Key Points
VTE is a frequent event in patients with adult-type diffuse glioma, but tools to assess VTE risk are not available.
Using clinical and pathologic variables, including molecular data, we generated a web-based VTE prediction tool for patients with glioma.
Abstract
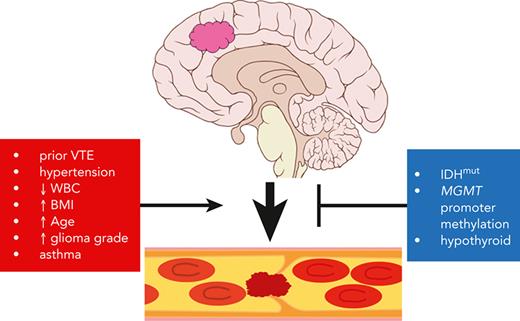
Venous thromboembolism (VTE) is a life-threating condition that is common in patients with adult-type diffuse gliomas, yet thromboprophylaxis is controversial because of possible intracerebral hemorrhage. Effective VTE prediction models exist for other cancers, but not glioma. Our objective was to develop a VTE prediction tool to improve glioma patient care, incorporating clinical, blood-based, histologic, and molecular markers. We analyzed preoperative arterial blood, tumor tissue, and clinical-pathologic data (including next-generation sequencing data) from 258 patients with newly diagnosed World Health Organization (WHO) grade 2 to 4 adult-type diffuse gliomas. Forty-six (17.8%) experienced VTE. Tumor expression of tissue factor (TF) and podoplanin (PDPN) each positively correlated with VTE, although only circulating TF and D-dimers, not circulating PDPN, correlated with VTE risk. Gliomas with mutations in isocitrate dehydrogenase 1 (IDH1) or IDH2 (IDHmut) caused fewer VTEs; multivariable analysis suggested that this is due to IDHmut suppression of TF, not PDPN. In a predictive time-to-event model, the following predicted increased VTE risk in newly diagnosed patients with glioma: (1) history of VTE; (2) hypertension; (3) asthma; (4) white blood cell count; (5) WHO tumor grade; (6) patient age; and (7) body mass index. Conversely, IDHmut, hypothyroidism, and MGMT promoter methylation predicted reduced VTE risk. These 10 variables were used to create a web-based VTE prediction tool that was validated in 2 separate cohorts of patients with adult-type diffuse glioma from other institutions. This study extends our understanding of the VTE landscape in these tumors and provides evidence-based guidance for clinicians to mitigate VTE risk in patients with glioma.
Introduction
Venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE), is one of the most common paraneoplastic conditions among patients with cancer, including patients with gliomas.1 Up to 30% of patients with glioma experience at least 1 VTE during their disease course.2-8 Anticoagulant prophylaxis reduces relative risk of VTE,3,9,10 but it is not known which patients should be treated after surgery, or for how long. This is complicated by possible increased risk of brain hemorrhage during anticoagulation,11-14 although this is controversial.15 Standard protocols for VTE prevention in patients with glioma are therefore needed.15,16
Effective VTE prophylaxis relies on knowing which patients are at highest risk. Models for VTE in other cancers utilize data like patient age, body mass index (BMI), cardiovascular disease, history of VTE, platelet counts, hemoglobin, and white blood cell (WBC) counts.2,6,17-20 The most widely used clinical prediction model for cancer-associated VTE is the Khorana score21; however, that focuses on VTE associated with other cancers, not glioma.
Over 70% of all gliomas are now designated as “adult-type diffuse gliomas” by the World Health Organization (WHO).22 The WHO recognizes 3 adult-type diffuse gliomas: (i) astrocytomas with mutations in the metabolic enzymes isocitrate dehydrogenase 1 (IDH1) or IDH2 (together “IDHmut”); (ii) IDHmut, 1p/19q-codeleted oligodendrogliomas; (iii) IDH wild-type (IDHwt) glioblastomas (GBMs) with TERT promoter mutation, EGFR amplification, and/or gain of chromosome 7 plus loss of chromosome 10.22 Compared with IDHwt GBM, IDHmut astrocytoma and oligodendroglioma have increased DNA hypermethylation.23
We previously showed that IDHmut astrocytomas and oligodendrogliomas have fewer intratumoral microthrombi, and are at lower risk of causing systemic VTE, than IDHwt GBM.24 One of the most hypermethylated and downregulated genes in IDHmut gliomas is F3, encoding tissue factor (TF).25,26 Another consistently hypermethylated and suppressed gene in IDHmut gliomas is PDPN, encoding podoplanin (PDPN).27,28 Both TF and PDPN are often present on cancer cells and microvesicles (MVs) shed by those cells into the circulation, and both TF and PDPN have been associated with increased VTE risk in a variety of cancers, although their roles in glioma-associated VTE are controversial.28-39 Furthermore, no predictive model of VTE risk in patients with adult-type diffuse glioma yet exists.
In the current prospective observational study, we report on a cohort of patients newly diagnosed with adult-type diffuse glioma who were tracked for VTEs during their disease courses. Clinical-, tumor-, and blood-based data from each patient, including TF and PDPN, were analyzed. Clinical, pathologic, and molecular data were used to develop a multivariable predictive model of VTE. This model was then tested in 2 external validation cohorts of patients with adult-type diffuse glioma.
Materials and methods
Patient cohorts
The discovery cohort consisted of 258 patients with newly diagnosed adult-type WHO grade 2 to 4 diffuse gliomas between December 2015 and February 2020, as well as 135 patients with recurrent gliomas at Northwestern Memorial Hospital (NMH; Chicago, IL) between December 2015 and February 2020. Only patients meeting the molecular criteria for IDHmut astrocytoma; IDHmut, 1p/19q-codeleted oligodendroglioma, and IDHwt GBM were included in the study. Astrocytomas were IDHmut gliomas lacking whole-arm 1p/19q codeletion. Oligodendrogliomas were IDHmut gliomas with whole-arm 1p/19q codeletion. Criteria for GBMs included IDHwt infiltrative gliomas with ≥1 of the following features, per 2021 WHO guidelines: (i) necrosis and/or microvascular proliferation; (ii) TERT promoter mutation; (iii) EGFR amplification; (iv) concomitant gain of chromosome 7 and loss of chromosome 10.22 Patients with known coagulation disorders (eg, factor V Leiden mutations), preexisting conditions requiring anticoagulation, and/or infections were excluded. All patients provided written informed consent before study initiation, as per the approved protocol at Northwestern University Feinberg School of Medicine Institutional Review Board, and in accordance with the Declaration of Helsinki.
Within the NMH discovery cohort, immediately before surgery, blood was drawn from an arterial line and banked for analysis of blood-based biomarkers (supplemental Material, available on the Blood website). Neurosurgical tumor tissue was processed for genetic alterations via genomic copy number arrays and either GlioSeq40 or whole exome sequencing (Tempus, Chicago, IL), as per routine clinical workup at NMH. Patients were followed up for at least 6 months following surgery until the occurrence of VTE and/or death.
When a patient developed symptoms of VTE (eg, extremity pain/swelling, chest pain, dyspnea, or tachycardia), a VTE was confirmed or ruled out by duplex ultrasound for DVT and computed tomography pulmonary angiogram for PE, as per standard criteria.41 Both upper and lower extremity DVTs, and all types of PEs, were included, but cerebral vein thrombosis was not. Incidental VTEs (eg, detecting a PE on a computed tomography scan) were also excluded. All judgments on what constituted a VTE were made by research staff blinded to the central hypotheses about potential VTE predictors.
External validation cohorts consisted of 157 cases from Duke University Medical Center between January 2003 and June 2020, and 68 from the Ronald Reagan UCLA Medical Center between February 2010 and January 2021, using the same inclusion/exclusion and VTE criteria as were applied for the NMH discovery cohort. Mean follow-up time, calculated from time of surgical resection for original glioma, was 20.5 months across all cohorts (NMH, 22.4 months; Duke, 18.7 months; UCLA, 17.8 months); end of follow-up for all 3 cohorts was September 2021. Data from each validation cohort were provided by investigators at those respective institutions in a deidentified format and uploaded to a database maintained by biobank staff, according to the Institutional Review Board–approved protocol. Only chart-based data were available from the validation cohort cases, not tissue or blood samples.
Statistical analyses
The primary objective was to identify predictors of VTE in patients with newly diagnosed glioma. Associations of the selected variables that contributed to predictive accuracy were also examined for independent associations with time to VTE. Time to VTE served as the primary end point, defined as time from surgical resection of original glioma to first VTE, calculated in months. Patients without VTE were censored at surgical resection of recurrent glioma or last follow-up. Analyses were performed on the subset of VTE events that occurred after surgical resection of the newly diagnosed glioma, and before surgical resection of recurrent glioma.
To determine an optimal predictive model for time to VTE, we used the Least Absolute Shrinkage and Selection Operator (LASSO) regularization method with the Cox family for variable selection using the discovery cohort. This was done along with fivefold cross-validation to choose the tuning parameter λ. Before LASSO, multiple imputation by chained equations treating missing data as missing at random was performed. Criteria used for removing variables before imputation were >10% missing and <5% in any level for categorical variables. After LASSO, 1 variable, hyperlipidemia, was removed from the final predictive model because it was selected in <2 of 10 imputed data sets. The final selected predictive model included 10 variables: patient age; BMI; WBC count; hypertension; asthma; hypothyroidism; history of VTE, IDHmut; MGMT promoter methylation; WHO glioma grade. Time-dependent receiver operating characteristic curves were used to assess the performance of the model using the CD2 nearest neighbor estimator.42 A Cox proportional hazards model was fit using the discovery cohort with 46 VTE events for the 10 selected variables. The estimated Cox model parameters were then used to obtain the predictor for time to VTE for validation in the Duke and UCLA cohorts.
A multivariable Cox model was used to estimate final model parameters in the discovery data set from NMH for the 10 variables that were selected by LASSO. Results were presented as hazard ratios (HRs) and associated 2-sided 95% confidence intervals (CIs). Unadjusted P values for the Wald test were presented. The Cox model parameters for the 10 selected variables estimated in the NMH data were then evaluated for predictive accuracy in the Duke and UCLA validation data sets.
The R statistical environment (R) version 4.0.2,43 along with packages mice (v 3.13.0), glmnet (v 4.1-2), caret (v 6.0-88), survival (v 3.2-13), and survivalROC (v 1.0.3), were used for statistical analyses. The proportional hazards assumption for all Cox models was checked using Schoenfeld residuals and satisfied.
Please see supplemental Material for blood- and tissue-based analytic methods, as well as the in vivo glioma VTE model.
Results
Patient cohort characteristics
A total of 628 patients with adult-type diffuse gliomas, divided into 3 separate cohorts across 3 institutions (NMH, Duke, and UCLA), composed the data set (supplemental Figure 1). Within that data set, 483 cases were new diagnoses, including 258 from NMH (Table 1). The remaining cases were recurrent gliomas, mostly from NMH (supplemental Figure 1). Among the newly diagnosed cases, most variables, such as patient age, sex, race, ethnicity, BMI, WHO grade, IDHmut, and treatment with temozolomide, were comparable across all 3 cohorts. Some differences included the frequency of MGMT promoter methylation, which was higher in the NMH cohort (53.9%) than in the Duke or UCLA cohorts (22.9% and 38.2%, respectively); that variable was missing at a higher rate in those latter two cohorts. At NMH, 11.2% of newly diagnosed patients were active smokers, compared with 7.6% at Duke and 4.4% at UCLA. History of VTE was also higher within the NMH cohort (8.5%) than in the Duke and UCLA cohorts (2.6% and 1.5%, respectively), and the average length of hospital stay was longer at NMH (5.4 days) relative to Duke (2.7 days) or UCLA (3.6 days).
