In this issue of Blood, Yu et al1 elucidate the mechanisms of iron homeostasis in β-thalassemia during pregnancy. The inherited disorder β-thalassemia is caused by disruptions to hemoglobin β-globin chain production, resulting in microcytic, hypochromic anemia. Individuals with β-thalassemia major have little to no production of β-globin chains, which leads to a more severe transfusion-dependent anemia than individuals with β-thalassemia intermedia. The severity of β-thalassemia intermedia is highly variable, with the severity of the specific globin mutation and the proportion of β-globin chain produced determining the severity of the condition.2 Iron overload is a common feature in both β-thalassemia major and intermedia due to increased erythropoietic demand and suppression of hepcidin expression.
Although transfusion contributes significantly to iron load in β-thalassemia major, enhanced gastrointestinal absorption is central to tissue iron accumulation β-thalassemia intermedia.3 Given this background, it is important to consider the potential impact of gestation on β-thalassemia. Patients with both transfusion- and non–transfusion-dependent thalassemia may experience complications during pregnancy, including cardiac and liver issues, increased risk of thrombosis and infection, endocrinopathies, and medication adverse effects.4,5
Using murine models of β-thalassemia intermedia (Th3/+), the article investigates changes in iron metabolism in both dams and fetal offspring during gestation. This study is unique as it provides an investigation into the pathophysiology of iron balance, which has not yet been reported in the literature. The authors discovered that hyperferremia in pregnant β-thalassemia mouse models resulted in differential iron loading of fetuses compared with pregnant wild-type counterparts. To understand the iron loading differential, expression of iron transporters and proposed possible mechanisms that could account for this disparity were investigated. Yu et al also examined the impact of fetuses’ genotype, thalassemia or wild type, on thalassemic dams, and their findings suggest a fetal influence on maternal iron homeostasis. Their experimental design included both wild-type and β-thalassemia (Th3/+) dams carrying fetuses of both genotypes, providing a unique perspective because the current literature primarily focuses on β-thalassemic mothers.
Yu et al discovered that both nonpregnant and pregnant Th3/+ mice have increased splenic iron load compared with wild-type controls, which aligns with the known functions of the spleen as a site of extramedullary erythropoiesis and red blood cell recycling. They also showed that pregnant wild-type and Th3/+ mice have enhanced iron absorption compared with nonpregnant mice of the same genotype. However, they found similar iron absorption in nonpregnant wild-type and β-thalassemia intermedia mice. The authors discuss that iron absorption normalizes in weanlings and adolescent Th3/+ mice, which may present a discrepancy between humans as iron loading increases with age.6
Yu et al found that Th3/+ fetuses from both wild-type and Th3/+ dams had iron overload, suggesting that iron loading occurs early in pregnancy. Late gestational iron absorption studies demonstrated lower absorption in both wild-type and Th3/+ fetuses of Th3/+ dams compared with those of wild type, suggesting that gestational iron loading mainly occurs early in pregnancy. The results presented in the article delineate a crucial gestational window for iron loading. However, the use of chelation medications during pregnancy is generally considered unsafe due to teratogenicity. Case studies and retrospective analysis have shown successful pregnancies in patients with unintentional use; however, due to lack of controlled studies in humans, discontinuation of these medications at the onset of pregnancy is recommended.4,7
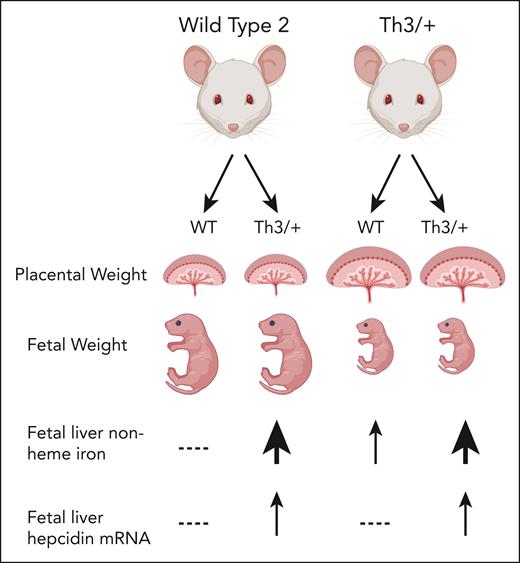
The authors also found significant changes in the expression of nonheme iron transport proteins (divalent metal transporter 1 [DMT1], transferrin receptor 1 [TFR1], and ferroportin 1 [FPN1]) in various organs of both pregnant and nonpregnant Th3/+ dams, compared with wild-type control mice. Their study revealed that the iron importer DMT1 and exporter FPN1 expression in the duodenum was lower in pregnant Th3+ dams than in nonpregnant Th3/+ dams. Furthermore, their data suggest that iron-loaded fetuses were able to mitigate iron loading through hepcidin transactivation and downregulation of placental FPN. These findings provide insight into potential innate protective mechanisms as there is still a gap in the understanding of iron metabolism in pregnancy and worsening iron overload is a concern. Furthermore, wild-type dams carrying Th3/+ fetuses did not have placentomegaly or hyperferritinemia, and Th3/+ fetuses from wild-type dams had increased placental nonheme iron as well as increased fetal liver nonheme iron compared with wild-type fetuses from the same litter. This suggests that fetal iron load is not solely based on maternal iron status.
Intrauterine growth restriction is a known complication of pregnancies affected by β-thalassemia. Yu et al found that placental weight and nonheme iron were increased in Th3/+ fetuses, whereas there was a decrease in fetal weight that was inversely proportional (see figure). Previous studies on β-thalassemia intermedia had suggested that intrauterine growth restriction may be due to ureteroplacental hypoxia, which may lead to the need for more transfusion support during pregnancy.8 However, the findings contradict this hypothesis by suggesting that iron overload contributes to intrauterine growth restriction.
Cellular and phenotypic outcomes in thalassemia during pregnancy. Placentomegaly, fetal growth restriction, and systemic iron homeostasis are altered in thalassemic dams and/or fetuses.
Cellular and phenotypic outcomes in thalassemia during pregnancy. Placentomegaly, fetal growth restriction, and systemic iron homeostasis are altered in thalassemic dams and/or fetuses.
Together, these findings highlight the importance of further studies to better understand the influence Th3/+ fetuses have on wild-type mothers. In addition, due to the phenotypic variability of β-thalassemia intermedia in humans, it will be important for further studies to confirm whether the findings from Yu et al remain consistent across patients with different disease presentations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal