transient ischemic attack (n = 5). Of the 106 patients who had a TE during the study period, 69.8% were receiving aspirin and 23.6% were receiving anticoagulant therapy at enrollment. A similar percentage of patients with TEs were enrolled in community (4.9%, n = 81) compared with academic (4.7%, n = 18) centers; of 236 patients enrolled at treatment centers of unspecified type, 7 had TEs.
Cumulative incidence of TEs occurring during the study period. Patients were censored after a TE; thus, only initial TEs on study are shown. (A) Venous events were more common than arterial events. (B) The majority of TEs occurred in patients categorized as high risk at study enrollment.
Cumulative incidence of TEs occurring during the study period. Patients were censored after a TE; thus, only initial TEs on study are shown. (A) Venous events were more common than arterial events. (B) The majority of TEs occurred in patients categorized as high risk at study enrollment.
Association between blood counts and TEs
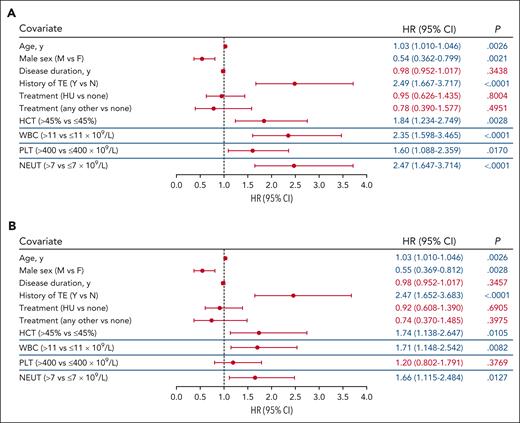
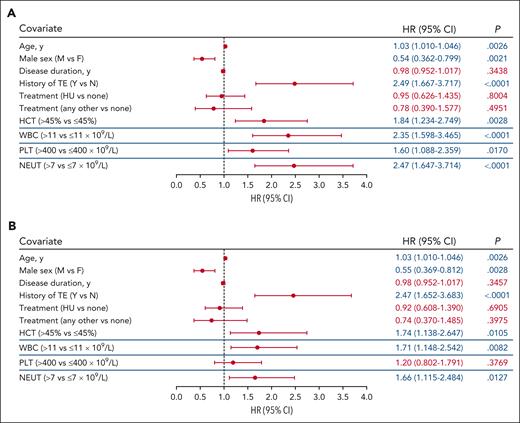
Time-dependent covariate analysis was conducted for first TE occurring during the postenrollment period. Each blood count type (hematocrit, WBC, platelet, and neutrophil) was modeled independently using the most recent laboratory value collected before the TE, together with covariates of age, sex, disease duration, history of TE, and treatment. Significant associations were identified between TE occurrence and hematocrit level >45% vs ≤45% (P = .0028), WBC count >11 × 109/L vs ≤11 × 109/L (P < .0001), platelet count >400 × 109/L vs ≤400 × 109/L (P = .0170), and absolute neutrophil count >7 × 109/L vs ≤7 × 109/L (P < .0001; Figure 2A). Alternative thresholds of WBC count (≥7 × 109/L to <8.5 × 109/L vs <7 × 109/L and ≤8.5 × 109/L to <11 × 109/L vs <7 × 109/L) and platelet count (>600 × 109/L) were also explored to further characterize the significance of the aforementioned observed associations, and no significant associations between blood count and TE occurrence were observed (supplemental Figure 1).
Multivariable analysis of TE risk. The blood count thresholds of hematocrit (HCT) level >45%, WBC count >11 × 109/L, platelet (PLT) count >400 × 109/L, and absolute neutrophil (NEUT) count >7 × 109/L were assessed in independent models together with the covariates of age, sex, disease duration, and history of TEs and PV-directed therapy. (A) Covariate analysis of blood count values adjacent (in time) to the event identified HCT, WBC, PLT, and NEUT thresholds each independently associated with TEs. (B) Covariate analysis of blood counts above threshold values for a sustained time period (1 year) identified HCT, WBC, and NEUT as independently associated with TEs. The blue lines separating rows demark blood count data that were obtained from independent models. In all models, the significance of the association for the covariates of age, sex, disease duration, history of TE, and treatment with TE risk were unchanged and representative data for these covariates from the HCT level >45% model are shown (data for all models shown in supplemental Figure 2). Significant values (P < .05) are indicated in blue font. Other treatments include ruxolitinib, anagrelide, interferon, busulfan, and chlorambucil. CI, confidence interval.
