In this issue of Blood, Alduaij et al1 report on the utility of a dark zone signature (DZsig) in the diagnosis of aggressive B-cell lymphoma with diffuse large B-cell lymphoma (DLBCL) morphology and show that DZsig has strong prognostic value.
The classification of aggressive B-cell lymphomas into clinically relevant entities (types) and subtypes based on the molecular characteristics of the tumor has been an active and ever-evolving area as a consequence of the molecular heterogeneity of the disease with its heterogeneous clinical behavior. Therefore, classification should aim to reflect both clinical behavior and biologic characteristics.
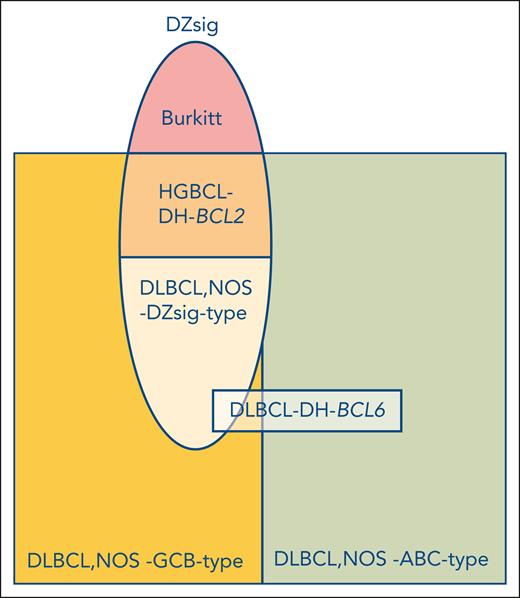
The “normal counterpart” model proposed by Karl Lennert for the Kiel Classification in the early 1980s was based on B-cell immunology and was the first to introduce a biological foundation for lymphoma classification.2 This normal counterpart model spawned the DLBCL grouping based on the RNA gene-expression-based “cell-of-origin” (COO) concept at the turn of the century. In the current paper, Alduaij et al renamed the previously reported gene-expression double hit signature (which was originally designed to recognize high-grade B-cell lymphoma with MYC and BCL2 rearrangement [HGBCL-DH-BCL2]),3 the dark-zone signature (DZsig). Thereby, also this RNA-based signature is now acknowledged for its “normal counterpart” nature (see figure). The DZsig captures not only HGBCL-DH-BCL2 tumors but also twofold more DLBCLs without this genetic alteration. The genetic makeup of these additional DZsig-positive/DH-BCL2-negative cases is still unclear as mere amplification of MYC and BCL2 as an alternative for translocation was excluded.
A COO-based classification of aggressive B-cell lymphomas. The newly described DZsig (oval), that is defined by a normal germinal center dark zone centroblast profile, identifies a group of DLBCLs that were originally predominantly included in the GCB gene-expression class (yellow). DZsig brings together cases with a dismal outcome (approximately 60% 2-year overall survival). The remaining DLBCL, not otherwise specified (NOS)-GCB-type cases are also distinct and characterized by an excellent outcome (approximately 90% 2-year overall survival). At the DNA level, however, DZsig is heterogeneous, containing approximately one-third lymphomas with concomitant MYC and BCL2 rearrangements (HGBCL-DH-BCL2, mid-oval, orange) and two-thirds without this genetic aberration (lower part of oval, light orange). The genetic composition of the latter group remains to be elucidated. It should be noted that the DZsig gene-expression profile is not specific for DLBCL, however, and extends beyond this entity since it is also a consistent feature of Burkitt lymphoma (top of oval, pink). How DLBCL with concomitant MYC and BCL6 rearrangements (DLBCL-DH-BCL6) may relate to this novel COO classification is not fully resolved.
A COO-based classification of aggressive B-cell lymphomas. The newly described DZsig (oval), that is defined by a normal germinal center dark zone centroblast profile, identifies a group of DLBCLs that were originally predominantly included in the GCB gene-expression class (yellow). DZsig brings together cases with a dismal outcome (approximately 60% 2-year overall survival). The remaining DLBCL, not otherwise specified (NOS)-GCB-type cases are also distinct and characterized by an excellent outcome (approximately 90% 2-year overall survival). At the DNA level, however, DZsig is heterogeneous, containing approximately one-third lymphomas with concomitant MYC and BCL2 rearrangements (HGBCL-DH-BCL2, mid-oval, orange) and two-thirds without this genetic aberration (lower part of oval, light orange). The genetic composition of the latter group remains to be elucidated. It should be noted that the DZsig gene-expression profile is not specific for DLBCL, however, and extends beyond this entity since it is also a consistent feature of Burkitt lymphoma (top of oval, pink). How DLBCL with concomitant MYC and BCL6 rearrangements (DLBCL-DH-BCL6) may relate to this novel COO classification is not fully resolved.
The findings in this article call to reflect on what might be the most meaningful way to classify DLBCL incorporating multiple factors including RNA gene-expression profiling (GEP), genetics (DNA mutations, chromosomal copy number aberrations and translocations), and epigenetics (methylation). A genetics approach in recent years has resulted in 2 largely similar next-generation sequencing (NGS)-based DNA classifications.4-6 Although the prognostic impact of these NGS-based classes may be limited, their strength lies in their potential to identify targetable genetic alterations. Two classes are notable for having the most defining DNA alterations. The C54/MCD5 class has among others genetic alterations leading to constitutive NF-kB activation and was recently shown to be 1 of the 2 unique classes (N1 being the other) benefiting from the addition of ibrutinib to R-CHOP in DLBCL patients under 60 years of age.7 This supports the predictive potential of the NGS-classification approach and thereby its clinical relevance. The other biologically more clearly defined group is C34/EZB,5 which is (almost) exclusively made up of COO-germinal center B cell-like (GCB)-DLBCL. The C3/EZB class splits into 2 subgroups with radically different outcomes, with the poor prognosis group enriched for HGBCL-DH-BCL2 tumors.4-6 This example demonstrates the limitations of the current NGS-based classifications. The results presented by Alduaij et al argue for a GEP-based approach to classify DLBCL, which includes 3 components: DZsig, germinal center-like (GCB), and activated B-cell-like (ABC) (see figure). The clinical relevance of this GEP classification is demonstrated by the homogeneous clinical behavior with a very poor prognosis and the far majority of relapse and fatal events occurring within the first year after diagnosis for thus defined DZsig-positive aggressive B-cell lymphomas (encompassing both DLBCL, NOS, and HGBCL-DH-BCL2). Here, GEP-based classification currently overrules NGS-based classification. Alas, GEP does not necessarily provide biological insights that may indicate potential treatment targets, which NGS does. Therefore, GEP may only be used to select patients for intensified treatment protocols, especially for the DZsig-positive patient population. How to proceed? Possible lessons could come from the neuro-oncology field, in which methylation profiling has proven to be successful to differentiate tumor entities and now serves as a basis for the new WHO classification.8 This approach has had limited exploration in the lymphoma field and awaits further research. An approach combining the strengths of NGS and GEP information may be feasible. A recent publication proposed a model incorporating gene-expression information derived from both tumor cells as well as from the reactive immune microenvironment, which may also be a treatable target.9
Recently, two updates to the fourth edition of the WHO Classification for Tumours of the Haematopoietic and Lymphoid Tissue were published and based on the same literature both revised the definitions for “DH-lymphomas” to include only those cases with concurrent MYC and BCL2 rearrangements, while excluding those with concurrent MYC and BCL6 rearrangements.10,11 The fifth edition of the WHO Classification chose to include DH-BCL6 cases as a subtype of DLBCL, NOS, while the International Consensus Classification proposal listed these as a provisional entity. The present study by Alduaij et al now provides a direction for further refinement of these classifications of aggressive B-cell lymphomas.
Although the findings in the study by Alduaij et al may not be perceived as entirely novel as the same British Columbia group had previously explored this area,3 the population-based nature of 1149 patients repositions these findings in an epidemiological context. Poor-risk and elderly patients are now better represented compared with trial or random cohorts, although bias remains due to lack of (remaining) biopsy material of the worst-performing patients. In this real-world series, it now seems that COO-ABC and DZsig are not enriched in elderly patients, as previously reported in other more biased series. The question still arises how unselected a population-based cohort from British Columbia really is. In the first place, British Columbia’s lymphoma care is exceptionally well organized with pathology review and molecular diagnostic support incorporated into routine care. Moreover, British Columbia is an example of a Western-world population with a highly diverse ethnic background but is not representative globally and hence only reflects their part of the real world.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal