Children with Down syndrome (DS) have a 20 times higher risk of developing acute lymphoblastic leukemia (DS-ALL). In this issue of Blood, Li et al1 report a comprehensive genomic analysis of 295 DS-ALLs, identifying 15 distinct molecular subtypes, 3 of which (see figure) have a much higher frequency in DS-ALL compared with ALL in children without DS (non-DS ALL).
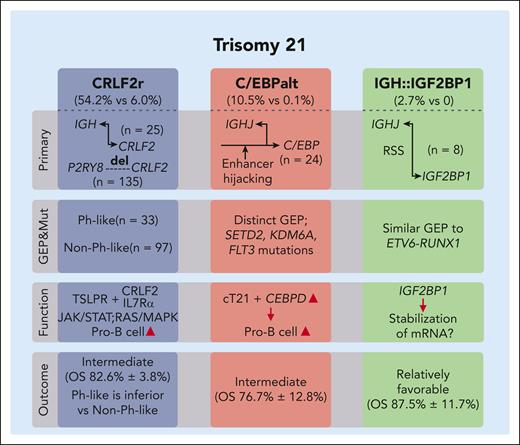
Molecular complexity of DS-ALL. Li et al identified 15 molecular subtypes, with 3 significantly novel subtypes enriched in DS-ALL compared with non-DS ALL (ratios shown in parentheses under subtype labels). CRLF2r, commonly resulting from IGH::CRLF2 translocations or P2RY8::CRLF2 microdeletions, leads to CRLF2 overexpression and activation of the JAK/STAT or RAS/MAPK signaling pathway, promoting pro–B-cell proliferation. Although CRLF2r is a signature event of a Ph-like subtype, this large DS-ALL cohort reveals further heterogeneity based on GEP, with Ph–like CRLF2r cases having worse clinical outcomes than non–Ph-like group. Another novel subtype is characterized by C/EBP gene family activation, primarily involving the CEBPD gene through genomic translocations (often with the IGHJ region) or enhancer hijacking mutations. This subtype displays a unique GEP, higher mutation rates in SETD2, KDM6A, and FLT3 genes, and intermediate risk levels. CEBPD overexpression promotes hematopoietic progenitor cell differentiation into pro-B cells, particularly in a cT21 genetic background. A minor subtype, characterized by IGHJ::IGF2BP1 gene rearrangement likely through RAG-mediated structural changes near RSS regions, has a relatively favorable clinical outcome. Although no distinct GEP is observed for this subtype, some cases share a similar GEP with the ETV6::RUNX1 subtype, potentially because of IGF2BP1 overexpression in ETV6::RUNX1 subtype resulting from ETV6 loss, which normally represses IGF2BP1 expression. The observed function of IGF2BP1 in stabilizing ETV6::RUNX1 messenger RNA further supports the potential association between these 2 genetic alterations in ALL. However, as ETV6::RUNX1 messenger RNA is not expressed in DS-ALL, the role of IGF2BP1 in DS-ALL remains unknown. ALL, acute lymphoblastic leukemia; CRLF2r, CRLF2 rearrangement; GEP, gene expression profile; RAG, recombination-activating gene; RSS, recombination signal sequences.
Molecular complexity of DS-ALL. Li et al identified 15 molecular subtypes, with 3 significantly novel subtypes enriched in DS-ALL compared with non-DS ALL (ratios shown in parentheses under subtype labels). CRLF2r, commonly resulting from IGH::CRLF2 translocations or P2RY8::CRLF2 microdeletions, leads to CRLF2 overexpression and activation of the JAK/STAT or RAS/MAPK signaling pathway, promoting pro–B-cell proliferation. Although CRLF2r is a signature event of a Ph-like subtype, this large DS-ALL cohort reveals further heterogeneity based on GEP, with Ph–like CRLF2r cases having worse clinical outcomes than non–Ph-like group. Another novel subtype is characterized by C/EBP gene family activation, primarily involving the CEBPD gene through genomic translocations (often with the IGHJ region) or enhancer hijacking mutations. This subtype displays a unique GEP, higher mutation rates in SETD2, KDM6A, and FLT3 genes, and intermediate risk levels. CEBPD overexpression promotes hematopoietic progenitor cell differentiation into pro-B cells, particularly in a cT21 genetic background. A minor subtype, characterized by IGHJ::IGF2BP1 gene rearrangement likely through RAG-mediated structural changes near RSS regions, has a relatively favorable clinical outcome. Although no distinct GEP is observed for this subtype, some cases share a similar GEP with the ETV6::RUNX1 subtype, potentially because of IGF2BP1 overexpression in ETV6::RUNX1 subtype resulting from ETV6 loss, which normally represses IGF2BP1 expression. The observed function of IGF2BP1 in stabilizing ETV6::RUNX1 messenger RNA further supports the potential association between these 2 genetic alterations in ALL. However, as ETV6::RUNX1 messenger RNA is not expressed in DS-ALL, the role of IGF2BP1 in DS-ALL remains unknown. ALL, acute lymphoblastic leukemia; CRLF2r, CRLF2 rearrangement; GEP, gene expression profile; RAG, recombination-activating gene; RSS, recombination signal sequences.
Chromosomal aneuploidy is common in cancer. Although DS is surprisingly associated with an overall decreased lifetime risk of cancer, the risk for childhood leukemias is markedly increased.2 Myeloid leukemias of DS have unique somatic genomic features; however, DS-ALLs are more heterogeneous, but with significant differences from non-DS ALL in children.3 We and others have discovered that approximately half of DS-ALLs have an abnormal expression of CRLF2, whose heterodimerization with IL7Rα forms the receptor to thymic stromal lymphopoietin. CRLF2 expression is caused by chromosomal rearrangement juxtaposing the upstream promoter of a constitutively expressed gene P2RY8 or by a translocation of CRLF2 into the immunoglobulin heavy chain locus (IGH). CRLF2 rearrangements (CRLF2r) are frequently accompanied by additional activating mutations in the receptors themselves or in the downstream signaling molecules of JAK/STAT or RAS/MAPK pathway (reviewed in Tal et al4). Interestingly, the same type of ALL has been detected in ∼5% of non-DS ALL.5
In addition to previously described CRLF2r DS-ALL, Li et al discovered 2 other subtypes of ALL enriched in DS-ALLs, altered C/EBP (C/EBPalt) and IGH::IGF2BP1. More than 10% of DS-ALL cases harbor alterations targeting C/EBP genes, with overexpression of CEBPD, or less commonly, CEBPA, or CEBPE. The C/EBP family of transcription factors regulate genes involved in multiple biological processes. They also play a crucial role in myeloid differentiation and pathogenesis of myeloid and lymphoid malignancies.6 The authors reported a significant concurrence of FLT3, SETD2, and KDM6A mutations (42.3%, 42.3%, and 30.8%, respectively) in C/EBPalt DS-ALL, compared with only 4.1%, 5.0%, and 5%, respectively, in the rest of DS-ALL subtypes. CEBPD, the most commonly altered gene in C/EBPalt subtype, enhanced the differentiation of mouse hematopoietic progenitor cells into pro-B cells in vitro, particularly in a DS genetic background.1 This finding is consistent with a specific role of CEBPD overexpression in the development of DS-ALL.
Another novel subtype defined by IGH::IGF2BP1 rearrangements was observed in 2.7% of DS-ALL cases. This subtype is characterized by deregulated expression of IGF2BP1 gene, which encodes a member of the insulin-like growth factor 2 messenger RNA (mRNA)–binding protein family. This protein is required for the transportation of certain mRNAs by affecting their stability, translatability, or localization. In ETV6::RUNX1 ALL, it binds and stabilizes ETV6::RUNX1 mRNA.7 IGF2BP1 is also activated in ETV6::RUNX1/-like ALL, owing to the loss of ETV6, a transcription repressor of IGF2BP1. Further research is needed to evaluate IGF2BP1 binding partners in non-ETV6::RUNX1 ALL and understand why this subtype is more frequent in DS-ALL.
The current and earlier studies raise 2 interesting questions. The first relates to the poor clinical outcome of DS-ALL. The worse prognosis of DS-ALLs has been attributed to both the high risk of chemotherapy associated infections in these patients and to the genomic subtypes of DS-ALLs. Here, Li et al demonstrate that this higher risk is limited to a subgroup of CRLF2r ALL that has a gene expression signature of Philadelphia chromosome (Ph)–like subtype of ALL. However, what distinguishes the Ph-like subtype that might explain the worse prognosis is still unknown.
Somatic alterations in CRLF2r ALL differed markedly between Ph-like and non–Ph-like subtypes with IKZF1 (76.9% vs 16.7%), XBP1 (26.9% vs 0%), USP9X (34.6% vs 2.8%), and EBF1 (53.8% vs 2.8%) alterations overrepresented in the former. However, by contrast to a previous study,8IKZF1 deletions were not independently associated with worse prognosis. Interestingly, we have shown that CRLF2r JAK2 mutated DS-ALLs are quite sensitive to chemotherapy, possibly because of “hypersignaling” by mutated JAK2 in the B-cell blasts.3 The USP9X mutant, which is more common in Ph-like subtype, reduces this hypersignaling and enhances the resistance of leukemic blasts to chemotherapy.
Perhaps the greatest mystery is why constitutive trisomy 21 (cT21) confers a significantly higher risk of B-cell precursor ALL. Recent analysis of hematopoietic development in human fetuses with DS revealed a marked B-cell developmental arrest.9 Li et al demonstrate that aberrant CEBPD expression enhances pro–B-cell development in the background of cT21. We have recently reported the same phenomenon with CRLF2 + IL7R expression in human cells.10 The relative block in B-cell differentiation may also explain the high rate of recombination–activating gene mediated genomic rearrangements identified by Li et al, leading to each of the genomic abnormalities (see figure). It is tempting to speculate that DS-ALL represents an unintended consequence of genomic events that drive differentiation toward the B-cell lineage, thereby rescuing the inherent developmental defect in DS. This phenomenon is somewhat similar to the myelodysplastic syndrome arising from the bone marrow “attempt” to correct the germ line SAMD9/SAMD9L mutation by deleting the chromosome 7 carrying the mutated gene.11 Could DS-ALL be a disease caused by an attempt to correct another disease, such as the B-cell developmental arrest caused by 3 copies of chromosome 21?
Conflict-of-interest disclosure: The authors declare no competing financial interests.