Multivariable analysis of TE risk. The blood count thresholds of hematocrit (HCT) level >45%, WBC count >11 × 109/L, platelet (PLT) count >400 × 109/L, and absolute neutrophil (NEUT) count >7 × 109/L were assessed in independent models together with the covariates of age, sex, disease duration, and history of TEs and PV-directed therapy. (A) Covariate analysis of blood count values adjacent (in time) to the event identified HCT, WBC, PLT, and NEUT thresholds each independently associated with TEs. (B) Covariate analysis of blood counts above threshold values for a sustained time period (1 year) identified HCT, WBC, and NEUT as independently associated with TEs. The blue lines separating rows demark blood count data that were obtained from independent models. In all models, the significance of the association for the covariates of age, sex, disease duration, history of TE, and treatment with TE risk were unchanged and representative data for these covariates from the HCT level >45% model are shown (data for all models shown in supplemental Figure 2). Significant values (P < .05) are indicated in blue font. Other treatments include ruxolitinib, anagrelide, interferon, busulfan, and chlorambucil. CI, confidence interval.
In each of these separate models, advanced age (by year) and a history of TE were significantly associated with increased TE occurrence (P < .01), whereas male sex was significantly associated with decreased TE occurrence (P < .02; Figure 2A; supplemental Figure 2A). Disease duration and treatment (including HU vs none, or other vs none) were not found to be associated with TE occurrence (P > .05; Figure 2A; supplemental Figure 2A).
The association of TEs with isolated laboratory values may be confounded by factors such as infection or interventions that may transiently alter blood counts. To address this, a second analysis was performed to assess whether TEs were associated with sustained elevated blood counts. A significant association with TE occurrence was observed for sustained hematocrit level >45%, WBC count >11 × 109/L, and absolute neutrophil count >7 × 109/L (P < .05); however, sustained platelet count elevation >400 × 109/L was not significantly associated with increased TE occurrence (P > .05; Figure 2B; supplemental Figure 2B). In addition, advanced age and history of TEs were again identified as significantly associated with increased TE occurrence (P < .01; Figure 2B; supplemental Figure 2B); male sex was identified as significantly associated with decreased TE occurrence (P < .01; Figure 2B; supplemental Figure 2B).
Association between blood counts and TEs by PV risk
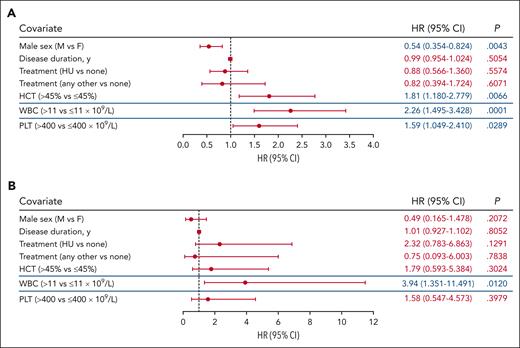
To identify variables associated with TE risk within established PV risk categories, a subgroup analysis of patients with high- and low-risk PV was performed. Because of their inclusion in the definition of PV risk, both age and history of TE were removed as variables in this analysis. In patients with high-risk PV (n = 1768), hematocrit level >45% and WBC count >11 × 109/L were significantly associated with increased TE risk (P < .01; Figure 3A; supplemental Figure 3A). Platelet count >400 × 109/L was also significantly associated with increased TE occurrence (P < .05), whereas male sex was significantly associated with decreased TE occurrence (P < .05; Figure 3A; supplemental Figure 3A). Analysis of TE risk factors in low-risk PV was limited by the relatively small cohort size (n = 503) and low number of events (n = 14). No significant associations were observed between TE occurrence and hematocrit level >45%, platelet count >400 × 109/L, and male sex in these patients (P > .05; Figure 3B; supplemental Figure 3B). However, WBC count >11 × 109/L remained associated with TE occurrence in patients with low-risk PV (P < .05; Figure 3B; supplemental Figure 3B).
Analysis of TE associations stratified based on PV risk group. (A) Subgroup analysis of high-risk PV identified HCT level >45%, WBC count >11 × 109/L, and PLT count >400 × 109/L as independently associated with TEs. (B) Only WBC count >11 × 109/L was identified to be associated with TE occurrence in patients with low-risk PV. The blue lines separating rows demark blood count data that were obtained from independent models. The significance of the association for sex, disease duration, and treatment with TE occurrence were unchanged and representative data for these covariates from the HCT level >45% model are shown (data for all models are shown in supplemental Figure 3). Significant values (P < .05) are indicated in blue font. Other treatments include ruxolitinib, anagrelide, interferon, busulfan, and chlorambucil.
Analysis of TE associations stratified based on PV risk group. (A) Subgroup analysis of high-risk PV identified HCT level >45%, WBC count >11 × 109/L, and PLT count >400 × 109/L as independently associated with TEs. (B) Only WBC count >11 × 109/L was identified to be associated with TE occurrence in patients with low-risk PV. The blue lines separating rows demark blood count data that were obtained from independent models. The significance of the association for sex, disease duration, and treatment with TE occurrence were unchanged and representative data for these covariates from the HCT level >45% model are shown (data for all models are shown in supplemental Figure 3). Significant values (P < .05) are indicated in blue font. Other treatments include ruxolitinib, anagrelide, interferon, busulfan, and chlorambucil.
Risk factors for venous vs arterial TEs
A second subgroup analysis was performed to investigate whether the clinical parameters identified as significantly associated with all TEs are common to both venous and arterial events. Interestingly, except for WBC count, venous and arterial TEs were found to be associated with distinct variables. In patients with venous TEs, history of TE was significantly associated with increased TE occurrence (P < .0001), whereas male sex was significantly associated with decreased TE occurrence (P < .002; supplemental Figure 4A). Alternatively, hematocrit level >45%, platelet count >400 × 109/L, and age were significantly associated with increased TE occurrence (P < .05) in patients with arterial TEs (supplemental Figure 4B). A WBC count >11 × 109/L was significantly associated with both venous and arterial TE occurrence.
Association between elevated WBC count and TEs with controlled hematocrit
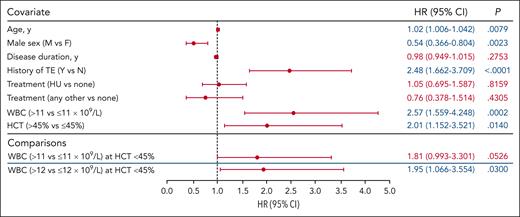
A hematocrit of 45% is an established treatment threshold in PV24; therefore, we investigated whether TE occurrence remained associated with elevated WBC count when hematocrit level was maintained at ≤45%. Associations between elevated WBC counts >11 × 109/L and >12 × 109/L and TE occurrence were evaluated in 2 separate interaction models in which the hematocrit level was controlled at ≤45% (Figure 4). Covariates of age, sex, disease duration, history of TE, and treatment were included. The association between WBC count >12 × 109/L and TE occurrence was significant compared with a WBC count ≤12 × 109/L (P = .0300); an elevated WBC count >11 × 109/L was not significantly associated with increased TE occurrence compared with WBC count ≤11 × 109/L (P = .05260).
WBC count association with TEs at HCT levels ≤45% (WBC count >11 × 109/L and >12 × 109/L). The model included the HR for WBC count (>11 × 109/L vs ≤11 × 109/L and >12 × 109/L vs ≤12 × 109/L) at HCT levels ≤45%; the association between WBC count elevation (>11 × 109/L and >12 × 109/L) and TEs was tested using separate models. Analyses did not identify an association between TEs and WBC count >11 × 109/L; however, a significant association between TEs and a WBC count >12 × 109/L was observed. The blue lines separating rows demark blood count data that were obtained from independent models. The significance for covariates of age, sex, disease duration, history of TE, and treatment with TE occurrence were unchanged across all models; representative data for these covariates from the WBC count >11 × 109/L model are shown (data for all models shown in supplemental Figure 5). Significant values are indicated in blue font.
WBC count association with TEs at HCT levels ≤45% (WBC count >11 × 109/L and >12 × 109/L). The model included the HR for WBC count (>11 × 109/L vs ≤11 × 109/L and >12 × 109/L vs ≤12 × 109/L) at HCT levels ≤45%; the association between WBC count elevation (>11 × 109/L and >12 × 109/L) and TEs was tested using separate models. Analyses did not identify an association between TEs and WBC count >11 × 109/L; however, a significant association between TEs and a WBC count >12 × 109/L was observed. The blue lines separating rows demark blood count data that were obtained from independent models. The significance for covariates of age, sex, disease duration, history of TE, and treatment with TE occurrence were unchanged across all models; representative data for these covariates from the WBC count >11 × 109/L model are shown (data for all models shown in supplemental Figure 5). Significant values are indicated in blue font.
Trends in associations between other covariates (age, male sex, disease duration, history of TEs, and treatment) and TE occurrence in these 2 interaction models were again similar to those observed for prior models: advanced age and history of TEs were significantly associated with increased TE risk (P < .01); male sex was significantly associated with decreased TE occurrence (P < .01; Figure 4; supplemental Figure 5). Disease duration and treatment (HU or other vs none) were not significantly associated with TE occurrence (P > .05).