Among 258 NMH patients newly diagnosed with adult-type diffuse gliomas, 46 (17.8%) eventually developed ≥1 VTEs (supplemental Table 1). The cumulative incidence of VTE was 12% at 6 months, 18% at 12 months, and 20% at 24 months (supplemental Figure 2A). VTEs were reported in 9 of 157 (5.7%) cases at Duke (supplemental Table 2; supplemental Figure 2B) and in 9 of 68 (13.2%) cases at UCLA (supplemental Table 3; supplemental Figure 2C).
TF and VTE risk in newly diagnosed adult-type diffuse gliomas
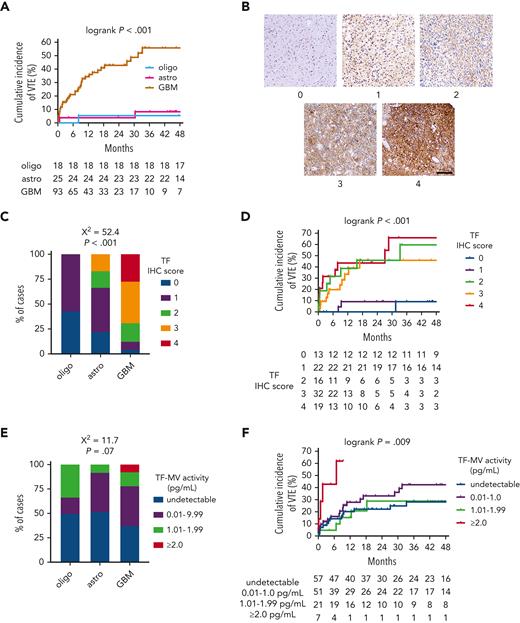
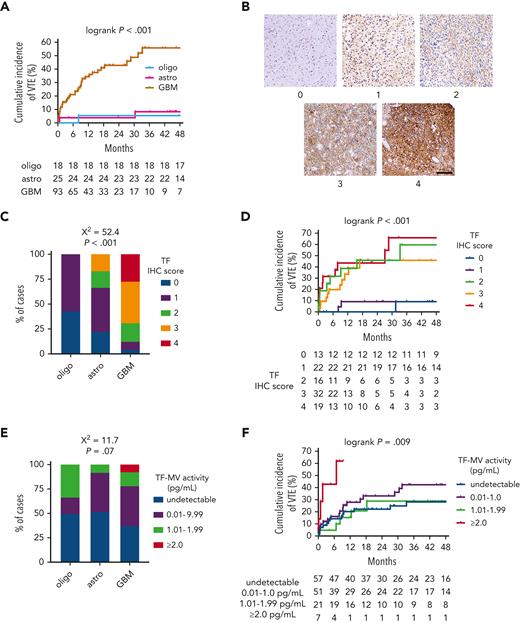
In the NMH discovery cohort, patients with IDHwt GBM had a substantially higher rate of cumulative VTE incidence (55.6%) than patients with either IDHmut astrocytomas (8.4%) or IDHmut, 1p/19q-codeleted oligodendrogliomas (5.6%; log-rank P < .001; Figure 1A; supplemental Table 1). This association was also observed in both validation cohorts, wherein 15 of 158 (9.5%) IDHwt GBM cases from both cohorts developed VTE, but none of the 47 IDHmut astrocytomas and oligodendrogliomas did (P = .025 by Fisher exact test; supplemental Tables 2 and3).
TF expression and circulating TF-MV activity in NMH newly diagnosed adult-type diffuse glioma cases. (A) Cumulative VTE incidence according to glioma genotype. (B) Representative photomicrographs of TF IHC, on a semiquantitative scale from 0 (negative) to 4 (strongest). Scale bar = 50 μm. (C) TF IHC scores across the 3 subsets of adult-type diffuse gliomas. (D) Cumulative VTE incidence according to glioma TF IHC score. (E) Circulating TF-MV activity, at the time of initial surgery, across the 3 subsets of patients with adult-type diffuse glioma. (F) Cumulative VTE incidence according to circulating TF-MV activity at the time of initial surgery. Astro, IDHmut grade 2 to 4 astrocytoma; GBM, IDHwt glioblastoma; oligo, IDHmut, 1p/19q-codeleted WHO grade 2 to 3 oligodendroglioma.
TF expression and circulating TF-MV activity in NMH newly diagnosed adult-type diffuse glioma cases. (A) Cumulative VTE incidence according to glioma genotype. (B) Representative photomicrographs of TF IHC, on a semiquantitative scale from 0 (negative) to 4 (strongest). Scale bar = 50 μm. (C) TF IHC scores across the 3 subsets of adult-type diffuse gliomas. (D) Cumulative VTE incidence according to glioma TF IHC score. (E) Circulating TF-MV activity, at the time of initial surgery, across the 3 subsets of patients with adult-type diffuse glioma. (F) Cumulative VTE incidence according to circulating TF-MV activity at the time of initial surgery. Astro, IDHmut grade 2 to 4 astrocytoma; GBM, IDHwt glioblastoma; oligo, IDHmut, 1p/19q-codeleted WHO grade 2 to 3 oligodendroglioma.
Within the NMH discovery cohort, TF expression was lower in IDHmut astrocytomas and IDHmut, 1p/19q-codeleted oligodendrogliomas compared with IDHwt GBM (χ2 = 52.4; P < .001; Figure 1B-C). TF expression also increased with WHO grade (χ2 = 52.5; P < .001; supplemental Figure 3A). Although cumulative incidence of VTE was lower in gliomas that were negative or weak for TF (9.1% for immunohistochemistry [IHC] score 0-1 vs 45.9%-66.1% for IHC score 2-4; log-rank P < .001; Figure 1D), there was no significant overall association between glioma TF IHC score and circulating TF-MV activity, even though the highest levels of TF-MV were only observed in gliomas with TF IHC scores of 3 or 4 (χ2 = 8.2; P = .76; supplemental Figure 3B). There was a trend toward higher circulating TF-MV activity in patients with IDHwt GBM, driven by the finding that the highest TF-MV activity—≥2.0 pg/mL—was only found in patients with IDHwt GBM (χ2 = 11.7; P = .07; Figure 1E). That level of TF-MV activity also had both the fastest time to VTE and highest cumulative incidence of VTE at 61.9%, compared with 28.9% to 42.3% for the other subsets (log-rank P = .009; Figure 1F).
PDPN and VTE risk in newly diagnosed adult-type diffuse gliomas
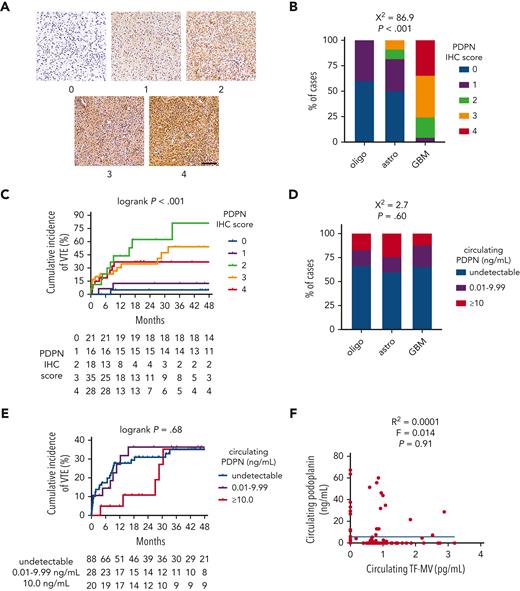
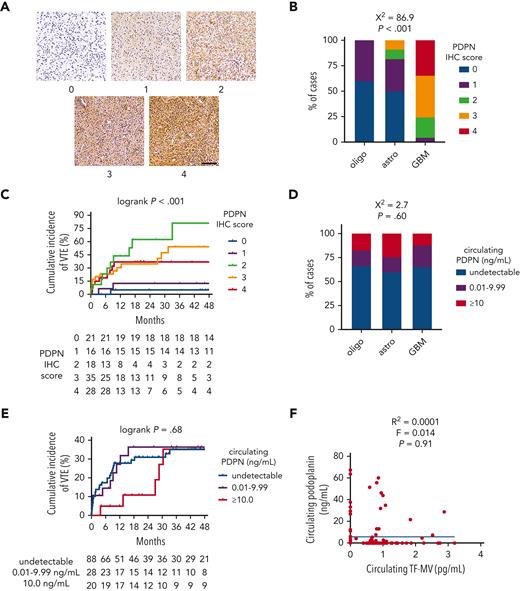
IDHwt GBMs from the NMH cohort showed significantly higher PDPN expression by IHC than IDHmut astrocytomas or IDHmut, 1p/19q-codeleted oligodendrogliomas (χ2 = 86.9; P < .001; Figure 2A-B). PDPN expression also increased with WHO grade (χ2 = 77.5; P < .001; supplemental Figure 3C). Although moderate-to-strong PDPN expression within gliomas was associated with higher cumulative incidence of VTE (36.9%-81.3% for IHC scores 2-4 vs 5.0%-12.5% for scores 0-1; log-rank P < .001; Figure 2C), there was no association between circulating PDPN and glioma molecular subtype (χ2 = 2.7; P = .60; Figure 2D), cumulative VTE incidence (log-rank P = .68; Figure 2E), glioma expression of PDPN (χ2 = 5.5; P = .70; supplemental Figure 3D), circulating TF-MV activity (R2 = 0.0001; F = 0.014; P = .91; Figure 2F), or circulating platelet counts (supplemental Figure 3E). Likewise, glioma PDPN expression showed no correlation with circulating platelet counts (supplemental Figure 3F).
PDPN expression and circulating PDPN in NMH newly diagnosed adult-type diffuse glioma cases. (A) Representative photomicrographs of PDPN IHC, on a semiquantitative scale from 0 (negative) to 4 (strongest). Scale bar = 50 μm. (B) PDPN IHC scores across the 3 subsets of adult-type diffuse gliomas. (C) Cumulative VTE incidence according to glioma PDPN IHC score. (D) Circulating PDPN, at the time of initial surgery, across the 3 subsets of patients with adult-type diffuse glioma. (E) Cumulative VTE incidence according to circulating PDPN at the time of initial surgery. (F) Linear regression plot of circulating TF-MV activity vs circulating PDPN at the time of initial surgery. Astro, IDHmut grade 2 to 4 astrocytoma; GBM, IDHwt glioblastoma; oligo, IDHmut, 1p/19q-codeleted WHO grade 2 to 3 oligodendroglioma.
PDPN expression and circulating PDPN in NMH newly diagnosed adult-type diffuse glioma cases. (A) Representative photomicrographs of PDPN IHC, on a semiquantitative scale from 0 (negative) to 4 (strongest). Scale bar = 50 μm. (B) PDPN IHC scores across the 3 subsets of adult-type diffuse gliomas. (C) Cumulative VTE incidence according to glioma PDPN IHC score. (D) Circulating PDPN, at the time of initial surgery, across the 3 subsets of patients with adult-type diffuse glioma. (E) Cumulative VTE incidence according to circulating PDPN at the time of initial surgery. (F) Linear regression plot of circulating TF-MV activity vs circulating PDPN at the time of initial surgery. Astro, IDHmut grade 2 to 4 astrocytoma; GBM, IDHwt glioblastoma; oligo, IDHmut, 1p/19q-codeleted WHO grade 2 to 3 oligodendroglioma.
Other blood-based biomarkers of VTE in newly diagnosed adult-type diffuse gliomas
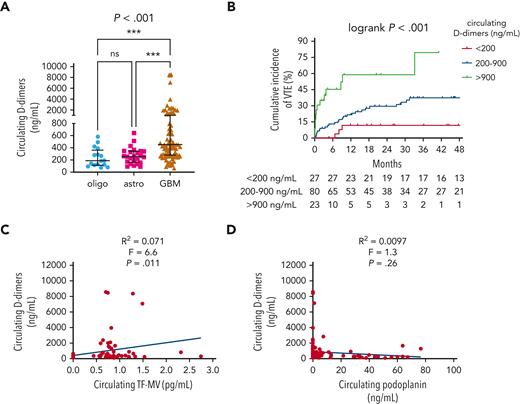
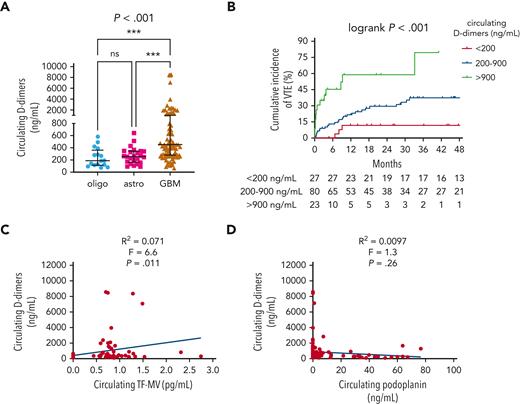
Median circulating D-dimers were higher in patients with IDHwt GBM (454.1 ng/mL) than in patients with IDHmut astrocytomas (256.9 ng/mL) or IDHmut, 1p/19q-codeleted oligodendrogliomas (188.9 ng/mL) (P < .001; Figure 3A). Higher D-dimers also positively correlated with higher cumulative VTE incidence (79.5% for >900 ng/mL, 37.2% for 200-900 ng/mL, and 11.5% for <200 ng/mL; log-rank P < .001; Figure 3B) and higher circulating TF-MV activity (R2 = 0.071; F = 6.6; P = .011; Figure 3C), but not with circulating PDPN (R2 = 0.0097; F = 1.3; P = .26; Figure 3D). In the univariable Cox proportional hazards model, the following log2 transformed blood-based markers were associated with VTE risk: D-dimers (HR, 1.61; 95% CI, 1.29-2.02; P = 3.27e-5); vascular cell adhesion molecule 1 (VCAM-1) (HR, 2.45; 95% CI, 1.2-5.01; P = .014); interleukin 10 (IL-10) (HR, 1.3; 95% CI, 1.05-1.62; P = .018); granzyme B (HR, 1.22; 95% CI, 1.01-1.47; P = .037) (supplemental Table 4; supplemental Figure 4). In the multivariable model adjusting for circulating D-dimer, VCAM-1, and IL-10 as additive effects, circulating D-dimer remained significant, whereas VCAM-1 and IL-10 did not (supplemental Table 4; supplemental Figure 4).
Circulating D-dimers in NMH patients with newly diagnosed adult-type diffuse glioma. (A) Circulating D-dimers according to glioma molecular subtype. P value was calculated by Kruskal-Wallis test, with Dunn multiple comparisons test, ∗∗∗P<.001. (B) Cumulative VTE incidence according to circulating D-dimers at the time of initial surgery. (C) Linear regression plot of circulating TF-MV activity vs circulating D-dimers. (D) Linear regression plot of circulating PDPN vs circulating D-dimers. Astro, IDHmut grade 2 to 4 astrocytoma; GBM, IDHwt glioblastoma; oligo, IDHmut, 1p/19q-codeleted WHO grade 2 to 3 oligodendroglioma.
Circulating D-dimers in NMH patients with newly diagnosed adult-type diffuse glioma. (A) Circulating D-dimers according to glioma molecular subtype. P value was calculated by Kruskal-Wallis test, with Dunn multiple comparisons test, ∗∗∗P<.001. (B) Cumulative VTE incidence according to circulating D-dimers at the time of initial surgery. (C) Linear regression plot of circulating TF-MV activity vs circulating D-dimers. (D) Linear regression plot of circulating PDPN vs circulating D-dimers. Astro, IDHmut grade 2 to 4 astrocytoma; GBM, IDHwt glioblastoma; oligo, IDHmut, 1p/19q-codeleted WHO grade 2 to 3 oligodendroglioma.
VTEs in recurrent adult-type diffuse gliomas
Please see supplemental Material, including supplemental Table 5 and supplemental Figures 5 to 8 with associated text, for information on VTEs in recurrent adult-type diffuse gliomas. The main findings are as follows: (i) intratumoral expression of TF tended to increase in recurrent gliomas compared to original tumors, whereas PDPN was unchanged (supplemental Figure 5); (ii) higher circulating TF-MV activity and D-dimers, but not PDPN, were associated with increased VTE incidence (supplemental Figures 6-8).
In vivo model of IDHwt vs IDHmut glioma-associated thrombosis
To directly compare the thrombogenic activity of IDHwt vs IDHmut glioma in an experimental setting, we used the inferior vena cava (IVC) stenosis model (supplemental Material).44 Athymic nude mice were subcutaneously engrafted with either high TF-expressing, IDH1wt, EGFR-amplified GBM12 or low TF-expressing, IDH1mut GBM164.25 Once flank tumors reached 0.5 mL in volume, the IVC in each engrafted mouse was ligated, followed by examination 24 hours later for IVC thrombus development (supplemental Figure 9A-D). Mice bearing IDH1wt flank patient-derived xenografts (PDX) developed larger IVC thrombi in response to the stenosis than those with IDH1mut PDX (10.3 vs 0.86 mg; P = .037; supplemental Figure 9E).
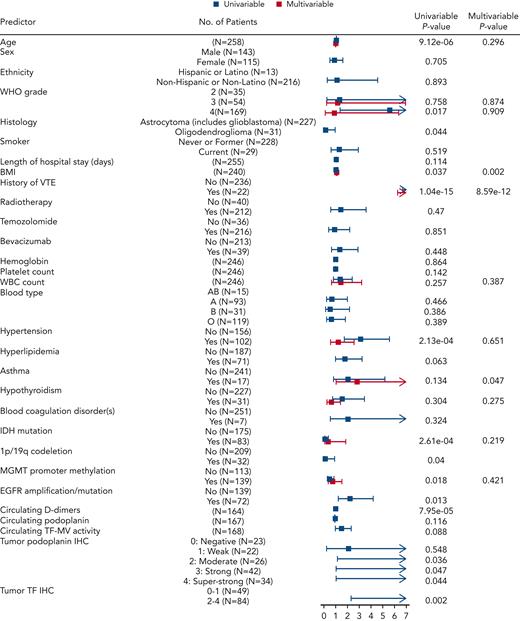
Univariable and multivariable VTE risk analyses in newly diagnosed adult-type diffuse gliomas
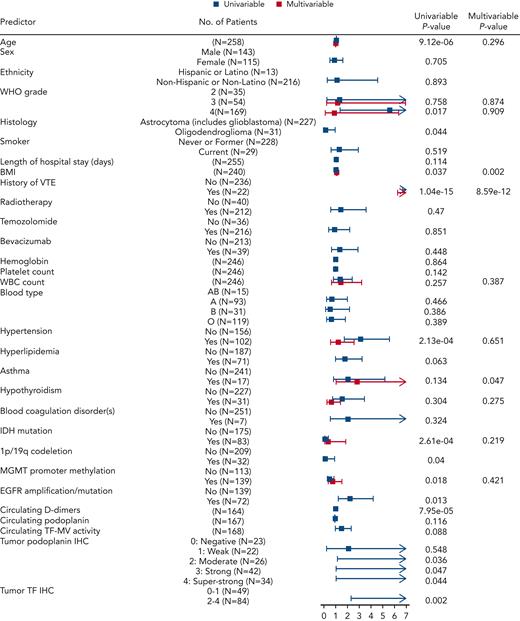
The discovery cohort of 258 patients with newly diagnosed glioma included 46 with VTE events and 212 censored patients. For an initial assessment of associations of candidate predictors, we performed univariable Cox proportional hazards models for baseline predictors and reported HRs along with corresponding 95% CIs to assess VTE risk (Figure 4; supplemental Table 6). The patient characteristics associated with significantly higher risk of VTE included age (HR, 1.05 for age higher by 1 year; 95% CI, 1.03-1.07; P = 9.12e-06), WHO tumor grade 4 vs 2 (HR, 5.62; 95% CI, 1.35-23.32; P = .017), BMI (HR, 1.05 for BMI higher by 1 kg/m2; 95% CI, 1-1.11; P = .037), history of VTE (HR, 12.24; 95% CI, 6.64-22.57; P = 1.04e-15), hypertension (HR, 3.09; 95% CI, 1.7-5.6; P = 2.13e-04), EGFR amplification and/or mutation (HR, 2.23; 95% CI, 1.19-4.2; P = .013), increased PDPN score (P = .04), and TF IHC 2 to 4 compared with 0 to 1 (HR, 9.58; 95% CI, 2.26-40.53; P = .002) (supplemental Table 6). Conversely, variables associated with lower risk of VTE included IDHmut (HR, 0.15; 95% CI, 0.05-0.41; P = 2.61e-04), 1p/19q codeletion (HR, 0.13; 95% CI, 0.02-0.91; P = .04), MGMT promoter methylation (HR, 0.49; 95% CI, 0.27-0.88; P = .018), and oligodendroglioma histology (HR, 0.13; 95% CI, 0.02-0.95; P = .044) (supplemental Table 6). Although age, WBC count, hypertension, hypothyroidism, IDHmut, MGMT promoter methylation, and glioma grade did not demonstrate individual statistical significance when considered jointly in the multivariable Cox model for time to VTE, they were contributors to predictive accuracy as determined by LASSO. BMI (HR, 1.09; 95% CI, 1.03-1.15; P = .002) and history of VTE (HR, 13.04; 95% CI, 6.24-27.26; P = 8.59e-12) were significantly independently associated with time to VTE in the multivariable model. In addition, patients with asthma had almost 3 times higher risk of VTE compared with patients without asthma, keeping all other variables constant (HR, 2.82; 95% CI, 1.02-7.81; P = .047) (supplemental Table 6).
Predictors of interest and time to VTE in the NMH cohort of newly diagnosed adult-type diffuse glioma cases. Forest plot showing univariable and multivariable Cox models with resulting hazard ratio effect estimates and corresponding P values for each predictor. EGFR, epidermal growth factor receptor; MGMT, O-6-methylguanine–DNA methyltransferase.
Predictors of interest and time to VTE in the NMH cohort of newly diagnosed adult-type diffuse glioma cases. Forest plot showing univariable and multivariable Cox models with resulting hazard ratio effect estimates and corresponding P values for each predictor. EGFR, epidermal growth factor receptor; MGMT, O-6-methylguanine–DNA methyltransferase.
Across 483 patients at 3 institutions (NMH, Duke, and UCLA), there were 64 VTE events, including 46 at NMH, 9 at Duke, and 9 at UCLA. In pooled analyses across all 3 institutions, characteristics associated with significantly higher risk of VTE remained consistent with what was found in the NMH cohort alone, with the addition of duration of hospital stay (HR, 1.06; 95% CI, 1.01-1.11; P = .03). IDHmut, 1p/19q codeletion, and oligodendroglioma histology continued to lower the risk of VTE, whereas MGMT promoter methylation was no longer significantly associated with lower VTE risk (HR, 0.71; 95% CI, 0.42-1.19; P = .19). In a multivariable model including all predictors selected by LASSO, the results were consistent with LASSO, except for asthma, which was no longer significant (HR, 2.01; 95% CI, 0.75-5.36; P = .162) (Table 2).
In a subset of newly diagnosed NMH patients (N=165, 32 VTE events), next-generation sequencing–based molecular alterations that composed >5% of the cohort were evaluated and analyzed alongside tumor TF and PDPN expression (supplemental Table 7; supplemental Figure 10). (Before analysis, alterations involving NRAS, JAK2, BRAF, CDK6, MYCN, and NOTCH were removed because of <5 VTE events for each.) In the univariable Cox model for time to VTE, the following markers were associated with increased VTE risk: RB1 (HR, 3.28; 95% CI, 1.22-8.78; P = .018); EGFR (HR, 2.4; 95% CI, 1.11-5.2; P = .027); tumor TF IHC of 2 to 4 vs 0 to 1 (HR, 17.34; 95% CI, 2.34-128.44; P = .005); tumor PDPN 2 vs 0 (HR, 8.31; 95% CI, 1.02-67.63; P = .048); tumor PDPN IHC of 4 vs 0 (HR, 8.52; 95% CI, 1.08-67.48; P = .042) (supplemental Table 7; supplemental Figure 10). Conversely, ATRX (HR, 0.23; 95% CI, 0.06-0.98; P = .048), MGMT promoter methylation (HR, 0.47; 95% CI, 0.23-0.96; P = .039), and IDHmut (HR, 0.1; 95% CI, 0.02-0.43; P = .002) were associated with lower risk of VTE.
