Key Points
Targeted ablation of OX40+ T cells with engineered T cells suppresses alloreactivity and preserves antiviral immunity.
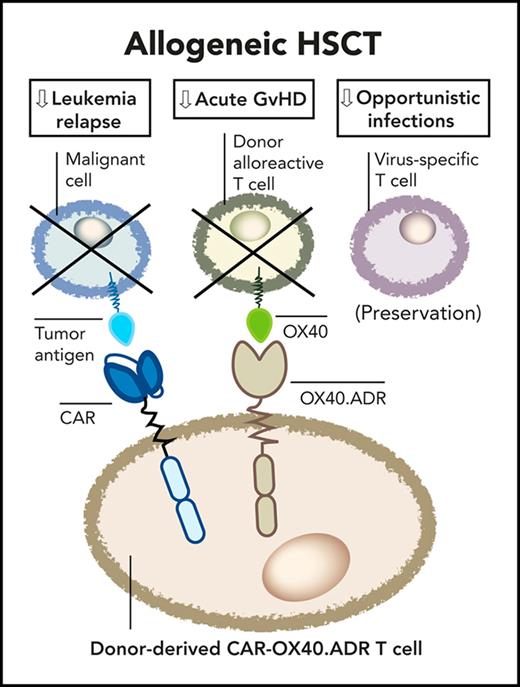
OX40-targeting T-cells co-expressing a tumor-specific chimeric antigen receptor protect animals from both acute GvHD and leukemia relapse.
Abstract
Acute graft-versus-host disease (aGVHD) limits the therapeutic benefit of allogeneic hematopoietic stem cell transplantation (allo-HSCT) and requires immunosuppressive prophylaxis that compromises antitumor and antipathogen immunity. OX40 is a costimulatory receptor upregulated on circulating T cells in aGVHD and plays a central role in driving the expansion of alloreactive T cells. Here, we show that OX40 is also upregulated on T cells infiltrating GVHD target organs in a rhesus macaque model, supporting the hypothesis that targeted ablation of OX40+ T cells will mitigate GVHD pathogenesis. We thus created an OX40-specific cytotoxic receptor that, when expressed on human T cells, enables selective elimination of OX40+ T cells. Because OX40 is primarily upregulated on CD4+ T cells upon activation, engineered OX40-specific T cells mediated potent cytotoxicity against activated CD4+ T cells and suppressed alloreactive T-cell expansion in a mixed lymphocyte reaction model. OX40 targeting did not inhibit antiviral activity of memory T cells specific to Epstein-Barr virus, cytomegalovirus, and adenoviral antigens. Systemic administration of OX40-targeting T cells fully protected mice from fatal xenogeneic GVHD mediated by human peripheral blood mononuclear cells. Furthermore, combining OX40 targeting with a leukemia-specific chimeric antigen receptor in a single T cell product provides simultaneous protection against leukemia and aGVHD in a mouse xenograft model of residual disease posttransplant. These results underscore the central role of OX40+ T cells in mediating aGVHD pathogenesis and support the feasibility of a bifunctional engineered T-cell product derived from the stem cell donor to suppress both disease relapse and aGVHD following allo-HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for several aggressive hematologic malignancies. However, the therapeutic benefit of allo-HSCT in cancer patients is partially offset by transplant-related mortality due to graft-versus-host disease (GVHD), leukemia relapse, and opportunistic infections.1-6 Acute GVHD (aGVHD) is mediated by alloreactive donor T cells that arise from the graft and produce systemic damage that can be fatal for the recipient. Standard GVHD prophylaxis and treatment rely on general immunosuppressants such as corticosteroids, methotrexate, inhibitors of calcineurin and mTOR, and other agents.2,5,7-13 The nonspecific immune suppression of these drugs further increases the risk of posttransplant infections and relapse due to weakened immunity,14-18 and current treatments do not fully prevent aGVHD in a subset of patients.8,12,19 Depleting T cells from the graft prior to transplant decreased the incidence of GVHD but significantly elevated the risk of leukemia relapse and infectious complications.20,21 Likewise, recent clinical evidence suggested that prophylactic use of posttransplant cyclophosphamide, an immunosuppressive agent targeting proliferating alloreactive T cells,22,23 reduced GVHD incidence and improved disease-free survival,24-26 but it also may increase the risk of viral infections early posttransplant.27-29 Despite these preventative methods, established steroid-resistant GVHD requires complex management and has a poor prognosis, significantly contributing to posttransplant mortality.16,19,30-32 Therefore, therapeutic strategies that selectively inhibit aGVHD without decreasing antitumor and antipathogen immunity are necessary to improve outcomes in patients post allo-HSCT.
The pathogenesis of aGVHD is complex and involves both helper and cytotoxic T-cell subsets that functionally interact.7-9 Donor-derived CD4+ T cells play a central role in initiating and propagating GVHD and supporting subsequent expansion of host-reactive CD8+ T cells.33-36 A high frequency of minimally differentiated CD4+ T cells in the graft has been associated with increased incidence of aGVHD following allo-HSCT,37,38 and approaches aimed at selective inhibition of CD4+ T cells have protected against aGVHD.39 One such approach is to block signaling from OX40, a costimulatory receptor upregulated primarily on CD4+ and a small subset of CD8+ human T cells, eliciting a costimulatory signal upon binding to OX40L on antigen-presenting cells.40,41 OX40 plays a pivotal role in driving expansion of host-reactive T cells.41 OX40 blockade or removal of OX40+ allo-stimulated donor T cells from the graft pretransplant controlled expansion of host-reactive T cells and can preserve protective T-cell immunity against tumor and viral antigens.42-44 Functional ablation of OX40+ T cells is thus emerging as an attractive strategy to mitigate aGVHD without globally suppressing productive T-cell immunity. However, therapeutic antibodies may not produce durable benefits, as other costimulatory signals may compensate for OX40 blockade and host-reactive T cells may emerge posttransplant due to the temporarily dysregulated mechanisms of central and peripheral tolerance.10
We hypothesized that selective elimination of OX40+ donor T cells after allo-HSCT could functionally ablate these cells to prevent or suppress aGVHD. Here, we engineered human donor T cells to recognize and eliminate OX40+ T cells and demonstrated their protective function in a xenogeneic model of aGVHD. Furthermore, we engineered the OX40-directed T cells to coexpress a tumor-specific chimeric antigen receptor (CAR), creating therapeutic T cells that protect from both aGVHD and leukemia relapse in vivo. These results highlight the importance of OX40+ T cells in driving GVHD pathogenesis and support the feasibility of manufacturing a donor-derived engineered T-cell product that protects against 2 major causes of failure after allo-HSCT.