Discussion
REVEAL represents the largest prospective observational cohort of patients with PV to date.19 This analysis of data from REVEAL helped evaluate the association of hematocrit level, WBC count, platelet count, and absolute neutrophil count with TE risk in patients with PV. The results demonstrate significant associations in individual models between elevated hematocrit levels, elevated WBC count, and elevated absolute neutrophil count and TE risk. Similar associations with TE risk were observed in models of sustained elevation of hematocrit levels, WBC count, and absolute neutrophil count, supporting the conclusion that these associations are not because of transient changes in blood counts. Although a significant association was observed between a platelet count elevation >400 × 109/L and TE risk, the association was not maintained for a platelet count >600 × 109/L or for a sustained platelet count >400 × 109/L. Importantly, when the hematocrit level was controlled at ≤45%, a WBC count >11 × 109/L was associated with an approximately twofold increase in TE risk vs WBC count ≤11 × 109/L; although this association was not significant, using a WBC count threshold of >12 × 109/L did result in a significant association with TE risk. Advanced age and a history of TEs were also significantly associated with increased TE risk across all models evaluated, whereas male sex was associated with decreased TE risk.
The present results are consistent with previous observations of hematocrit, advanced age, and history of TE as risk factors for TE, and support the inclusion of WBC count as an additional risk factor.4,17 Multiple prior studies have investigated the association of elevated WBC counts with the risk of TE with mixed results (summarized in supplemental Table 5).16-18,20,25 A recent systematic review and meta-analysis of 40 studies indicated that associations between leukocytosis and risk of thrombosis are inconclusive, which was suggested to be because of inconsistency among the studies in how WBC thresholds were selected with respect to prognostic accuracy.26 Although a randomized, controlled trial investigating the efficacy of WBC count control on TE reduction has yet to be performed, the recent prospective, interventional MAJIC-PV study found that patients who achieved normalization of all blood counts had significantly longer TE-free survival than patients who did not reach this end point.27 These observations, together with the present results from REVEAL, support the notion that TE risk can be reduced by controlling both hematocrit level and WBC count.
Results from the subgroup analysis of patients with high-risk PV support the conclusions from the full analysis, with significant associations observed between hematocrit level >45% and WBC count >11 × 109/L, and increased TE risk. A significant association was also observed between male sex and decreased TE risk. However, in the subset analysis of patients with low-risk PV, the only significant association observed was between WBC count >11 × 109/L and increased TE risk. Interpretation of results from the low-risk PV subgroup is limited by the small number of TEs that occurred in this population as well as by the fact that enrollment in REVEAL was biased toward patients with high-risk PV.
Additional subgroup analysis according to the TE type identified WBC count as a shared risk factor as well as distinct risk factors for venous compared with those for arterial TEs. Venous events were found to be associated with female sex as well as the previously validated risk factor of a prior TE.7-9 Arterial events were found to be associated with increased age, a known risk factor for cardiovascular disease, as well as hematocrit and platelet count. The observation that male sex is associated with a decreased risk of venous thrombosis is supported by the results of previous studies and may be because of the increased frequency of splanchnic venous thrombosis in females.9,28 Similarly, the association of arterial events with hematocrit level > 45% is supported by the results of the Cytoreductive Therapy in Polycythemia Vera study.5
The association of elevated absolute neutrophil count >7 × 109/L with increased TE risk may provide insight into the mechanism underlying the relationship between WBC count and TE risk. Elevations in absolute neutrophil count and WBC count have been shown by previous studies to approximately correlate with JAK2 p.V617F mutant allele burden.29,30 Furthermore, the presence of a somatic JAK2 mutation is known to be positively associated with risk of venous TEs and leukocytosis in the general population.31,32 An association between JAK2 p.V617F allele burden and TE risk has also been implicated in prior studies,33 and the present data from REVEAL provide additional indirect evidence to support this notion.
Additional explanations for the role of leukocytosis in thrombogenesis have been provided in the literature. Activated WBCs have an established role in regulating the coagulation cascade by expressing coagulation and fibrinolytic factors, as well as producing cytokines and other proteins that modulate normal hemostasis, including platelet and endothelial physiology. Under pathologic conditions in which elevated blood counts exist, increased levels of cytokines create a proinflammatory environment, stimulating coagulation and promoting thrombosis.34-36 Therefore, maintaining WBC counts within a normal range (between 4.0 × 109/L and ≤11.0 × 109/L) may be an independent and critical consideration for PV risk stratification and management. However, the observed association between elevated WBC counts and TE risk does not prove the therapeutic efficacy of maintaining WBC counts below a certain threshold.