Given our data showing that TF and PDPN expression are lower within IDHmut gliomas (Figures 1C and 2B), we investigated the relationship between IDHmut, tumor TF IHC, and tumor PDPN via multivariable Cox model with these 3 variables as additive effects. In that model, IDHmut and tumor PDPN were no longer associated with time to VTE, whereas the association with tumor TF IHC persisted (HR, 10.71; 95% CI, 1.21-94.95; P = .033) (supplemental Table 7; supplemental Figure 10).
Glioma risk assessment modeling and prediction
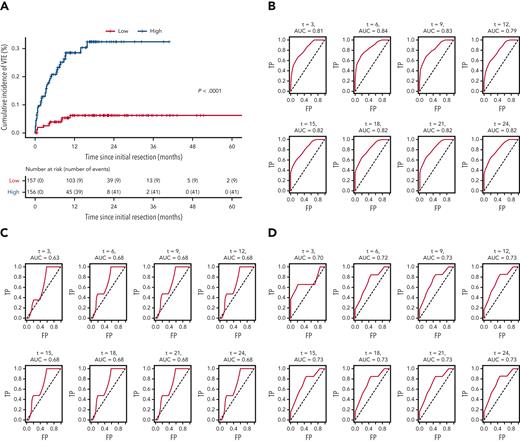
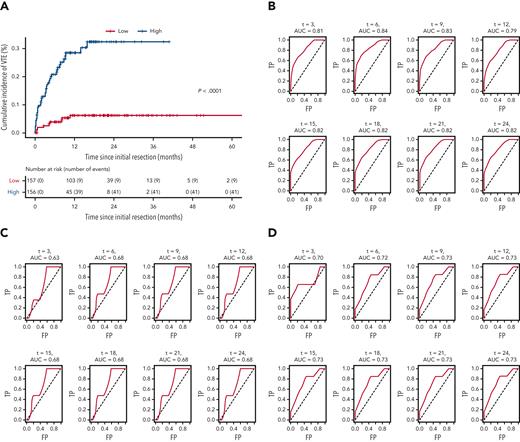
LASSO regression analysis (see “Statistical analyses” subsection within the “Materials and methods”) identified 10 variables as influential in predicting VTE risk in patients with newly diagnosed adult-type diffuse gliomas. Mean β values indicated relative strength and directionality of each variable to the final predictive model, where positive β values indicated higher risk, and negative β values indicated lower risk: history of VTE (1.992); hypertension (0.252); asthma (0.082); increasing WBC count (0.020); increasing WHO grade (0.013); increasing patient age (0.008); increasing BMI (0.005); MGMT promoter methylation (−0.026); hypothyroidism (−0.033); IDHmut (−0.461) (supplemental Table 6). Cox prediction scores showed strong risk stratification across all 3 cohorts pooled together (P < .0001; Figure 5A). Estimated areas under the curves ranged between 0.79 and 0.84 for the NMH discovery cohort, 0.63 and 0.68 for the Duke validation cohort, and 0.70 and 0.73 for the UCLA validation cohort, presented for 3 to 24 months (Figure 5B-D).
Receiver operating characteristic curves for the LASSO time-to-VTE prediction model across all 3 cohorts. (A) Kaplan-Meier curves of all 3 cohorts pooled together, stratified by low vs high risk, as defined by the prediction model. (B) NMH discovery cohort. (C) Duke validation cohort. (D) UCLA validation cohort. AUC, area under the curve; FP, false positive; TP, true positive.
Receiver operating characteristic curves for the LASSO time-to-VTE prediction model across all 3 cohorts. (A) Kaplan-Meier curves of all 3 cohorts pooled together, stratified by low vs high risk, as defined by the prediction model. (B) NMH discovery cohort. (C) Duke validation cohort. (D) UCLA validation cohort. AUC, area under the curve; FP, false positive; TP, true positive.
The 10-variable model was used to generate a web-based application that predicts probability of VTE event in patients with newly diagnosed adult-type diffuse gliomas (https://kbellburdett.shinyapps.io/GliomaPredictVTE/).
Discussion
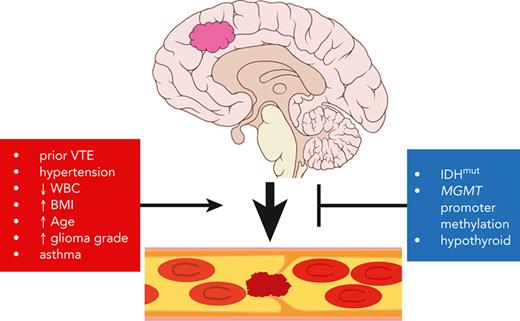
Any cancer can trigger a VTE, but the ones most likely to do so are pancreatic, uterine, pulmonary, gastric, renal, and glial.45 Although molecular characterization of cancers has proceeded at an extraordinary rate over the past decade, with evidence of genotype modulating VTE risk among several cancers, none of those markers has yet been incorporated into VTE prediction tools. Herein, we studied VTE risk in adult-type diffuse gliomas, stratified according to the most recent molecular-based guidelines.22 That led to the integration of 2 key (and frequently tested) alterations, IDHmut and MGMT promoter methylation, into a new multivariable VTE prediction tool for adult-type diffuse gliomas that was validated in 2 separate, independent cohorts.
Our 2016 study was the first to show that IDHmut gliomas produced fewer intratumoral microthrombi compared with IDHwt gliomas, and that patients with IDHmut glioma were at reduced VTE risk compared with patients with IDHwt glioma.26 Since then, some have not confirmed that association,46-49 whereas others have.50,51 The 3 cohorts from our current study are in alignment with our prior study of 2 unrelated cohorts,26 representing 5 institutions in total, all of which showed a markedly reduced risk of VTE in patients with IDHmut glioma. In our prior and current studies, only tumors that were adult-type diffuse gliomas proven by genetic profiling were included, using strict molecular criteria that are now part of the newest WHO classification system.22 By definition, neither circumscribed astrocytic gliomas nor benign mimickers, like ganglioglioma, are IDHmut, yet they typically do not cause VTE. Thus, inclusion of such cases could potentially skew studies of VTE in patients with glioma.
Although IDHmut is associated with reduced VTE risk in patients with glioma, the reasons why remain a subject of investigation. The 2 proteins most heavily studied within the context of cancer-induced VTE are TF and PDPN, and both are strongly hypermethylated and suppressed in IDHmut gliomas.25,27,29,31 Glioma-derived PDPN has been shown to promote intratumoral platelet aggregation and thrombosis,28 which would help explain why IDHwt GBMs contain far more microthrombi compared with IDHmut astrocytomas and oligodendrogliomas.26 However, we found no significant association between circulating PDPN and glioma subtype (including IDHmut status or WHO grade), or between circulating PDPN and VTE risk. Multivariable analysis showed that tumor TF expression was a stronger correlate with time to VTE than either PDPN or even IDHmut. Recent work by others showed that, although glioma-derived PDPN-MVs caused platelet activation in vivo, glioma-derived TF-MVs were responsible for actually triggering the clotting cascade.31 Thus, the inverse connection between IDHmut and VTE may be due more to IDHmut suppressing TF than PDPN.
Circulating TF-MV has consistently been linked to increased VTE risk in other malignancies,52 although studies in gliomas have generated conflicting results.38,53,54 Our data suggest that it is associated with increased VTE risk, but only when activity is ≥2.0 pg/mL, the same cutoff previously determined by Hisada et al55 as conferring the highest VTE risk in other cancers.
We found that high TF-expressing IDH1wt GBM12 PDX facilitated more and larger thrombi than low TF-expressing IDH1mut GBM164 PDX. In our previously published tail vein bleeding assay, IDH1wt glioma-engrafted mice stopped bleeding within 90 seconds, whereas those engrafted with IDH1mut gliomas exsanguinated (supplemental Figure 10 of Unruh et al, 2016).26 Thus, in addition to suppressing TF expression, IDHmut may also inhibit hemostasis via other unknown mechanisms. Others have reported that patients with IDHmut glioma may be more likely to experience hemorrhage as an adverse effect of anticoagulation,56 and mice engineered to express IDHmut in the brain die of perinatal intracerebral hemorrhage.57 Antithrombotic prophylaxis may therefore not only be unnecessary in most patients with IDHmut glioma, but IDHmut might even be a relative contraindication, although prospective studies would be needed to confirm that.
Besides IDHmut, another molecular variable associated with VTE in time to event LASSO modeling was MGMT promoter methylation, although 2 other studies did not show a connection between MGMT promoter methylation and VTE risk.50,58 To the extent that MGMT promoter methylation predicts response to temozolomide—the most widely used chemotherapy in adult-type diffuse gliomas59—such an association between MGMT promoter methylation and lower VTE risk is plausible.
As concerns study limitations, time to VTE was censored at surgical resection of recurrent glioma or last follow-up, which included death in many cases. Validating the model in a competing risk framework is an important future objective. Second, only a single preoperative arterial blood sample was available per patient. It is entirely possible that TF-MV activity fluctuates substantially throughout the course of disease, and that later surges in TF-MV might also portend imminent VTEs. Third, circulating TF and PDPN assays are sensitive, and results can be highly variable between laboratories. Fourth is the variability in VTE rates among the 3 cohorts, including comparatively few VTE cases in the validation cohorts. Our discovery NMH cohort had an overall VTE incidence of 17.8%. This was higher than the VTE incidences from the Duke (5.7%) and UCLA (13.2%) cohorts, but such heterogeneity is common in the glioma literature, with reported VTEs ranging from 3% to 30%.2-8 Still, our prediction model for VTE risk in patients with adult-type diffuse glioma worked in both the discovery and validation cohorts, consistently generating areas under the curves in the 0.7 to 0.8 range.
In sum, we performed a comprehensive analysis of VTE in patients with molecularly defined adult-type diffuse glioma. We developed a new web-based tool to predict VTE risk in these patients (https://kbellburdett.shinyapps.io/GliomaPredictVTE/), focusing on patient- and tumor-based variables that are routinely available among all adult diffuse-type glioma cases. This study provides more insights as to the drivers of VTE risk in patients with adult-type diffuse glioma, with the goal of improving VTE prevention.
Acknowledgments
The authors thank Qazi F. Haider, Yuping D. Li, and Anh N. Tran for assistance with data procurement and organization.
D.U. was supported by the National Institutes of Health (NIH) National Cancer Institute (NCI) grant F32CA216996. C.H. was supported by NIH National Institute of Neurological Disorders and Stroke grants R01NS102669, R01NS117104, and R01NS118039; the Northwestern University SPORE in Brain Cancer grant P50CA221747; and the Lou and Jean Malnati Brain Tumor Institute. Histology and microscopy services were provided by the Mouse Histology and Phenotyping Laboratory, supported by NCI grant P30CA060553, awarded to the Robert H. Lurie Comprehensive Cancer Center.
Authorship
Contribution: C.H. conceptualized the project, designed the study, scored all immunostains, and wrote and edited the manuscript; K.B.B. and D.U. performed statistical and blood-based analyses, respectively, and drafted corresponding parts of the manuscript; A.S. performed all immunohistochemistry; M.D., J.L., J.J., M.S., R.J., C.A., E.S.L., B.S.C.E, and K.M. provided deidentified patient data; K.B.P., A.L., A.B.H., and D.M.S. edited the manuscript; and all authors reviewed the draft and approved the final version for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Craig Horbinski, Northwestern University, SQ 6-518, 303 East Superior St, Chicago, IL 60611; e-mail: craig.horbinski@northwestern.edu.
References
Author notes
∗K.B.B. and D.U. are joint first authors.
For original data, contact craig.horbinski@northwestern.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.