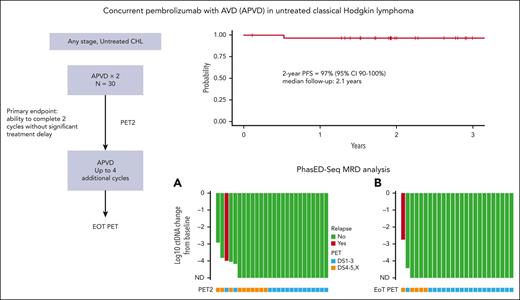
Key Points
Concurrent APVD was safe and effective in untreated HL without clinically significant treatment delays.
CR rates by PET were lower than expected at all time points despite only one relapse, and no patient who cleared ctDNA has relapsed to date.
Abstract
Concurrent administration of pembrolizumab with chemotherapy in untreated classic Hodgkin lymphoma (CHL) has not been studied previously. To investigate this combination, we conducted a single-arm study of concurrent pembrolizumab with AVD (doxorubicin, vinblastine, and dacarbazine; APVD) for untreated CHL. We enrolled 30 patients and met the primary safety end point with no observed significant treatment delays in the first 2 cycles. Twelve patients experienced grade 3 or 4 nonhematologic adverse events (AEs), most commonly febrile neutropenia and infection/sepsis. Grade 3 or 4 immune-related AEs, including alanine aminotransferase elevation and aspartate aminotransferase elevation were observed in 3 patients. One patient experienced an episode of grade 2 colitis and arthritis. Six patients missed at least 1 dose of pembrolizumab because of AEs, primarily grade 2 or higher transaminitis. Among 29 response-evaluable patients, the best overall response rate was 100% and the complete response rate was 90%. With a median follow-up of 2.1 years, the 2-year progression-free survival (PFS) and overall survival were 97% and 100%, respectively. To date, no patient who has withheld or discontinued pembrolizumab because of toxicity has progressed. Clearance of circulating tumor DNA (ctDNA) was associated with superior PFS when measured after cycle 2 and at the end of treatment (EOT). None of the 4 patients with persistent uptake by fluorodeoxyglucose positron emission tomography (PET) at EOT yet negative ctDNA have relapsed to date. Concurrent APVD shows promising safety and efficacy but may yield spurious PET findings in some patients. This trial was registered at www.clinicaltrials.gov as #NCT03331341.
Introduction
ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) as well as combinations with brentuximab vedotin (BV; AAVD), have been the standard frontline treatment regimen for classic Hodgkin lymphoma (CHL) in North America, with the duration of treatment and use of consolidative radiotherapy dependent on baseline risk factors and stage.1-5 Despite high cure rates, many patients will still relapse and require more intensive second-line therapies6-8 followed by high-dose chemotherapy and autologous stem cell transplant to achieve a long-term remission.
Three drugs have been approved for CHL in the United States since 2011 (BV9, nivolumab,10 and pembrolizumab11), based on significant activity in the relapsed/refractory setting. Pembrolizumab was found to have superior progression-free survival (PFS) vs BV in relapsed/refractory patients who were ineligible for autologous transplant.12 Subsequent studies using these agents in the frontline setting have been promising, and recent long-term follow-up of the ECHELON-1 study suggests a potential overall survival (OS) benefit with BV-AVD vs ABVD in patients who are at advanced stages.5 Programmed cell death protein 1 (PD1) inhibitor–based combinations so far have focused on performing a lead-in of PD1 inhibitor monotherapy followed by chemotherapy alone13,14 or concurrent PD1 inhibitor + chemotherapy.14,15 A phase 3 randomized study comparing concurrent nivolumab + AVD vs BV + AVD is ongoing, and results are not yet available.16
Interim positron emission tomography after 2 cycles of ABVD (PET2) has been identified as a prognostic marker17 and subsequently validated using the Deauville 5-point scoring system,18-20 leading to the development of several PET-adapted trials.2,21,22 Although initial results of these studies suggested that treatment intensification may improve outcomes compared with historical controls, the lack of a control arm has limited the interpretation of this data. Prospective data from uniformly treated patients with interim PET (PET2) results but who did not undergo treatment intensification or de-escalation have been made available through long-term results from the ECHELON-1 study.23 These findings suggest that improved surrogate markers are needed beyond PET2 for both risk stratification and early identification of treatment efficacy. Circulating tumor DNA (ctDNA) can be sequenced in plasma samples from patients with CHL,24,25 and response assessments may be enhanced with newer ultrasensitive minimal residual disease (MRD) techniques.26
We hypothesized that concurrent pembrolizumab with AVD (APVD) chemotherapy would be safe, feasible, and effective therapy for untreated patients with CHL and would be delivered more efficiently than similar sequential approaches. In exploratory analyses, we aim to examine the role of ctDNA in response assessment and the examination of MRD compared with traditional radiographic approaches.
Methods
Trial conduct
This was a single-center, open-label, investigator-initiated clinical trial of APVD in untreated CHL. The protocol was approved by the institutional review board at our institution. The trial was conducted in accordance with the good clinical practice guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and the principles of the Declaration of Helsinki.27 All the patients provided written, informed consent. Drug-only support was provided by Merck, with research funding provided by philanthropic sources, including the Richard Hotes Foundation. Our institutional data safety monitoring committee reviewed safety data on a regular basis. The first draft of the manuscript was written by the first author. All the authors reviewed the data and contributed to the preparation of the final version of the manuscript. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol and statistical analysis plan.
Patients
Eligible patients were at least 18 years of age having any stage of CHL with no prior systemic treatment for lymphoma, measurable disease of at least 1 cm in extranodal sites and 1.5 cm in nodal sites, an Eastern Cooperative Oncology Group performance status of 0 or 1, an ejection fraction of at least 50%, and adequate hematologic and organ function. Notable exclusion criteria include current or prior autoimmune disease (except vitiligo), current or prior history of pneumonitis/interstitial lung disease requiring corticosteroids, or current use of supplemental oxygen. Details of trial methods, including complete eligibility criteria, are provided in the protocol in the supplemental Appendix, which is available on the Blood website.
Treatment and assessments
A cycle of therapy was given every 28 days, with doses of AVD (doxorubicin [IV, 25 mg/m2], vinblastine [IV, 6 mg/m2], and dacarbazine [IV, 375 mg/m2]) on days 1 and 15, per the standard dosing.21 Steroid premedication was allowed per institutional standard of care. Pembrolizumab (IV, 200 mg) was given every 21 days starting on cycle 1 day 1 (eg, cycle 1 day 1, cycle 1 day 22, cycle 2 day 15, etc). Treatment was allowed regardless of absolute neutrophil count with or without granulocyte colony-stimulating factor (GCSF) support, which was optional based on previous publication and institutional practice.28 We recommended primary GCSF prophylaxis for patients who were older (ie, aged ≥60 years), with comorbidities, or with prior episodes of febrile neutropenia, at the discretion of the investigator. All patients were required to receive a minimum of 2 cycles of study therapy, with up to 6 total cycles allowed, at the discretion of the investigator, based on stage and baseline risk factors. Preplanned consolidative radiotherapy was allowed. Growth factor support was allowed and optional. Treatment responses were collected per Lugano criteria20 after 2 cycles of therapy, and if >2 cycles were administered, at the completion of all systemic therapy. Adverse events (AEs) were noted per Common Terminology Criteria for Adverse Events version 5.0 at the end-of-treatment (EOT) visit. Additional follow-up beyond the EOT visit was not specified in the protocol and was up to the institutional standard of care.
End points and statistical considerations
The primary objective of this study was to estimate the safety and tolerability of 2 cycles of the study treatment. At the time of study conception, there were concerns regarding the toxicity observed in the phase 1 study of BV + ABVD or AVD.29 We did not want the addition of pembrolizumab to the chemotherapy backbone to lead to delays in chemotherapy because of immune-related toxicity. Using the phase 1 BV + chemotherapy study as a benchmark, we determined that we would deem the regimen safe if >85% of the patients are able to complete 2 cycles of treatment without a dose delay due to toxicity of >3 weeks. This was used as a stopping rule and served as the primary end point. Criteria for interruption or discontinuation of pembrolizumab because of immune-related AEs (IRAEs) was outlined in the protocol and available in the supplemental Material. Dose modification or interruption of AVD were performed per the institutional standard of care.
Our secondary objective was to estimate the number of patients in a complete response (CR; Deauville 1-3) via PET2/computed tomography (CT). Exploratory objectives include markers of efficacy, including overall response rate (ORR), CR rate, PFS, and OS, for the intention-to-treat population. We also examined the predictive capacity of PET2 with long-term outcomes in a prespecified analysis. Additional exploratory analyses, including survival analyses, were performed using ctDNA collected at baseline as well as after cycle 1, cycle 2, and the EOT. Using a separate institutional retrospective protocol, we were able to perform a post hoc tumor metabolic tumor volume (TMTV) analysis for all patients at baseline and for each response assessment.
