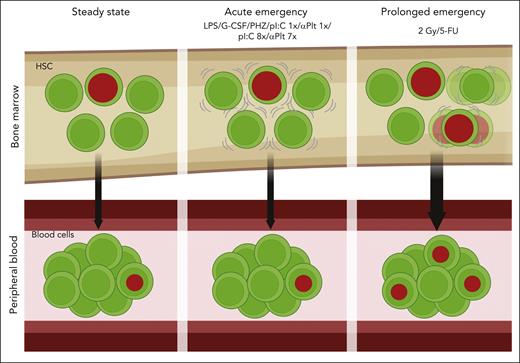
In this issue of Blood, Munz et al1 demonstrate that the contribution of hematopoietic stem cells (HSCs) to fresh, new blood cells from hematopoiesis induced by a sudden emergency is negligible.
New blood cells are generated at astonishing rates of hundreds of billions per day in humans. Current dogma is that all of these cells are coming from a rare population of HSCs that may count about one-millionth of the productive clones. These orders of magnitude of production are made possible by hard-working progenitors, in contrast to the highly quiescent HSCs in the bone marrow. Although the basic concept of the hematopoietic tree is being reevaluated,2 HSCs have been mostly defined and evaluated at homeostasis. But what happens in an emergency state due to sudden blood loss?
Various perturbations, such as irradiation, myeloablations, inflammation, loss of red blood cells (RBCs), or loss of platelets, can drive emergency hematopoiesis to increase production. HSCs' dormancy protects long-term potency, but stimulation can drive them into proliferation.3 A series of seminal studies reported how various inflammatory stimuli do effectively activate HSCs. The regulatory network is fascinatingly complex, yielding a fine balance of proliferation and differentiation, as was recently reviewed for effects of interferons.4 It was shown that reentry into quiescence protects HSCs from deleterious impact of chronic stimulation.5 Moreover, activation of HSCs might drive clonal hematopoiesis,6 further highlighting the need to better understand and possibly modulate immune aging, and the increased risk of hematological malignancies. Nevertheless, it is commonly thought that HSCs' activation is important for enhancing the production of new blood cells.7
Munz et al used murine reporter systems that provided quantitative data from HSCs within the host animal. Histone-2B green fluorescent protein (H2B-GFP) labels histones with a fluorescent reporter, which is diluted by cell division. Fgd5-driven lineage tracing provides a fate map from HSCs to all progeny. The authors used these 2 systems to quantitatively measure the response of HSCs to severe perturbations. Total-body irradiation (2 Gy), or myeloablation (5-fluorouracil), caused a significant increase in the fraction of fate-labeled progenitors and mature blood cells, demonstrating an enhanced contribution of HSCs to new blood cells.
Surprisingly, in an impressive series of experiments, they found virtually no increase in the contribution of HSCs to mature blood cells following physiological stimuli using lipopolysaccharide (a bacterial cell wall), granulocyte colony-stimulating factor (used for HSC mobilization), or polyinosinic:polycytidylic acid (pIpC; synthetic RNA that induces interferons). None of these caused a significant increase of fate-labeled mature blood cells. RBC depletion using phenylhydrazine or platelet depletion using antibodies (αPlt) also did not cause substantial increase in the contribution of HSCs. Little to no additional cell divisions were counted, except after repetitive pIpC (see figure). Taken together, the authors argue that primitive HSCs are not contributing to the increased generation of new blood cells following physiological emergency situations, such as inflammation or blood loss.
HSCs do not contribute to sudden emergency hematopoiesis. Fate-mapping HSCs of the bone marrow showed no increase following various physiological stimuli, such as inflammation or depletion of blood cells. Harsh perturbations, like irradiation or myeloablation, did show an increase in the contribution of HSCs to blood cells. Progenitors may play a larger role than previously appreciated.
HSCs do not contribute to sudden emergency hematopoiesis. Fate-mapping HSCs of the bone marrow showed no increase following various physiological stimuli, such as inflammation or depletion of blood cells. Harsh perturbations, like irradiation or myeloablation, did show an increase in the contribution of HSCs to blood cells. Progenitors may play a larger role than previously appreciated.
This study agrees with the recently published results of another study, by Fanti et al.8 Using a different fate-map system with different perturbations, no increase in HSC differentiation was found from stimuli, including sepsis, bleeding, or depletion of granulocytes or B cells. Fanti et al also added transcriptome data, showing a complex activation response within HSCs, suggesting a link with self-renewal and maintenance.8 Both studies agree on the main point: emergency hematopoiesis is not reliant on HSCs. Intriguingly, another recent study discovered that HSC-independent multilineage progenitors (termed embryonic multi-potent progenitors, [eMPP]) contribute a substantial portion of blood cells during development.9 Thus, progenitors may handle homeostasis and recovery from some perturbations without help from the HSCs.
So, what do we need HSCs for? First, they (still) keep all the potency that enables stem cell transplantation. Second, HSCs take an active part in normal hematopoiesis and are called for duty following major perturbations. You may perhaps think of them as “senior physicians on call”: going home to preserve potency (in supportive niche). In case of a manageable emergency, you may call and wake them up; but only in a major crisis are they awoken and arrive to help address the crisis. Such a model highlights several important remaining questions, such as identification of communication mechanisms, and defines the threshold of each activation step.
The current study of Munz et al has an interesting peculiarity: the 8 × pIpC perturbation caused additional cell divisions, but no increase in labeled blood cells. This agrees with Fanti et al,8 who also saw an increased early proliferation. One explanation is that not all HSC divisions make productive progenitors. Another issue is that both studies quantify relative portions of fate-labeled cells; if the hematopoiesis rate is increased by progenitors, it might be hard to measure a relative low increase by rare HSCs. Robust increase following nonphysiological irradiation or chemotherapy is partially enhanced by the destruction of proliferating progenitors.
Thanks to this study, we have to change our concept of emergency hematopoiesis. Additional tools, such as endogenous markers of HSC activation,10 can help to identify and isolate HSCs following perturbations. HSCs demonstrate smart behavior that helps preserve their long-term potency. It is reasonable to speculate that other adult stem cells may utilize a similar strategy. Understanding the regulatory mechanisms will open novel opportunities to either enhance hematopoiesis at times of great need or restrain unnecessary activations for longer and healthier life.
Conflict-of-interest disclosure: The authors declare no competing financial interests.