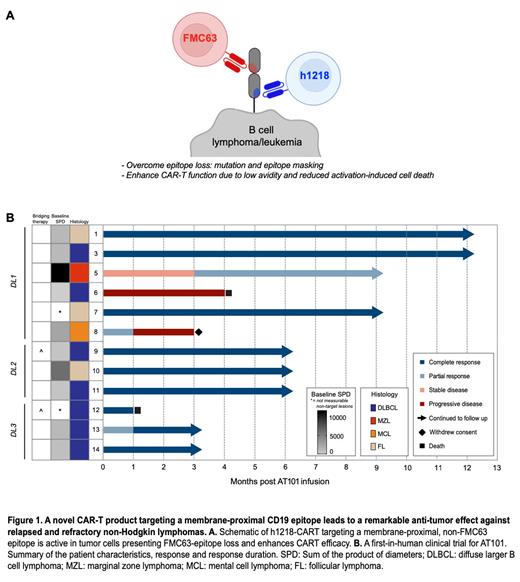
Introduction: Anti-CD19 chimeric antigen receptor T-cell therapies (CART19) are highly efficacious against advanced B cell non-Hodgkin lymphoma (NHL) but the majority of patients (pts) ultimately fail. Several mechanisms contribute to CART19 failure, including CD19-neg escape and CART dysfunction. Notably, all four commercial CART19 products utilize the FMC63 single chain variable fragment (scFv) with high-avidity specificity to a CD19 membrane-distal epitope. Interestingly, loss of the FMC63-recognized epitope due to CD19 mutations ( Zhang Z, JITC, 2020) or epitope-masking (CAR19:CD19) ( Ruella M, Nat Med, 2018) has been described. We hypothesized that a novel anti-CD19 scFv that engages an alternative CD19 membrane-proximal epitope independent of FMC63 with low avidity could: 1. mitigate CD19 epitope loss; but also 2. enhance CART functions ( Fig. 1A) .
Methods and results: We developed an autologous, CART19 product with 4-1BB co-stimulation using a novel humanized chicken antibody (h1218) specific to a membrane-proximal epitope of CD19 (amino acids T51,S53, E55,K59 and K63). We previously reported initial preclinical data ( Patel R., ASH, 2021 #2798) that demonstrated h1218-CART19 recognize and kill B-cell leukemia that aberrantly expresses the FMC63-CAR19. More recently, we demonstrated that differently than FMC63-based CART, h1218-CART19 could respond to and kill tumor cells expressing point mutations of CD19 (L174V and R163L). Of note, h1218-CART19 had enhanced control of CD19+ B-cell neoplasms as compared to FMC63-CART19 both in vitro and in vivo. Mechanistically, thanks to the lower avidity and faster on/off rate, h1218-CART19 had decreased T cell exhaustion and activation-induced cell death.
Given the promising preclinical results, we tested the clinical h1218-CART19 product (AT101) in a first-in-human, multi-center, phase I/II, dose-escalation clinical trial for pts with histologically confirmed relapsed or refractory B cell NHL (NCT05338931). For the phase I study, pts were treated with a single intravenous infusion of AT101 (day 0) in three dose levels (DL) based on a standard 3+3 design: DL1 (0.2 x 10 6 cells/kg), DL2 (1.0 x 10 6 cells/kg), or DL3 (5.0 x 10 6 cells/kg). All pts received lymphodepletion with intravenous fludarabine (250 mg/m 2) and cyclophosphamide (25 mg/m 2) on days -4, -3, and -2. The primary objective was to determine the maximum tolerated dose (MTD) of AT101. The secondary objectives were to evaluate AT101 pharmacokinetics and preliminary efficacy.
At the July 3, 2023 data cut, 12 pts (median age 62.5 years (range 39-84); diffuse large B cell lymphoma (DLBCL, n=7, 58.3%), follicular lymphoma (FL, n=3, 25.0%), mantle cell lymphoma (MCL, n=1, 8.3%), and marginal zone lymphoma (MZL, n=1, 8.3%)) received AT101 ( Fig. 1B). The median follow-up after AT101 was 6.5 months (1.5-13.7 months), the median number of previous lines of treatment 3 (range 1-8); no prior cellular therapies. Five pts (41.7%) had previous autologous stem cell transplantation. Two pts (16.7%) had refractory disease and two pts (16.7%) received bridging therapy.
Three pts (2 pts at DL2 and 1 pt at DL3) developed a grade (G) 1 cytokine-release syndrome (CRS) and one pt at DL3 developed a G3 CRS that resolved within 24 hours. Three pts (1 pt at each DL) developed immune-cell-related neurotoxicity syndrome (ICANS), of which one G4 dose-limiting toxicity (DLT) was reported at DL1 that corrected within 6 days. One DL3 pt experienced G3 sepsis that promptly resolved; the same pt ultimately developed fatal neutropenic septic shock outside the DLT period. Four additional patients experienced ≥G3 neutropenia without evidence of infection.
Complete response (CR) occurred in 8/12 pts (66.6%) and overall response (CR+partial response) in 83.3% (95%CI, 51.6-97.9) at day 28 post-AT101 infusion ( Fig. 1B). Remarkably, in DL2 and DL3, the CR rate was 100.0%. Of the 8 pts in CR none has relapsed (median follow-up 6.0 months). AT101 cells expanded (peak in DL3 113.6 ± 6.50 x10 4 CAR gene copies/ug DNA) and persisted in patients' blood.
Conclusion: In this first-in-human phase I trial, AT101 was tolerable with limited and manageable toxicities. Notable preliminary efficacy was observed at the RP2D with no evidence of disease relapse after achieving a CR. These results warrant the pursuit of the planned phase II expansion cohort.
Disclosures
Patel:viTToria biotherapeutics: Consultancy. Kim:Abclon: Current Employment. Lee:Takeda: Consultancy; Janssen: Consultancy; Takeda: Honoraria; Janssen: Honoraria. Ghilardi:viTToria biotherapeutics: Consultancy. Kim:Abclon: Current Employment. Kim:Abclon: Current Employment. Lee:Abclon: Current Employment. Park:Abclon: Current Employment. Cui:Abclon: Current Employment. Lee:Abclon: Current Employment. Hwang:Abclon: Current Employment. Lee:Abclon: Current Employment. Lee:Abclon: Current Employment. Lee:Abclon: Current Employment. Kim:Abclon: Current Employment. Lee:Abclon: Current Employment. Yoon:GC cell: Consultancy; GI cell: Consultancy; Abclon: Consultancy; Pharos iBio: Consultancy; Beigene: Consultancy; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Samyang: Research Funding; Roche: Honoraria, Research Funding, Speakers Bureau; Boryung: Research Funding; BMS: Honoraria, Speakers Bureau; Kirin Pharm: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau. Ruella:GlaxoSmithKline: Consultancy; Bayer: Consultancy; Beckman Coulter: Research Funding; NanoString: Consultancy, Research Funding; viTToria biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Founder, Research Funding; AbClon: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy.