Introduction:
Despite the numerous significant advances in leveraging actionable genetic biomarkers, targeted therapies continue to exhibit limited success in AML. This may be attributed to the genetic heterogeneity and plasticity of cancer cells, which ultimately overrides the driving force of individual mutations and their resulting phenotypes. In the face of this well-known challenge, there is a growing promise of functional assays that can leverage the integrated phenotypic response of cancer patients' cells to determine treatment schemes in a personalized manner. In addition, functional testing holds the potential to identify elusive responders to targeted therapies in cohorts of patients who do not present the actionable mutations that are used as biomarkers for prescription.
Aim:
To perform pre-clinical correlation studies between FLT3 mutational status and the ex-vivo response to Gilteritinib using a functional assay of Patient Micro Avatars (PMAs) developed at OncoPrecision.
Materials and Methods:
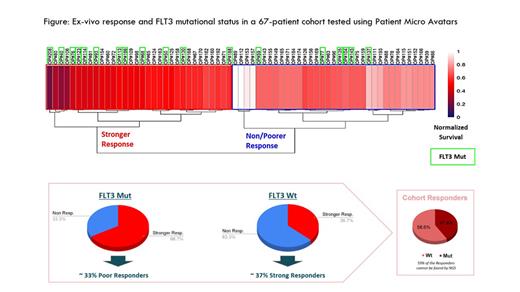
Ex-vivo testing with PMAs was performed to evaluate the ex-vivo response to Gilteritinib in 67 bone marrow or peripheral blood samples from patients diagnosed with different AML subtypes (de novo, relapsed/refractory and secondary). Pathological subpopulations were analyzed using lineage markers and survival of blasts was assessed using flow cytometry. Samples were classified as “responders” and “non-responders” using in-house supervised and non-supervised machine learning tools. In parallel, samples were analyzed by NGS and molecular biology to identify mutations and internal tandem duplications in the FLT3 gene. Finally, FLT3 alterations were correlated with the phenotypic response obtained in the functional assay using statistical analysis to compare mutant and non-mutant frequencies in the two groups.
Results:
The results of the phenotypic screening revealed that ~70% of patients with FLT3 mutations exhibited a strong response to Gilteritinib, which is in line with the presence of the actionable genetic biomarker (Figure). The fact that the remaining ~30% of mutated patients presented a poor response to Gilteritinib suggests the existence of acquired mechanisms of resistance that are dominant over the mutational status of FLT3. Interestingly, we also identified a large group of FLT3wt patients (~30%) which showed a phenotypic response to Gilteritinib equivalent to the one observed in FLT3mut patients (Figure). This finding unveils the existence of a subpopulation of FLT3wt patients who may benefit from Gilteritinib treatment and cannot be identified using conventional genetic testing.
Conclusions:
The present study highlights the reach of functional testing as a powerful tool to predict Gilteritinib resistance in FLT3mut backgrounds and to identify exceptional responders in FLT3wt backgrounds, creating opportunities for AML patients beyond the currently available genetic biomarkers.
OffLabel Disclosure:
Garcia:OncoPrecision: Current Employment, Current holder of stock options in a privately-held company. Bertoldi:OncoPrecision: Current Employment. Scaglia:OncoPrecision: Current Employment. Carbajosa:OncoPrecision: Current Employment. Cavallo:OncoPrecision: Current Employment. Garcia-Melani:OncoPrecision: Current Employment. Guantay:OncoPrecision: Consultancy. Piaggio:OncoPrecision: Current Employment. Ferreira:OncoPrecision: Current Employment. Pavlovsky:Astra Zeneca: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau. Fernandez:Pfizer: Honoraria; Bristol Myers Squibb, ARG: Honoraria; Astellas Pharma Latin America: Honoraria; Novartis, ARG: Honoraria. Pavlovsky:Novartis: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Pfizer: Speakers Bureau. Solimano:OncoPrecision: Consultancy, Honoraria. Bernaschini:OncoPrecision: Consultancy, Ended employment in the past 24 months. Andino:OncoPrecision: Consultancy, Ended employment in the past 24 months, Honoraria. Conrrero:OncoPrecision: Current Employment. Drocchi:OncoPrecision: Honoraria. Zaki:OncoPrecision: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Gatti:OncoPrecision: Current Employment, Current equity holder in private company. Llorens:OncoPrecision: Current Employment, Current equity holder in private company. Soria:OncoPrecision: Current Employment, Current equity holder in private company.
Identification of Gilterinitinb responders beyond FLT3 mutant patients in AML using ex-vivo testing.