Methods
Constructs
The OX40-specific alloimmune defense receptor (OX40.ADR) contains ligand-binding fragment of human OX40L (polymerase chain reaction–amplified from a human complementary DNA library) fused with an immunoglobulin G Fc spacer, CD28 transmembrane region, 4-1BB, and CD3ζ intracellular domains using InFusion cloning (Takara Bio). A first-generation construct (OX40.ADR-1G) in supplemental Figure 7, available on the Blood website, had the same structural design except for the 4-1BB costimulatory domain and an mEmerald tag linked at the end of CD3ζ. The final construct was verified by Sanger sequencing. A second-generation CD19.CAR, consisting of a CD19-specific scFv (FMC63), immunoglobulin G4 hinge, CD28 transmembrane domain, a 4-1BB costimulatory domain, and a CD3ζ chain, was previously developed and evaluated in the laboratory.45 The CD19.CAR construct also contains IRES-tNGFR (NGFR without the intracellular signaling domain) sequence downstream of CD19.CAR. For the xenogeneic GVHD mouse model, we used a control vector encoding the mEmerald fluorescent protein to distinguish Ctrl T cells from donor-matched peripheral blood mononuclear cells (PBMCs) for flow cytometric analyses.
PBMC isolation and T-cell transduction
To obtain activated T cells, PBMCs were isolated from healthy donors using Lymphoprep (Axis-Shield PoC AS) or Ficoll-Paque PLUS (GE Healthcare) after informed consent on protocols approved by the Institutional Review Board at the Baylor College of Medicine (H-15152, H-45017) or Boston Children’s Hospital (IRB-P00032515) and conducted in accordance with the Declaration of Helsinki, Belmont Report, and US Common Rule. In some experiments, deidentified buffy coats were purchased from Gulf Coast Regional Blood Center and used as a source for human PBMCs. T-cell activation and transduction by gammaretroviral vectors have been previously described.46 See detailed procedures in supplemental “Methods.”
Mouse strains and study approval
Breeder pairs of NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (NSG, stock no. 005557) were purchased from The Jackson Laboratory and bred in the Baylor College of Medicine animal facility. Both female and male littermates (aged 6 to 12 weeks) were used for experiments. All animal experiments were conducted in compliance with the Baylor College of Medicine Institutional Animal Care and Use Committee (Protocol #AN-4758).
Mouse xenogeneic GVHD model
NSG mice were irradiated at 1.2 Gy and on the following day received 5 × 106 freshly isolated human PBMCs. Four days later, mice received 5 × 106 control mEmerald-expressing or ADR T cells from the same PBMC donor intravenously. At specified time points, 50 μL of peripheral blood was obtained by tail-vein bleeding. After red blood cell lysis, samples were sequentially stained with antihuman Fcγ (for ADR detection) antibody and washed, followed by antihuman CD45, CD3, CD8, and CD4 antibodies for flow cytometry analysis. Clinical scoring (on a scale of 0 to 10) was performed based on previously described methods.47 Body weights were measured at specified time points. Mice were euthanized when they developed signs of severe distress or when weight loss exceeded 20% of baseline.
Mouse xenogeneic GVHD with residual B-cell leukemia model
NSG mice were irradiated at 1.2 Gy and received intravenously 5 × 106 freshly isolated human PBMCs and 5 × 105 NALM6-GFP.FFluc (β2mKO) (or 3 × 106 BV173-GFP.FFluc [β2mKO]) on the following day, followed by 5 × 106 control mEmerald-expressing or ADR/CAR-expressing T cells generated from the same PBMC donor 4 days later. Tumor progression was monitored by injecting mice intraperitoneally with 100 μL D-luciferin (30 mg/mL, PerkinElmer Inc) followed by bioluminescence imaging using an IVIS Lumina II imaging system and analyzed by Living Image 4.5 software (Caliper Life Sciences). Body weights were measured at specified time points, and clinical scoring (on a scale of 0 to 10) was based on previously described methods.47 Mice were euthanized when they developed signs of excessive tumor burden or when weight loss exceeded 20% of baseline.
Statistics
Statistical tests are indicated in the figure legends and were performed using Prism 6 software (GraphPad).
Results
Expression of OX40 on activated pathogenic T cells
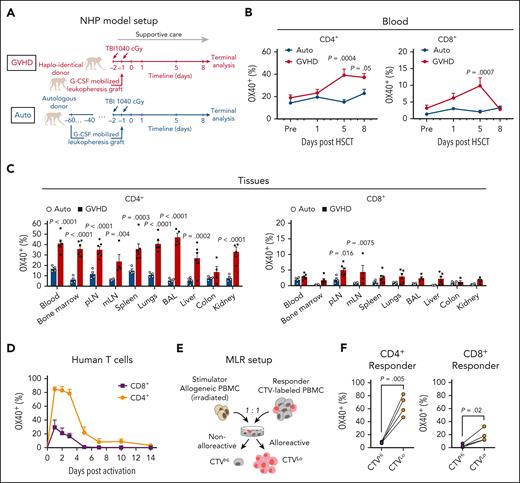
OX40 upregulation has been detected in peripheral blood T cells in GVHD patients following allo-HSCT.48,49 However, its expression on T cells that infiltrate target organs in aGVHD has not been well documented. To validate OX40-targeting in the context of aGVHD, we measured its expression on T cells isolated from rhesus macaques that underwent allo-HSCT without GVHD prophylaxis (Figure 1A), which allowed us to study GVHD biology in an immunosuppression-free setting and obtain data from multiple peripheral tissues that are inaccessible in patients. Allo-HSCT in this NHP model induces aGVHD that closely recapitulates human disease.44,50,51 Compared with control animals receiving autologous bone marrow (Auto), animals in the allogeneic transplantation group developed aGVHD with concomitant OX40 upregulation on T cells in peripheral blood (Figure 1B, supplemental Figure 1A). We also detected elevated levels of OX40 on T cells in lymphoid and nonlymphoid tissues that are commonly infiltrated by host-reactive T cells (Figure 1C). OX40 expression was more prevalent on CD4+ T cells compared with CD8+ T cells (Figure 1B-C) and was predominantly restricted to effector and memory T-cell subsets, reflecting their activated functional state (supplemental Figure 1B-E).
Upregulation of OX40 on activated T cells. (A) Nonhuman primate (NHP) model setup for panels B-C. (B-C) Percentage of OX40+ CD4+ or CD8+ T cells detected in (B) peripheral blood (Auto: n = 6; GVHD: n = 10) at specified time points or (C) tissues upon terminal analysis (Auto: n = 4; GVHD: n = 6). Mean ± standard error of the mean values are shown. In panel C, each dot represents data from an individual animal. P values were calculated using 2-way analysis of variance (ANOVA) followed with Holm-Sidak correction. (D) Percentage of OX40+ primary human T cells within total CD4+ or CD8+ T cell population at specified time points after OKT3/αCD28 stimulation (n = 6). Mean ± standard deviation (SD) values are shown. (E) MLR model setup for panel F. (F) Percentage of OX40+ cells within the specified T cell population on day 7 after MLR setup. Each line connects data from the same pair of stimulator and responder. P values were calculated using paired t test. BAL, bronchoalveolar lavage; mLN, mesenteric lymph node; pLN, popliteal lymph node; TBI, total body irradiation.
Upregulation of OX40 on activated T cells. (A) Nonhuman primate (NHP) model setup for panels B-C. (B-C) Percentage of OX40+ CD4+ or CD8+ T cells detected in (B) peripheral blood (Auto: n = 6; GVHD: n = 10) at specified time points or (C) tissues upon terminal analysis (Auto: n = 4; GVHD: n = 6). Mean ± standard error of the mean values are shown. In panel C, each dot represents data from an individual animal. P values were calculated using 2-way analysis of variance (ANOVA) followed with Holm-Sidak correction. (D) Percentage of OX40+ primary human T cells within total CD4+ or CD8+ T cell population at specified time points after OKT3/αCD28 stimulation (n = 6). Mean ± standard deviation (SD) values are shown. (E) MLR model setup for panel F. (F) Percentage of OX40+ cells within the specified T cell population on day 7 after MLR setup. Each line connects data from the same pair of stimulator and responder. P values were calculated using paired t test. BAL, bronchoalveolar lavage; mLN, mesenteric lymph node; pLN, popliteal lymph node; TBI, total body irradiation.
Next, we evaluated the kinetics of OX40 expression on primary human T cells. OX40 was not detected on resting human T cells but was rapidly upregulated upon in vitro stimulation with plate-bound CD3 and CD28 monoclonal antibodies (Figure 1D, supplemental Figure 2A). Following removal of stimulatory antibodies on day 2, OX40 expression gradually decreased (Figure 1D) and was upregulated again following restimulation (supplemental Figure 2B). Consistent with the observations in the NHP model, CD4+ T cells had a higher magnitude and duration of OX40 upregulation compared with CD8+ T cells (Figure 1D). OX40 expression was also elevated on alloreactive human T cells stimulated in a mixed lymphocyte reaction (MLR) assay in which CellTrace Violet (CTV)-labeled PBMCs (Responder) were mixed with irradiated allogeneic PBMCs (Stimulator) (Figure 1E). OX40 was upregulated on actively dividing (CTVLo) responder T cells compared with minimally divided (CTVHi) T cells, with a higher level of expression on stimulated/proliferating CD4+ T cells compared with CD8+ T cells (Figure 1F). These results suggest that OX40 is a suitable marker for selective targeting of activated pathogenic T cells in aGVHD.
Generation and characterization of OX40-specific alloimmune defense receptor T cells
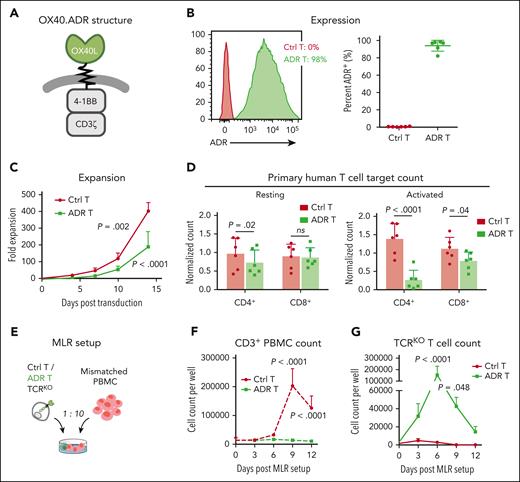
To enable specific targeting of OX40-expressing human T cells, we designed a cytotoxic OX40-specific alloimmune defense receptor (OX40.ADR) consisting of the extracellular domain of OX40L (the natural OX40 ligand) as a binder, connected via spacer and transmembrane regions with intracellular signaling domains of 4-1BB and CD3ζ to elicit T-cell cytotoxicity (Figure 2A). Through retroviral transduction, OX40.ADR was successfully expressed on the surface of activated primary human T cells, with a mean transduction efficiency of 94% (Figure 2B). OX40.ADR T cells expanded following transduction, albeit more slowly than control nontransduced T cells, likely reflecting mild fratricide of OX40.ADR T cells and/or toxicity from ADR-derived tonic 4-1BB signaling.52 This is evidenced by their transient reduction in viability during week 1 (supplemental Figure 3A), minimal detectable surface OX40 expression (supplemental Figure 3B), and decreased percentage of CD4+ T cells (supplemental Figure 3C), likely reflecting their preferential elimination due to higher expression of OX40 (Figure 1D). As a result of 4-1BB and CD3ζ signaling, OX40.ADR T cells were enriched with central memory T cells (TCM)53 and had increased expression of inhibitory receptors LAG-3 and TIM-3 (supplemental Figure 3D-E). OX40.ADR T cells produced a mean 183-fold expansion over 14 days, indicating the feasibility of generating sufficient quantities of ADR T cells for functional analyses or clinical use (Figure 2C). In vitro coculture assays confirmed that OX40.ADR T cells produced specific and robust cytotoxicity against model target CCRF-CEM cells modified to express OX40, with minimal activity against parental (OX40−) CCRF-CEM cells (supplemental Figure 4).
Generation of OX40.ADR T cells and their activity in vitro. (A) Schematic of OX40.ADR structure. (B) Surface expression of OX40.ADR on primary human T cells on day 8 post transduction. Mean ± SD values are shown. Each dot represents data from an individual donor. (C) Ex vivo expansion kinetics of OX40.ADR T cells compared with donor-matched Ctrl T cells. Mean ± SD values from 6 individual donors are shown. P values were calculated using 2-way ANOVA followed with Sidak correction. Only P values < .05 (days 10 and 14) are shown. (D) Normalized residual counts of resting (left) or activated (right) primary human T cell targets after 48 hours of coculture with autologous Ctrl or ADR T cells. Mean ± SD values are shown. Each dot represents data from an individual donor. P values were calculated using 2-way ANOVA followed with Holm-Sidak correction. Not significant (ns), P ≥ .05. (E) Schematic of the MLR model for panels F-G. (F-G) Absolute cell counts of (F) CD3+ PBMC or (G) T cell receptor–edited (TCRKO) T cells measured at indicated time points. Mean ± SD values are shown. P values were calculated using 2-way ANOVA followed with Sidak correction. Ctrl, control nontransduced T cells; ADR, OX40.ADR.
Generation of OX40.ADR T cells and their activity in vitro. (A) Schematic of OX40.ADR structure. (B) Surface expression of OX40.ADR on primary human T cells on day 8 post transduction. Mean ± SD values are shown. Each dot represents data from an individual donor. (C) Ex vivo expansion kinetics of OX40.ADR T cells compared with donor-matched Ctrl T cells. Mean ± SD values from 6 individual donors are shown. P values were calculated using 2-way ANOVA followed with Sidak correction. Only P values < .05 (days 10 and 14) are shown. (D) Normalized residual counts of resting (left) or activated (right) primary human T cell targets after 48 hours of coculture with autologous Ctrl or ADR T cells. Mean ± SD values are shown. Each dot represents data from an individual donor. P values were calculated using 2-way ANOVA followed with Holm-Sidak correction. Not significant (ns), P ≥ .05. (E) Schematic of the MLR model for panels F-G. (F-G) Absolute cell counts of (F) CD3+ PBMC or (G) T cell receptor–edited (TCRKO) T cells measured at indicated time points. Mean ± SD values are shown. P values were calculated using 2-way ANOVA followed with Sidak correction. Ctrl, control nontransduced T cells; ADR, OX40.ADR.
To further evaluate the selectivity of targeting activated T cells by OX40.ADR T cells, we cocultured them with autologous resting or activated T cells. OX40.ADR T cells had minimal activity against resting autologous CD8+ T cells (Figure 2D left). We detected a 25% reduction in mean resting CD4+ T-cell counts upon coculture with OX40.ADR T cells (Figure 2D left), likely due to OX40 upregulation on targets induced by nonspecific stimulation in the presence of control or OX40.ADR T cells (supplemental Figure 5). In contrast, after coculturing OX40.ADR T cells with T-cell targets preactivated with anti-CD3/anti-CD28 monoclonal antibodies, mean CD4+ and CD8+ target counts were reduced by 80% and 29%, respectively (Figure 2D right). Increased cytotoxicity against activated CD4+ T cells is consistent with the pattern of OX40 expression on human T cells (Figure 1D). OX40.ADR T cells produced similar cytotoxicity against CD3/CD28-stimulated conventional CD4+ T cells and regulatory T cells but had minimal activity against resting subsets (supplemental Figure 6).
To assess the ability of OX40.ADR T cells to suppress alloreactive T-cell expansion, we adopted an MLR model in which alloreactive T cells were stimulated with HLA-mismatched T cell receptor–edited control or OX40.ADR T cells (Figure 2E). At the end of the assay, unmodified control T cells were eliminated by expanding alloreactive T cells, whereas OX40.ADR-armed T cells suppressed the expansion of alloreactive T cells and resisted their cytotoxicity (Figure 2F-G). These results suggest that OX40.ADR T cells selectively inhibit the expansion and function of alloreactive T cells.
OX40.ADR T cells prevent progression of fatal xenogeneic GVHD in vivo
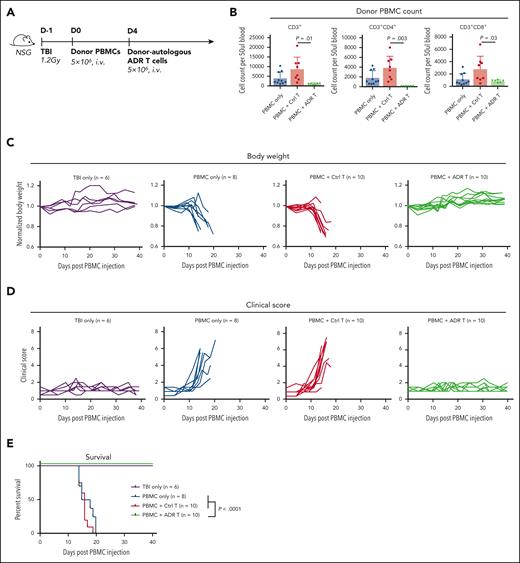
To assess whether OX40.ADR T cells protect against systemic aGVHD in vivo, we adopted an established xenogeneic GVHD model that recreates many aspects of human disease.54,55 Moreover, previous work suggested that OX40 expression was enriched in human T cells from this model compared with unstimulated T cells.55 Here, immunodeficient NSG mice were sublethally irradiated and injected intravenously with human PBMCs to induce rapid-onset, lethal GVHD mediated by xenoreactive human T cells (Figure 3A). Four days after PBMC injection, animals were split into 3 experimental groups, with 2 groups receiving donor-matched T cells either transduced with a control vector (control T) or OX40.ADR. One week following treatment, mice receiving OX40.ADR T cells had significantly lower numbers of CD3+ donor PBMCs in circulation, compared with mice receiving control T cells (Figure 3B). Consistent with our in vitro findings (Figure 2D), OX40.ADR T cells produced higher cytotoxicity against CD4+ T cells in vivo (Figure 3B). Mice receiving OX40.ADR T cells maintained body weights similar to mice without GVHD (that did not receive PBMCs), whereas control untreated mice or animals treated with control T cells rapidly lost weight due to GVHD progression (Figure 3C). We further examined the severity of GVHD symptoms using an established clinical scoring system. Injection of human PBMCs rapidly induced clinical symptoms in all experimental groups, except for animals treated with OX40.ADR T cells (Figure 3D). OX40.ADR T-cell treatment significantly prolonged animal survival (Figure 3E), indicating potent protection against xenogeneic GVHD.
OX40.ADR T cells suppress fatal xenogeneic GVHD in vivo. (A) Schematic of model setup for panels B-E. (B) Absolute counts of specified populations on day 11 post PBMC injection. Mean ± SD values are shown. Each dot represents data from an individual animal. P values were calculated using unpaired t test. (C) Animal body weight (normalized to weight on day 0 of the same animal) at specified time points. Each line represents data from an individual animal. (D) GVHD clinical scores at specified time points. Each line represents data from an individual animal. (E) Animal survival over time. P values were calculated using a log-rank test. TBI, total body irradiation.
OX40.ADR T cells suppress fatal xenogeneic GVHD in vivo. (A) Schematic of model setup for panels B-E. (B) Absolute counts of specified populations on day 11 post PBMC injection. Mean ± SD values are shown. Each dot represents data from an individual animal. P values were calculated using unpaired t test. (C) Animal body weight (normalized to weight on day 0 of the same animal) at specified time points. Each line represents data from an individual animal. (D) GVHD clinical scores at specified time points. Each line represents data from an individual animal. (E) Animal survival over time. P values were calculated using a log-rank test. TBI, total body irradiation.
4-1BB costimulation in the OX40.ADR construct improved in vivo expansion and persistence of ADR T cells (supplemental Figure 7A-B), resulting in better protection against fatal xenogeneic GVHD compared with studies using a first-generation construct (OX40.ADR-1G, supplemental Figure 7C-F). Although OX40.ADR-1G T cells reduced GVHD pathology in target organs, increased infiltration and activity of (second-generation) OX40.ADR T cells resulted in elevated GVHD scores on day 12 (supplemental Figure 7G-H). However, the activity of OX40.ADR T cells did not manifest in any physical signs of GVHD, suggesting the increased pathology scores were, at least in part, driven by increased infiltration and transient local inflammation produced by ADR T cells in target tissues. As the animals receiving the second-generation OX40.ADR T cells were fully protected from GVHD for the duration of the experiment, we used this construct for all remaining studies.
OX40.ADR T cells preserve cytotoxic antiviral T-cell immunity
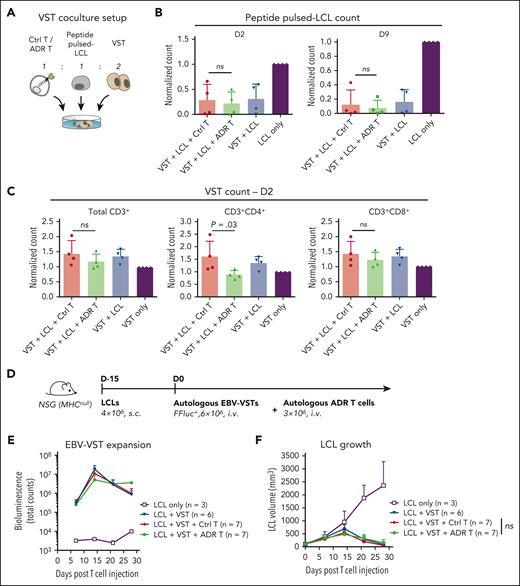
To produce the greatest clinical benefit, treatment for aGVHD should not impair protective donor T-cell responses and preserve antiviral immunity posttransplant. Therefore, we evaluated the activity of OX40.ADR T cells against autologous multivirus-specific T cells (VSTs) that recognize immunodominant epitopes from Epstein-Barr virus (EBV),56,57 cytomegalovirus (CMV),58,59 and adenovirus (AdV),60,61 3 of the most common viruses affecting posttransplant patients62 (Figure 4A). VSTs were expanded through 2 rounds of stimulation with overlapping peptide pools containing immunodominant epitopes of EBV, CMV, and AdV antigens. Autologous EBV-transformed lymphoblastoid cell lines (LCLs) were generated for each donor and pulsed with the same peptide mix, thus modeling virus-infected target cells. When cocultured with autologous VSTs and LCLs (Figure 4A), OX40.ADR T cells did not reduce the ability of VSTs to eliminate LCLs presenting viral antigens (Figure 4B). Expansion of activated VSTs in the presence of ADR T cells was largely preserved, with only a slight reduction observed in CD4+ compartment (Figure 4C), which correlates with the OX40 expression profile in activated VSTs during coculture (supplemental Figure 8).
OX40.ADR T cells preserve cytotoxic antiviral T-cell immunity. (A) Schematic of model setup for panels B-C. Ctrl or OX40.ADR T cells were cocultured with autologous peptide pulsed-lymphoblastoid cell lines (LCLs) (EBV-immortalized lymphoblastoid cell lines pulsed with a mix of EBV, CMV, and AdV peptides) and autologous VSTs. (B) Relative counts of residual peptide pulsed-LCL for specified conditions on day 2 (left) and day 9 (right) post coculture. Counts were normalized to LCL-only conditions. (C) Relative counts of residual VSTs for specified conditions on day 2 post coculture. Counts were normalized to VST-only conditions. In panels B-C, mean ± SD values are shown. Each dot represents data from an individual donor. P values were calculated using 1-way ANOVA followed with Sidak correction. (D) Schematic of model setup for panels E-F. (E) Change of EBV-VST bioluminescence signals overtime. (F) Change of LCL tumor volumes overtime. P value was calculated using 2-way ANOVA followed with Tukey correction. Statistical difference on day 28 is shown. In panels E-F, mean ± SD values are shown. Not significant (ns), P ≥ .05. Ctrl, control nontransduced T cells; FFluc, firefly luciferase; i.v., intravenous injection; s.c., subcutaneous injection.
OX40.ADR T cells preserve cytotoxic antiviral T-cell immunity. (A) Schematic of model setup for panels B-C. Ctrl or OX40.ADR T cells were cocultured with autologous peptide pulsed-lymphoblastoid cell lines (LCLs) (EBV-immortalized lymphoblastoid cell lines pulsed with a mix of EBV, CMV, and AdV peptides) and autologous VSTs. (B) Relative counts of residual peptide pulsed-LCL for specified conditions on day 2 (left) and day 9 (right) post coculture. Counts were normalized to LCL-only conditions. (C) Relative counts of residual VSTs for specified conditions on day 2 post coculture. Counts were normalized to VST-only conditions. In panels B-C, mean ± SD values are shown. Each dot represents data from an individual donor. P values were calculated using 1-way ANOVA followed with Sidak correction. (D) Schematic of model setup for panels E-F. (E) Change of EBV-VST bioluminescence signals overtime. (F) Change of LCL tumor volumes overtime. P value was calculated using 2-way ANOVA followed with Tukey correction. Statistical difference on day 28 is shown. In panels E-F, mean ± SD values are shown. Not significant (ns), P ≥ .05. Ctrl, control nontransduced T cells; FFluc, firefly luciferase; i.v., intravenous injection; s.c., subcutaneous injection.
To evaluate the effect of OX40.ADR activity against antiviral T-cell immunity in vivo, we subcutaneously engrafted immunodeficient NSG (MHCnull) mice with LCLs and then treated them with autologous EBV-specific VSTs (EBV-VSTs) along with ADR T cells (Figure 4D). Here, we used an NSG strain that lacks murine major histocompatibility complex expression to minimize ADR T-cell activation due to xenoreactivity against the murine host. Although OX40.ADR T cells slightly reduced the initial EBV-VST expansion, we observed sustained EBV-VST expansion in mice receiving OX40.ADR T cells at later time points (Figure 4E) and comparable anti-LCL activity between groups receiving unmodified Ctrl T cells and OX40.ADR T cells (Figure 4F). These results suggest OX40.ADR T cells do not ablate antiviral responses, at least in the short term.
OX40.ADR T cells coexpressing a CD19.CAR retain high cytotoxic activity through both chimeric receptors in vitro
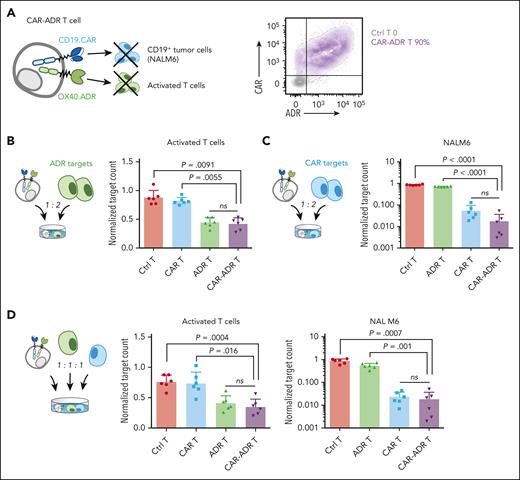
Because relapse and GVHD account for most patient deaths post allo-HSCT,63 we engineered donor T cells to produce both antitumor and anti-GVHD activity directed against recipient malignancy and donor alloreactive T cells, respectively. To enable dual recognition of leukemia and alloreactive T cells, we coexpressed a second-generation CD19-specific CAR (CD19.CAR) on OX40.ADR T cells (CAR-ADR T cells, Figure 5A). We then cocultured CAR-ADR T cells with either autologous activated T cells (OX40.ADR targets) or CD19+OX40− pre-B ALL cell line NALM6 (CD19.CAR targets, supplemental Figure 9) at a 1:2 effector-to-target ratio (Figure 5B-C). CAR-ADR T cells produced robust cytotoxicity against activated T-cell targets, similar to that with singly transduced OX40.ADR T cells (Figure 5B). Likewise, CD19.CAR-mediated T-cell cytotoxicity against NALM6 tumor cells was not inhibited by coexpression of OX40.ADR (Figure 5C). To investigate the activity of CAR-ADR T cells when both receptors were stimulated simultaneously, we cocultured effector T cells with a mixture of activated T cells and tumor targets at a 1:1:1 cellular ratio. Again, CAR-ADR T cells showed cytotoxicity against respective target cells that was as potent as T cells expressing either receptor alone (Figure 5D). Comparable activity between CAR-ADR T cells and singly transduced T cells was also observed upon titrating effector-to-target ratios in a stress-test model (supplemental Figure 10). These results indicate that OX40.ADR-armed CD19.CAR T cells possess the dual-targeting capability and that both receptors elicit cytotoxicity independently.
CAR-ADR T cells effectively eliminate both activated T cells and tumor cells. (A) Schematic of a CAR-ADR T cell (left) and a representative flow plot showing expression of both receptors on primary human T cells (right). (B) Effector T cells and autologous activated T cell targets were cocultured at a 1:2 ratio. Residual target counts (normalized to target-only condition) at 24 hours post coculture are shown. (C) Effector T cells and NALM6 tumor targets were cocultured at a 1:2 ratio. Residual target counts (normalized to target-only condition) at 24 hours post coculture are shown. (D) Effector T cells, autologous activated T cells, and NALM6 were cocultured at a 1:1:1 ratio. Residual target counts (normalized to target-only condition) at 24 hours post coculture are shown. In panels B-D, mean ± SD values are shown. Each dot represents data from an individual donor. P values were calculated using 1-way ANOVA followed with Tukey correction.
CAR-ADR T cells effectively eliminate both activated T cells and tumor cells. (A) Schematic of a CAR-ADR T cell (left) and a representative flow plot showing expression of both receptors on primary human T cells (right). (B) Effector T cells and autologous activated T cell targets were cocultured at a 1:2 ratio. Residual target counts (normalized to target-only condition) at 24 hours post coculture are shown. (C) Effector T cells and NALM6 tumor targets were cocultured at a 1:2 ratio. Residual target counts (normalized to target-only condition) at 24 hours post coculture are shown. (D) Effector T cells, autologous activated T cells, and NALM6 were cocultured at a 1:1:1 ratio. Residual target counts (normalized to target-only condition) at 24 hours post coculture are shown. In panels B-D, mean ± SD values are shown. Each dot represents data from an individual donor. P values were calculated using 1-way ANOVA followed with Tukey correction.
CAR-ADR T cells protect against both xenogeneic GVHD and leukemia progression in vivo
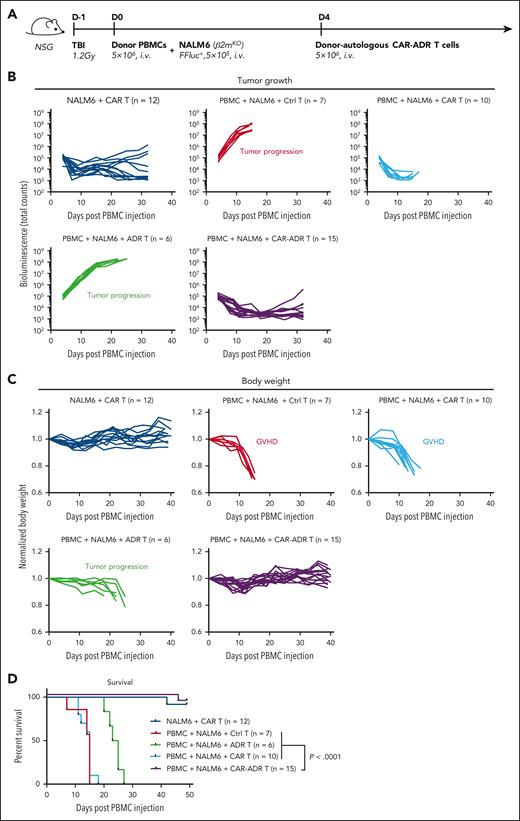
Relapse posttransplant arises from residual disease that survives allo-HSCT conditioning. We modeled minimal residual leukemia post allo-HSCT by co-injecting irradiated NSG mice with human PBMCs and CD19+ NALM6 leukemia. Four days later, we treated animals with T cells expressing CD19.CAR or OX40.ADR or both (Figure 6A). In the absence of human PBMCs, CD19.CAR T cells produced robust antileukemic activity protecting mice from fatal tumor progression (Figure 6B). However, in mice engrafted with human PBMCs, CD19.CAR T cells rendered no protection against GVHD, resulting in rapid weight loss in those animals compared with mice not receiving human PBMCs (Figure 6C light and dark blue lines, respectively). Conversely, mice receiving OX40.ADR T cells had no early onset weight loss but succumbed to rapid leukemia progression (Figure 6C green lines). However, infusion of CAR-ADR T cells prevented both weight loss and leukemia progression (Figure 6C purple lines), prolonging animal survival (Figure 6D).
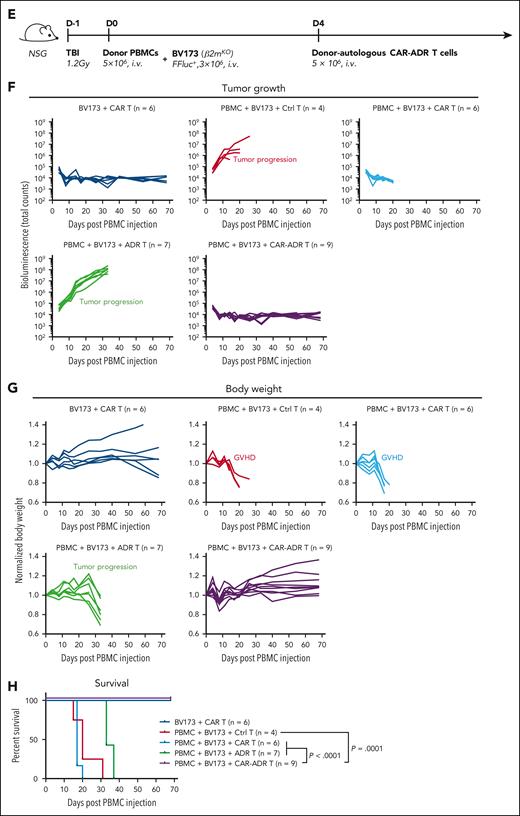
To validate our findings in the NALM6 leukemia model, we evaluated the activity of CAR-ADR T cells in a second model using a CD19+OX40− B cell leukemia line BV173 (Figure 6E, supplemental Figure 9). In line with previous findings, CAR-ADR T cells effectively suppressed both leukemia progression and GVHD development, resulting in sustained tumor eradication (Figure 6F), maintained body weight (Figure 6G), lack of signs of GVHD (supplemental Figure 11), and extended survival (Figure 6H).
Taken together, these results illustrate the dual protective function of OX40.ADR-armed CD19.CAR T cells against both aGVHD and leukemia relapse in vivo.
Discussion
We show that targeting OX40+ T cells with ADR-modified T cells suppresses alloreactivity in vitro and prevents fatal xenogeneic aGVHD in vivo. OX40.ADR T cells coexpressing CD19.CAR provide simultaneous protection against both aGVHD and leukemia relapse in vitro and in vivo while preserving T-cell–mediated antiviral immunity. These results support the feasibility of a dual-pronged cell therapy to mitigate the 2 major complications following allo-HSCT without substantially increasing the risk of infectious complications.
aGVHD has a complex pathogenesis involving multiple cell types at different stages of disease progression. Both CD4+ and CD8+ T cells play important roles in initiating and propagating disease in models of aGVHD.7-9 As both T-cell subsets also are involved in critical protective immunity posttransplant, including tumor- and pathogen-specific responses, untangling T cells that mediate aGVHD from the beneficial T-cell subsets has become a major challenge in the field. Purging alloreactive T-cell subsets from the graft after a brief incubation with the recipient’s cells has been evaluated, both preclinically64-68 and in the clinic,69-73 and achieved a lower frequency and severity of alloreactive responses. However, risks of GVHD, relapse, and infections remained. Similarly, using the posttransplant cyclophosphamide protocol as prophylaxis, which relies on preferential targeting of rapidly proliferating effector T cells in response to alloantigens,22,23 substantially reduced the risk of GVHD and relapse24-26 but predisposed patients to some viral infections.27-29 These studies highlight the need for more selective targeting.
Using engineered donor T cells for targeted elimination of autologous host-reactive T-cell subset is an appealing way to selectively and permanently ablate the pathogenic population. Recent evidence highlighted the ability of engineered T cells, originally developed as cancer treatments, to target pathogenic T cells mediating allo-immune complications such as immune rejection and GVHD. For example, we previously reported the development of a 4-1BB–specific ADR (4-1BB.ADR) that, when expressed on human T cells, enabled selective elimination of alloreactive T cells responsible for immune rejection.45 Another group demonstrated that CAR T cells targeting CD83, an antigen mainly expressed on mature dendritic cells and activated lymphocytes, successfully inhibited the progression of xenogeneic GVHD.74 These approaches prove the feasibility of using engineered T cells to prevent or suppress diseases driven by pathogenic T cells. However, targeting 4-1BB or CD83 with engineered T cells in the context of aGVHD may result in a broader ablation of activated T cells and/or myeloid cells, potentially affecting protective immune responses posttransplant. Therefore, we sought to determine whether more selective ablation of pathogenic T cells produced therapeutic benefit without significantly damaging other components of protective immunity.
The pattern of OX40 expression in activated T cells and its limited expression on cell surface of other critical hematopoietic or nonhematopoietic tissues40 makes it a suitable target for adoptive cell therapy. T cells expressing an OX40-specific ADR were easy to manufacture and had limited fratricide, likely due to both the cis-masking of OX40 by the ADR construct (similar to that observed with the 4-1BB.ADR45) and the minimal expression of OX40 on cytotoxic CD8+ T cells. Indeed, OX40.ADR T cells were enriched for CD8+ T cells at the end of expansion, supporting self-selection for a fratricide-resistant population. Reflecting the pattern of OX40 expression, OX40.ADR T cells predominantly target activated CD4+ T cells with limited activity against activated CD8+ T cells and preserved the broad anti-viral activity of VSTs, in line with previous studies42 and clinical evidence that inherited OX40 deficiency did not compromise immunity against most common childhood pathogens including EBV and CMV.75
Apart from GVHD and opportunistic infections, leukemia relapse is another major complication and a leading cause of death after allo-HSCT. CAR T cells have demonstrated potent antitumor activity against hematologic malignancies resistant to conventional treatments and have been used to mitigate posttransplant relapse in clinical trials.76 Several clinical reports showed reduced GVHD from donor-derived CD19.CAR T-cell transplant recipients,77-79 which has been modeled in subsequent mechanistic studies in mice.80 In the posttransplant setting, CAR T-cell administration requires immediate tapering of immunosuppressive GVHD prophylaxis to ensure maximal potency, which may increase the risk of aGVHD. Therefore, arming CAR T cells with OX40.ADR should enable dual protection against donor alloreactivity and host malignancy and thus may serve as posttransplant prophylaxis for GVHD and relapse in a broad patient cohort.
Despite its tightly regulated specificity, OX40.ADR T cells may produce unwanted “on-target, off-alloreactive T cell” cytotoxicity against subsets of activated Tregs that suppress alloreactivity and helper CD4+ T cells involved in clearance of tumor or pathogens. As we observed strong GVHD protection in our animal model, it is possible that concomitant depletion of pathogenic T cells by OX40.ADR T cells would offset potential suppression of Treg function. Furthermore, the effect of OX40+ T-cell depletion on the incidence of chronic GVHD81 warrants additional studies as OX40 expression is increased on T cells in that condition. Although the xenogeneic GVHD mouse model allows the recreation of many aspects of human disease,54,55 additional studies are needed in immunocompetent models, such as NHPs,44,50,51 which may more closely resemble human physiology. Using Tregs themselves as a cellular platform for ADR targeting may help avoid excessive inflammatory responses and compensate for the potential systemic Treg targeting via OX40. Multiple preclinical studies have described the development of CAR-expressing Tregs82 to treat T-cell–driven diseases, such as multiple sclerosis83 and xenogeneic GVHD,84 thus supporting the feasibility of this approach. Aside from potential activity against activated Tregs, OX40.ADR T cells may damage protective antileukemia and antipathogen responses with a helper T cell component, especially in lymphodepleted patients posttransplant.34,85 Several strategies can be used to overcome these limitations. As shown in this study, coexpressing a tumor-specific CAR on OX40.ADR T cells enables a graft-versus-leukemia effect with simultaneous protection against aGVHD. Similarly, these receptors can be expressed on VSTs manufactured from the stem cell donor to achieve triple-pronged activity. Long-term damage can be minimized by limiting the persistence of OX40.ADR-expressing T cells either by configuring CAR/ADR signaling (eg, by modifying costimulation86,87) or by incorporating a suicide switch.88
In conclusion, we showed that a single engineered T-cell product can mitigate both aGVHD and leukemic relapse after allo-HSCT. Depletion of OX40+ activated T cells with OX40.ADR T cells effectively prevents fatal xenogeneic GVHD, and T cells coexpressing OX40.ADR and a CAR provide dual protection against GVHD and leukemia relapse. Our studies support the feasibility of using donor-derived therapeutic T cells to reduce transplant-related mortalities and improve outcomes in patients post allo-HSCT. More broadly, the current study and our previous work using 4-1BB.ADR to prevent immune rejection demonstrate the effectiveness of ADR T cells against different types of pathogenic T cells. Through careful selection of the target molecule, the application of ADR T cells may be further expanded to T-cell–mediated autoimmune diseases such as multiple sclerosis and type 1 diabetes. Furthermore, ADR T cells can be used as a tool to selectively target specific populations of pathogenic T cells to better understand their role in alloimmune or autoimmune disease pathogenesis and inform targeted therapy approaches in T-cell–driven pathologies.
Acknowledgments
The authors thank David Quach, Sandhya Sharma, and Naren Mehta in the Cliona Rooney lab for the K562-CS cell line, LCLs, and instructions on VST generation; Cheng-Yen Chang in the Farrah Kheradmand lab and M. Sayeeduddin, Shahida Salar, and Zahida Sayeeduddin in the Baylor College of Medicine Pathology and Histology Core for tissue processing and H&E staining; and Catherine Gillespie for editing the manuscript.
This project was supported by the Leukemia and Lymphoma Society Translational Research Award #6566, ASTCT New Investigator Award, CIBMTR/Be the Match Foundation Amy Strelzer Manasevit Research Program, and the National Institute of Health (F99CA253757, P50CA126752, P30 CA125123, 2U19 AI051731, 2R01 HL095791).
Authorship
Contribution: F.M. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. N.W. performed experiments and provided feedback on experimental design and the manuscript. K.I.O. and P.M.B. designed and performed experiments and analyzed data. X.D. provided guidance on mouse models and aided in data collection. E.H. offered technical guidance on mouse models and provided feedback on the manuscript. A.P.-M. performed histopathology scoring and helped with data interpretation. R.R.J., H.E.H., and L.S.K. advised on the study and edited the manuscript. M.K.B. provided guidance and feedback on the study and edited the manuscript. V.T. provided feedback, designed experiments, and wrote the manuscript. M.M. conceptualized, directed, and funded the study; designed ADR constructs; designed experiments; analyzed and interpreted data; and wrote the manuscript.
Conflicts-of-interest disclosure: H.E.H. is a cofounder with equity in Allovir and Marker Therapeutics; serves on advisory boards for Gilead Sciences, GSK, Tessa Therapeutics, Fresh Wind Biotherapeutics, Novartis, and Kiadis; and has received research funding from Tessa Therapeutics and Kuur Therapeutics. M.K.B. is a cofounder with equity in Allovir, Marker Therapeutics, and Tessa Therapeutics and serves on advisory boards for Tessa Therapeutics, Allogene Therapeutics, Memgen, Kuur Therapeutics, Walking Fish Therapeutics, Tscan, Abintus, and Turnstone Biologics. M.M., F.M., N.W., and M.K.B. are coinventors on a patent related to ADRs and methods of their use that is licensed to Fate Therapeutics and Allogene Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Maksim Mamonkin, Baylor College of Medicine, 1102 Bates Ave, Suite 1770.02, Houston, TX 77030; e-mail: mamonkin@bcm.edu.
References
Author notes
All data generated for this manuscript will be made available upon reasonable request to the corresponding author, Maksim Mamonkin.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.