Limitations of this study include a potential reporting bias of TEs introduced by subjectivity or misclassification associated with physician-reported events. In addition, the total TE rate observed in this study (4.7%) is lower than reported in previously published retrospective studies,37 which is likely explained by the REVEAL study design. REVEAL is a prospective study and collected TE data throughout the study period following enrollment. Collection of TE data outside of the study period was not prespecified and such TEs were not routinely recorded. Because TE incidence in PV is highest around the time of diagnosis,38 retrospective studies reporting TEs during the entire disease course of a patient, including shortly before or at the time of diagnosis, are likely to report higher rates of TEs. Of note, the percentage of patients enrolled in REVEAL with a history of TEs (20.1%) before enrollment was similar to the TE rates reported previously.37 In addition, inclusion in this analysis required patients to have ≥3 laboratory values in the period after enrollment and a laboratory value <6 months before a postenrollment TE. Therefore, the observed rate of TEs is likely an underestimation of the true rate over the course of disease in the overall REVEAL population.
This analysis from REVEAL, the largest prospective study in PV to date, provides further evidence regarding the association between leukocytosis and TE risk. These data, combined with those from previous prospective studies such as ECLAP17,18 as well as from multiple retrospective analyses, suggest that control of both hematocrit and WBC count are important in PV disease management. The results of this study may be used to improve the design of clinical studies that recruit patients with PV. In addition, they provide evidence to move beyond the conventional risk model in PV7 and improve risk stratification and treatment guidelines for patients.
Acknowledgments
The authors thank the patients and their families, the investigators, and the site personnel who participated in this study. The authors also thank Evan M. Braunstein for his insights and guidance in the preparation of this manuscript. Medical writing and editorial assistance were provided by Rachel Shparberg and Sophie Pickles of Envision Pharma Group (Fairfield, CT) and were funded by Incyte Corporation.
This study was supported by Incyte Corporation (Wilmington, DE).
Authorship
Contribution: J.E.H.-M. validated the results/experiments; J.E.H.-M., J.Y., and P.S. analyzed and curated the data and wrote the initial draft of the manuscript; all authors conceptualized and designed the trial, conducted the trial, provided critical review of the manuscript, and created the visualization of data for the manuscript; and P.S. provided supervision.
Conflict-of-interest disclosure: A.T.G. reports consulting fees from AbbVie, Bristol Myers Squibb, Constellation Pharmaceuticals, Novartis, PharmaEssentia, and Sierra Oncology; and research support from Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, and Kratos Pharmaceuticals. R.M. reports consultancy fees from AOP Health, Blueprint Medicines, Bristol Myers Squibb, Geron, Incyte Corporation, La Jolla Pharma, Novartis, Roche, Samus Therapeutics, Sierra Oncology; and research support from AbbVie, Celgene, CTI BioPharma, Genentech, Gilead Sciences, Imago BioSciences, Incyte Corporation, Promedior, and Sierra Oncology. J.M.B. reports consulting fees from AbbVie, Adaptive Biotechnologies, AstraZeneca, BeiGene, Bristol Myers Squibb, Epizyme, Kura Oncology, Kymera, MorphoSys AG, Roche/Genentech, Seagen, Verastem Oncology, and X4 Pharmaceuticals; and participated in speaker’s bureaus for BeiGene and Seagen. M.R.G. reports consulting fees from AbbVie, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI BioPharma, Daiichi Sankyo, Gamida Cell, Genentech, Gilead Sciences, GlaxoSmithKline, Incyte Corporation, Invitae, Jazz Pharmaceuticals, Karius, Novartis, Ono Pharmaceutical, Pfizer, Premier Pharmaceuticals, Servier, and Stemline Therapeutics; research support from Genentech, Incyte Corporation, and Janssen; and stock ownership in Medtronic. B.L.S. participated in an advisory board for Constellation Pharmaceuticals; and an advisory board and steering committee for PharmaEssentia. P.S., J.Y., and J.E.H.-M. are employed by and have stock ownership in Incyte Corporation. S.T.O. reports consulting fees from AbbVie, Blueprint Medicines, Constellation Pharmaceuticals, CTI BioPharma, Disc Medicine, Geron, Incyte Corporation, PharmaEssentia, and Sierra Oncology.
The current affiliation for R.M. is Levine Cancer Institute, Atrium Health, Charlotte, NC.
Correspondence: Aaron T. Gerds, Hematology and Medical Oncology, Cleveland Clinic Taussig Cancer Institute, 9500 Euclid Ave CA60, Cleveland, OH 44195; email: gerdsa@ccf.org.
References
Author notes
The sponsor (Incyte Corporation, Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized data sets owned by Incyte for the purpose of conducting legitimate scientific research through the Incyte platform at https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.