ctDNA profiling and MRD detection
Phased variant enrichment and detection sequencing (PhasED-seq) for tracking MRD and cancer personalized profiling by deep sequencing for ctDNA profiling were performed at Stanford University as previously described,26,30 with exceptions noted here. Whole blood was collected in either K2 EDTA tubes and processed within 6 hours or Streck BCT tubes and processed within 72 hours. Tubes were centrifuged twice at 1600g for 10 minutes at room temperature. After centrifugation, plasma was stored at −80°C in 1.8 mL aliquots until cell-free DNA (cfDNA) was isolated. Plasma-depleted whole blood was stored at −80°C until DNA was isolated from leukocytes. cfDNA was extracted from 1 to 11 mL of plasma (median, 5 mL) using the QIAamp Circulating Nucleic Acid Kit (Qiagen), following the manufacturer’s instructions. After isolation, cfDNA was quantified using the Qubit dsDNA High Sensitivity Kit (Thermo Fisher Scientific) and the High Sensitivity NGS Fragment Analyzer Kit (Agilent). Genomic DNA (gDNA) from matched plasma-depleted whole blood was extracted using the Qiagen Dneasy Blood and Tissue Kit, quantified using the Qubit dsDNA High Sensitivity Kit, and fragmented to a target size of 170 base pairs using the Covaris S2 sonicator. Fragmented gDNA was purified using the QIAquick PCR Purification Kit (Qiagen). For cfDNA, a median of 32 ng (range, 6-50 ng) was inputted for library preparation. For gDNA from leukocytes, 75 ng of fragmented gDNA was inputted for library preparation. After sequencing on Illumina HiSeq4000/X-ten or NovaSeq6000 machines, data preprocessing, alignment, identification of single nucleotide variants and phased variants and detection of MRD were performed as previously described.26,30 Quantitative levels of ctDNA were measured in haploid genome equivalents per mL and determined as the product of total cfDNA concentration and the mean allele fraction of somatic mutations expressed on a logarithmic scale (log10 haploid genome equivalents per mL).30,31 ctDNA change from baseline was calculated as log10 (on-treatment ctDNA level/pretreatment ctDNA level) for visualization in waterfall plots. Wilcoxon rank-sum test and Spearman correlation were used to correlate ctDNA levels with stage and TMTV, respectively. Plasma was prospectively banked at serial time points, with ctDNA MRD analysis performed later in batches, but not in real time.
Assessment of TMTV and correlation with ctDNA levels
TMTV was analyzed using PET/CTs performed at baseline, after cycle 2, and at the EOT. Native support for the calculation of TMTV was performed using MIM Encore (version 7.1.3; Cleveland, OH), which uses a threshold-based, semiautomated technique to identify any tumor with a standardized uptake value above the liver background of maximum standardized uptake value.32 TMTV analyses were performed retrospectively.
Results
Patients
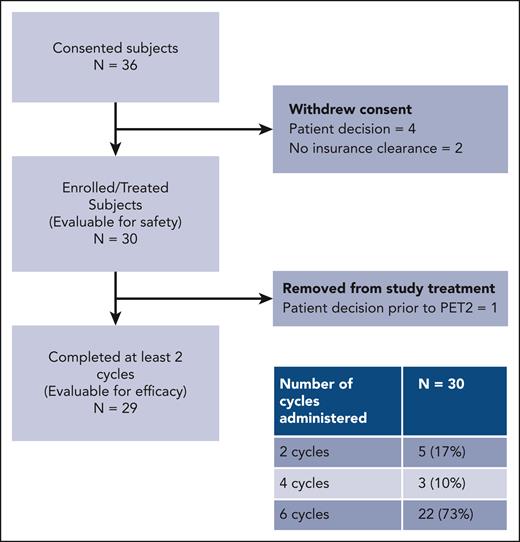
Between 1 February 2019 and 18 June 2021, 30 patients were enrolled on the study (Figure 1). Six additional patients consented but subsequently withdrew because of patient decision (n = 4) and lack of insurance clearance (n = 2). Enrollment ended when the accrual goal of 30 patients was met.
Patient characteristics are presented in Table 1. The median age was 33 years (range, 18-69 years), and most patients were females (18; 60%). Patients at all stages enrolled on the study, but most were in the advanced stage (18; 60%). At diagnosis, 13 patients (43%) had B symptoms, 6 (20%) had mediastinal bulk (>10 cm), 11 (37%) had an elevated erythrocyte sedimentation rate, and 7 (23%) had spleen involvement. Among the 12 patients at early stages, 6 (50%) were unfavorable per the National Comprehensive Cancer Network criteria. Among patients at advanced stages, 5 (28%) had an international prognostic score ranging from 4 to 7. Most patients received 6 cycles of treatment (22; 73%), with others receiving 2 cycles (5; 17%) and 4 cycles (3; 10%). Nine patients (30%) used growth factors for at least 1 cycle of treatment, including 4 (13%) for primary prophylaxis and 5 (17%) for secondary prophylaxis of febrile neutropenia.
Treatment exposure and safety
Thirty patients received at least 1 dose of study therapy and were evaluable for safety. A list of treatment-emergent AEs, observed in >10% of patients, as well as all grade 3 and 4 AEs are listed in Table 2. No deaths were observed in any study patient, either during treatment or during long-term follow-up. Serious AEs were observed in 6 patients (20%), including 5 patients with febrile neutropenia and 1 patient with a grade 4 large bowel obstruction/abscess in the setting of diverticulitis with no evidence of colitis. The most common grade 3 or 4 nonhematologic AEs observed include febrile neutropenia (5; 17%), hyponatremia (3; 10%), syncope (3; 10%), and sepsis (2; 7%). One secondary malignancy was observed, a diffuse large B-cell lymphoma (DLBCL) in a 69-year-old patient that presented 11 months after completion of the study therapy. In this patient, at diagnosis, the CHL was tested as Epstein-Barr encoding region in situ hybridization–positive, whereas the secondary DLBCL was Epstein-Barr virus–negative. For this reason, we felt this was more likely to represent a distinct malignancy and unlikely to represent a relapse of a composite lymphoma, though comparative clonality studies are not available.
There were no treatment delays >21 days observed during the first 2 cycles of therapy, meeting the study’s primary end point. Three patients had treatment delays because of AEs, including 2 patients with grade 3 febrile neutropenia (2 and 5 days, respectively). One patient with a grade 4 large bowel obstruction/abscess in the setting of diverticulitis had treatment delay and doxorubicin omitted from cycle 3 onward because of newly onset grade 1 heart failure and discontinued all study treatment from cycle 4 day 22 onward because of recurrent postoperative complications. Six patients (20%) missed at least 1 dose of pembrolizumab because of AEs, primarily grade 2 or higher transaminitis (5; 17%). Three patients (10%) experienced grade 3 or higher aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation, which required permanent discontinuation of pembrolizumab. One patient with grade 3 ALT elevation who was required to discontinue pembrolizumab was transitioned to AAVD therapy at investigator discretion but did not have evidence of progressive disease. One other patient who omitted at least 1 dose of pembrolizumab did so because of a newly onset grade 1 pleural effusion (subsequently found to be due to reduced ejection fraction), which was attributed to doxorubicin because there was no elevation of troponins or evidence of myocarditis observed. After several cycles with pembrolizumab and doxorubicin being held because of toxicity, all treatment was discontinued before cycle 5, and the patient has remained in remission for >1 year after completing all therapy.
We have outlined all grades of AEs deemed to be IRAEs in Table 3. In addition to the previously mentioned elevations of AST and ALT, we also observed grade 2 hypothyroidism (2; 7%), rash (any grade, 13 [43%] and grade 2+, 3 [10%]), grade 2 arthritis (1; 3%), and grade 2 colitis (1; 3%). All elevations of AST or ALT are transient and reversible. Five patients with grade 2 or higher AST or ALT elevation were managed with holding pembrolizumab and corticosteroids as required by the protocol, including 3 patients with grade 3 or 4 transaminitis that required permanent discontinuation of pembrolizumab. All patients who required corticosteroids during the study treatment were able to discontinue them before the completion of chemotherapy. The episode of grade 2 colitis occurred 1 month after the completion of study treatment, in which all expected doses of pembrolizumab were received. The patient was able to discontinue all steroids ∼3 months later. No patient who required any dose modification, discontinuation, or treatment delay has experienced progression to date. No pneumonitis or other grade ≥3 IRAEs were observed. All IRAEs resolved by the EOT or during long-term follow-up, except those in patients who continued thyroid hormone supplementation.
Efficacy
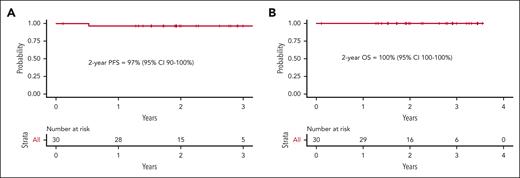
Twenty-nine patients had at least 1 response assessment and were evaluable for efficacy. The best ORR was 100%, including 90% in CR. A PET2/CT showed an ORR of 100% and CR rate of 66%. In the 23 patients with early-stage unfavorable or advanced-stage disease, there was a PET2 CR rate of 57%. Three patients at early-stage disease (all favorable per the German Hodgkin Study Group criteria) with a PET2 CR proceeded with preplanned consolidative radiotherapy. At the EOT, 82% of patients were in complete remission. Five patients at the EOT had persistent or new fluorodeoxyglucose (FDG) uptake, 1 of whom ultimately developed biopsy-proven progressive disease. Two patients remained in partial response (Deauville 4) throughout the study treatment, without disease progression to date. One of these patients was followed up with surveillance CT and has remained progression-free 28 months later. Another patient had persistent FDG uptake upon follow-up PET/CT 3 months later, underwent a mediastinoscopy, showed the presence of benign thymic tissue, and has remained progression-free 28 months after treatment completion. Two other patients achieved a PET2 CR but developed FDG uptake upon EOT PET/CT. One of these patients had an additional PET/CT 4 months later that remained FDG-avid but with stable lymph node size. The investigator opted to transition to CT-based surveillance, which has not demonstrated progressive lymphoma to date, 36 months after the completion of therapy. Another patient’s new FDG uptake resolved by the subsequent PET/CT, and the patient has remained progression-free 33 months after completion of therapy. These 4 patients were followed up with serial imaging and/or biopsies, and, to date, none have developed recurrent HL 28, 28, 33, and 36 months after completion of study treatment. With a median follow-up of 2.1 years, only 1 patient has suffered a relapse, resulting in a 2-year PFS and OS of 97% (95% confidence interval, 90%-100%) and 100% (95% confidence interval, 100%-100%), respectively (Figure 2).
Kaplan-Meier estimates. PFS (A) and OS (B) for the response-evaluable study population. CI, confidence interval.
Kaplan-Meier estimates. PFS (A) and OS (B) for the response-evaluable study population. CI, confidence interval.
MRD analysis with PhasED-Seq and correlation with TMTV
Banked plasma samples for all 29 response-evaluable patients were analyzed for ctDNA at baseline. Twenty-six patients had sufficient ctDNA detected at baseline and were eligible for MRD monitoring. One patient did not have detectable ctDNA at baseline, and 2 others had insufficient mutation reporters at baseline to allow for sensitive MRD detection. Of note, all 3 patients with insufficient baseline ctDNA at baseline had early-stage disease. Of the 26 patients evaluable for MRD assessment, all banked plasma samples could successfully be profiled at cycle 3 day 1 (n = 26) and the EOT (for those with >2 total cycles, n = 24).
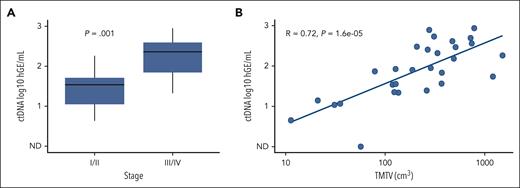
Baseline quantitative ctDNA levels and tumor volume were compared with those at baseline stage and total metabolic tumor volume (Figure 3). ctDNA levels were lower in patients at early stage compared with patients at advanced stage (P = .001). In addition, TMTV was strongly correlated with ctDNA at baseline (RS = 0.72; P < .0001).
Total metabolic tumor volume and ctDNA analyses. (A) Comparison of baseline ctDNA levels in patients at early-stage I or II compared with in those at stage III or IV. (B) Scatterplot comparing baseline ctDNA levels to TMTV. hGE, haploid genome equivalents.
Total metabolic tumor volume and ctDNA analyses. (A) Comparison of baseline ctDNA levels in patients at early-stage I or II compared with in those at stage III or IV. (B) Scatterplot comparing baseline ctDNA levels to TMTV. hGE, haploid genome equivalents.
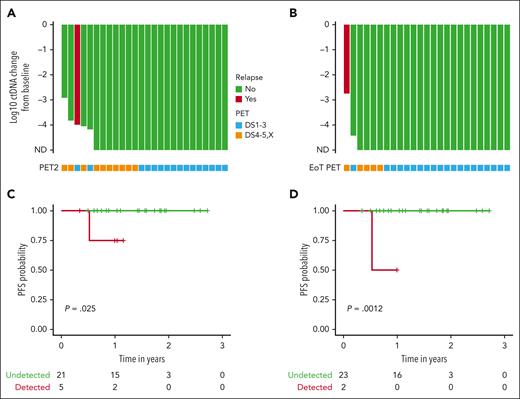
We compared the log reduction in ctDNA levels with the baseline levels at both cycle 3 day 1 and the EOT (Figure 4). Undetectable ctDNA levels were common at both time points. At cycle 3 day 1, 21 patients (81%) were tested as ctDNA-negative, including 7 of 8 (88%) with early-stage favorable disease and 14 of 18 (78%) with early unfavorable/advanced-stage disease. At the EOT, ctDNA clearance increased to 24 of 26 (92%) in all patients, with 8 of 8 (100%) patients who were early-stage favorable and 16 of 18 patients who were early-stage unfavorable or advanced stage showing no detectable disease. The only known patient who relapsed had detectable ctDNA at all time points, whereas 4 patients with positive EOT PETs had undetectable ctDNA. None of these 4 patients have had recurrent disease at 28, 28, 33, and 36 months since the completion of treatment. One additional patient had detectable ctDNA at the EOT. However, this patient had discontinued treatment during cycle 4 after developing a cardiomyopathy, so the EOT sample was drawn nearly 2 months earlier than expected. This patient remains without evidence of recurrence more than a year after discontinuing therapy. We performed a survival analysis comparing patients who were tested as ctDNA-positive vs those who were tested as ctDNA-negative at both cycle 3 day 1 and the EOT. ctDNA clearance was associated with improved PFS at both cycle 3 day 1 (P = .025) and the EOT (P = .0012).
Exploratory analyses of ctDNA and PET with clinical outcomes. Waterfall plot with x-axis plotted from treatment initiation representing log change in ctDNA levels at cycle 3 day 1 (A) and EOT (B). Green bars represent patients with no evidence of relapse and the red bar represents the only patient with evidence of recurrence. Below each bar is a square that indicates the PET result at the same time point. Blue squares represent a complete metabolic response (Deauville 1-3) and orange squares indicate FDG uptake (Deauville 4, 5, or X). Of note, 2 patients with detectable ctDNA at baseline that only received 2 total cycles of treatment on study were represented on both waterfall plots. One patient who came off study after cycle 3 day 1 because of toxicity and received alternate frontline treatment was represented only on the cycle 3 day 1 waterfall plot. Survival analyses comparing PFS in patients with detectable or undetectable ctDNA at cycle 3 day 1 (C) and the EOT (D). ND, not detected.
Exploratory analyses of ctDNA and PET with clinical outcomes. Waterfall plot with x-axis plotted from treatment initiation representing log change in ctDNA levels at cycle 3 day 1 (A) and EOT (B). Green bars represent patients with no evidence of relapse and the red bar represents the only patient with evidence of recurrence. Below each bar is a square that indicates the PET result at the same time point. Blue squares represent a complete metabolic response (Deauville 1-3) and orange squares indicate FDG uptake (Deauville 4, 5, or X). Of note, 2 patients with detectable ctDNA at baseline that only received 2 total cycles of treatment on study were represented on both waterfall plots. One patient who came off study after cycle 3 day 1 because of toxicity and received alternate frontline treatment was represented only on the cycle 3 day 1 waterfall plot. Survival analyses comparing PFS in patients with detectable or undetectable ctDNA at cycle 3 day 1 (C) and the EOT (D). ND, not detected.
Discussion
The therapeutic landscape for newly diagnosed CHL continues to evolve, with additional data on the use of various novel agents in combination with chemotherapy. In long-term follow-up, BV-AVD has shown continued PFS and now an OS benefit when compared with ABVD.5 However, the risks of peripheral sensory neuropathy, febrile neutropenia, and the mandatory use of growth factors support the need for further improvements. In the relapsed setting, pembrolizumab has been shown to be superior to BV in a head-to-head comparison, leading to its exploration in the frontline setting by our study.
To our knowledge, these initial results of APVD in untreated CHL are the first to demonstrate the tolerability and activity of concurrent PD1 inhibitor anthracycline-based chemotherapy in patients at advanced stages without a PD1 inhibitor lead-in, building on the work of other trials using a sequential approach that demonstrated single-agent frontline anti-PD1 activity.13-15 We were able to show that the APVD regimen showed promising safety and efficacy with similar overall outcomes over a much shorter time course than those of sequential PD1 inhibitor regimens. This shortened timeline not only resulted in a diminished total treatment duration for patients but also allowed for a more rapid achievement of disease control. This approach also avoids the possibility of experiencing an IRAE during the PD1 monotherapy portion that could delay or prevent the administration of combination chemotherapy.
The APVD regimen was well tolerated, and the IRAEs observed were manageable and reversible in all cases (excluding hypothyroidism). The concurrent approach saw increased rates of elevated AST and ALT compared with previously published monotherapy and sequential chemotherapy combinations, including cases of grade 3 or higher. Our findings are comparable with the results of using pembrolizumab with liposomal doxorubicin, gemcitabine, and vinorelbine and may indicate the potential for low-grade hepatotoxicity with concurrent chemotherapy and anti-PD1 strategies.8 Although our protocol did call for up to 8 total doses of pembrolizumab (vs 3 total in the sequential approach13), most toxicity occurred early and likely was not a reflection of an increased cumulative dose of pembrolizumab. Fortunately, all patients remained asymptomatic, had no elevations of total bilirubin, and did not suffer lower remission rates.
We also observed a febrile neutropenia rate of 17%, compared with 10% observed in approaches with a PD1 inhibitor lead-in (Checkmate-20515 and sequential pembrolizumab + AVD13) and 8% seen in ABVD.33 Our patients experienced higher rates of at least 1 episode of neutropenia (93% any grade and 83% grade 4) than reported in these other frontline combination regimens. Despite this, we observed only 3 patients (10%) with grade 3 or 4 infections, in line with ABVD findings,34 and below the 18% grade 3 or higher infection rate observed with AAVD that led to the mandatory use of GCSF. More importantly, we did not observe any pneumonitis in our study, which is said to affect up to 7% of patients treated with bleomycin. Careful attention is needed in larger PD1 and chemotherapy combination studies to determine whether checkpoint inhibitors continue to contribute to higher rates of febrile neutropenia and thus determine whether GCSF use should be mandated and also to assess the risk of rare IRAEs more intricately.
The role of PET-adapted therapy in the era of novel agent chemotherapy combinations remains undefined. Long-term follow-up from the ECHELON-1 study found that patients who were tested as PET2+ and continued receiving AAVD and ABVD had a 5-year PFS of 60.6% and 45.9%, respectively.23 These results exceed those shown in previously published retrospective reports,17,19 and in the case of AAVD, they are very similar to the outcomes observed among patients who escalated to BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) in prior PET-adapted studies.21,22 PET2-negativity was also an imperfect prognostic marker in both AAVD- and ABVD-treated patients, with a 5-year PFS of 84.9% and 78.9%, respectively, in these groups. APVD yielded a lower PET2 CR rate (66%) as well as EOT CR rate (82%) compared with historical regimens, yet only 1 out of 30 patients enrolled ultimately relapsed. Importantly, no patient who tested PET2+ has relapsed to date, and only 1 out of 5 patients with FDG uptake on EOT imaging ultimately relapsed. It is not clear why APVD was associated with increased rates of presumably false-positive results, but we have observed high rates of false-positive FDG-PET in other settings of pembrolizumab-chemotherapy combinations for lymphoma (PR-CHOP [pembrolizumab, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone]).35 These false-positive findings were not reported in the sequential pembrolizumab + AVD approach13 but were observed in the Checkmate-20515 and NIVAHL14 studies, suggesting that perhaps the concurrent use of these drugs may increase the likelihood of spurious FDG uptake. These results imply that most of the patients who test PET2+ and are treated with APVD will achieve a long-term remission, countering the use of PET2-adapted escalation approaches with this or similar regimens. These findings broadly call into question the utility of an interim PET in patients without signs of clinical progression treated with similar approaches.
To improve response assessment beyond FDG-PET, we explored ctDNA as a potential tool for measuring both tumor burden before therapy and residual disease during and after therapy. Similar to findings in DLBCL,26,30,31,36,37 we found baseline ctDNA levels in HL to reflect tumor burden, with ctDNA negativity after treatment being associated with favorable treatment outcomes. Previous efforts to demonstrate the utility of MRD in CHL have used immunoglobulin sequencing,38 droplet polymerase chain reaction,39 or capture-based strategies tracking point mutations,24,25,40,41 generally associated with sensitivities of ∼1:10 000 or less when using typical volumes of plasma. However, as with radiographic responses, deep molecular responses in CHL seem to be achieved substantially faster than in DLBCL, with most prior techniques demonstrating limited clinical sensitivity for the early detection of residual disease. Accordingly, highly sensitive assays such as PhasED-Seq26 that allow for ultrasensitive MRD detection at levels ∼10−6 are likely to be critical for measuring MRD after therapy of CHL. Remarkably, >80% of patients with HL in this study tested negative for ctDNA MRD after just 2 cycles of APVD therapy, using PhasED-seq. In contrast, less than half of the patients with DLBCL appear to clear ctDNA MRD after 2 cycles of therapy, using this same assay.42
Here, no patients who cleared ctDNA had recurrent CHL, and those with persistent FDG uptake received additional scans and biopsies. If PD1 inhibitor–based chemotherapy combinations are associated with increased false-positive PET scans,14,15 then they may represent a tool to help avoid the anxiety and morbidity of additional scans and/or biopsies or potentially obviate the need for follow-up FDG imaging. Given the poor performance of interim PET in our and similar studies, a better understanding of ctDNA dynamics in patients treated for CHL may inform the design of future response-adapted clinical trials for treatment de-escalation. The negative predictive value of ctDNA MRD appears high in this study as well as in a pooled analysis,43 which could inform future abbreviation strategies. In contrast, although the positive predictive value of ctDNA for treatment failure seems somewhat lower, particularly on interim assessments, the positive predictive value of interim ctDNA appears to be at least as high as that of interim PET in the context of APVD therapy. Nevertheless, future strategies for leveraging the positive predictive value ctDNA MRD for treatment escalation are likely to be most efficient when considering patients who test ctDNA MRD+ at the end-of-induction landmark.
The generalizability of our results to date is limited given the small sample size, but our APVD data provide early insight into the safety and efficacy of this strategy. This was a single-arm study that enrolled a heterogeneous patient population, including limited and advanced stage patients who received different treatment durations based on their stage and baseline risk factors. Our study was not enriched for male patients (who are at higher-risk) or those having an international prognostic score ranging from 4 to 7. An additional cohort has since been added after completing this study to treat exclusively patients who are advanced stage to better define efficacy and further examine the dynamics of response assessment with PET and ctDNA. Another limitation is the every 3-week pembrolizumab dosing, which adds 4 additional visits over the course of 6 cycles and was required by the Merck Investigator Studies Program at the time of study conception despite the availability of every 6-week dosing outside of a clinical trial. The program has since allowed future studies to incorporate every 6-week dosing, which would allow for future studies to use a dosing schedule that would avoid additional infusion visits outside of the chemotherapy administration days.
In conclusion, concurrent APVD represents a time-efficient regimen with promising safety and efficacy as frontline therapy for CHL. However, a positive posttreatment FDG-PET after this approach was poorly predictive of persistent ctDNA and relapse. ctDNA may represent a more sensitive and specific treatment-agnostic response assessment tool that merits further study in larger data sets.
Acknowledgments
The authors acknowledge the clinical nurse coordinators, patient care coordinators, as well as the participating patients and their families at the Fred Hutch Cancer Center.
The authors acknowledge the philanthropic support of the Richard Hotes Foundation, Frank and Betty Vandermeer, Sonya and Tom Campion, and Seattle Translational Tumor Research. This work was supported in part by a research grant (drug-only supply of pembrolizumab without research funding) from the Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. Correlative studies were funded by National Institutes of Health, National Cancer Institute grant R01CA257655-01. Additional support was provided by a Lymphoma Research Foundation Career Development Award.
The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC.
Authorship
Contribution: R.C.L. and A.K.G. designed the research and wrote the manuscript; R.C.L., C.S.U., C.P., E.H.W., S.D.S., M. Shadman, B.T., V.M.R., H.C., M. Shelby, S.O., S.K., K.V., Y.T., P.M., and E.M. performed the research; S.A., A.A.A., A.G., and D.L.C. contributed vital new reagents or analytical tools; R.C.L., H.D., J. Vandermeer, A.G., and H.R. collected data; R.C.L., S.A., A.A.A., A.G., D.L.C., J. Voutsinas, and A.K.G. analyzed and interpreted the data; and J. Voutsinas, S.A., and A.A.A. performed statistical analysis.
Conflict-of-interest disclosure: R.C.L. reports research funding from TG Therapeutics, Incyte, Bayer, Cyteir, Genentech, Seagen, and Rapt and consultancy at Cancer Study Group and Seagen. C.S.U. reports consultancy at Genentech. S.D.S. reports research funding from ADC Therapeutics, AstraZeneca, Ayala (spouse), Bayer, BeiGene, Bristol Myers Squibb (BMS; spouse), De Novo Biopharma, Enterome, Genentech, Ignyta (spouse), Incyte Corporation, Kymera Therapeutics, Merck Sharp and Dohme Corp, MorphoSys, Nanjing Pharmaceuticals Co, Ltd, Portola Pharmaceuticals, and Viracta Therapeutics and consultancy or membership on advisory board at ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Karyopharm, KITE pharma, Incyte, Numab Therapeutics AG, AbbVie, and Coherus Biosciences. B.T. reports consultancy at Mustang Bio and Proteios Technology; patents and royalties at Mustang Bio; and research funding from Mustang Bio and BMS/Celgene. M. Shadman reports consulting, being on advisory boards, steering committees, or data safety monitoring committees at AbbVie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, BMS, Morphosys/Incyte, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate Therapeutics, MEI pharma, and Atara Biotherapeutic and research funding from Mustang Bio, Celgene, BMS, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, MorphoSys/Incyte, and Vincerx. A.A.A. reports ownership interest in CiberMed, Forty Seven Inc, and Foresight Diagnostics; patent filings related to cancer biomarkers; research funding from BMS and Celgene; and paid consultancies from Genentech, Karyopharm, Roche, Chugai, Gilead, and Celgene. A.G. reports research funding from Merck, I-Mab Biopharma, IgM Bio, Takeda, Gilead, AstraZeneca, Agios, Janssen, BMS, Seagen, Teva, and Genmab; consultancy/honoraria from Incyte, Kite, Morphosys/Incyte, ADC Therapeutics, Acrotech, Merck, Karyopharm, Servier, BeiGene, Cellectar, Janssen, Seagen, Epizyme, I-Mab Biopharma, Gilead, Genentech, Lilly, Caribou, and Fresenius-Kabi; and equity ownership at Compliment Corporation. The remaining authors declare no competing financial interests.
Correspondence: Ajay K. Gopal, Division of Medical Oncology, Department of Medicine, University of Washington, 825 Eastlake Ave E LG-650 Seattle, WA 98109; e-mail: agopal@uw.edu.
References
Author notes
Proposals for collaborations using patient-level data are available on request from the corresponding author, Ajay K. Gopal (agopal@uw.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.